Micro- and Nanostructures of Agave Fructans to Stabilize Compounds of High Biological Value via Electrohydrodynamic Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Polymer Solutions
2.3. Obtaining Fibers by Electrospinning
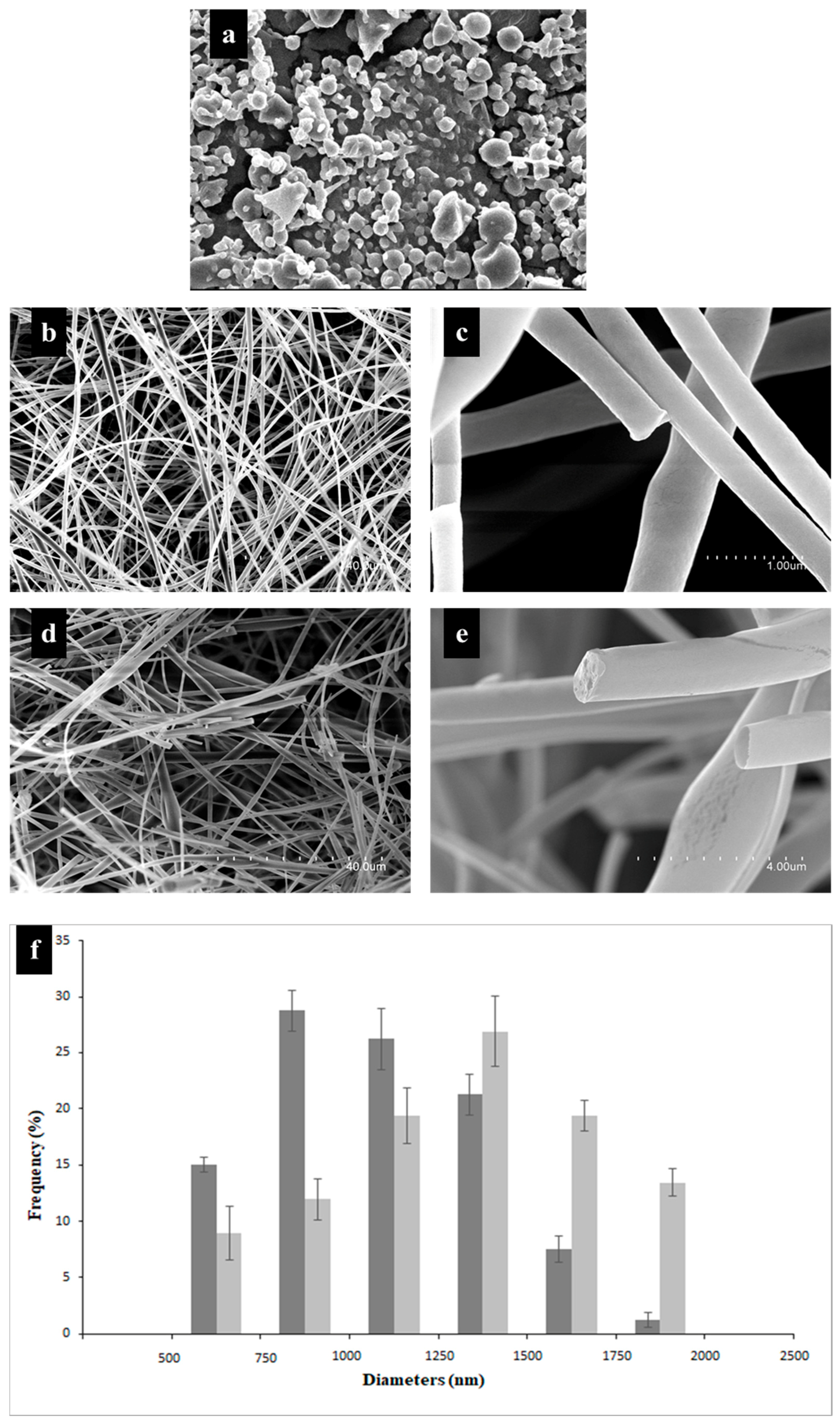
2.4. Morphology Analysis through SEM
2.5. Loading and Encapsulation Efficiency
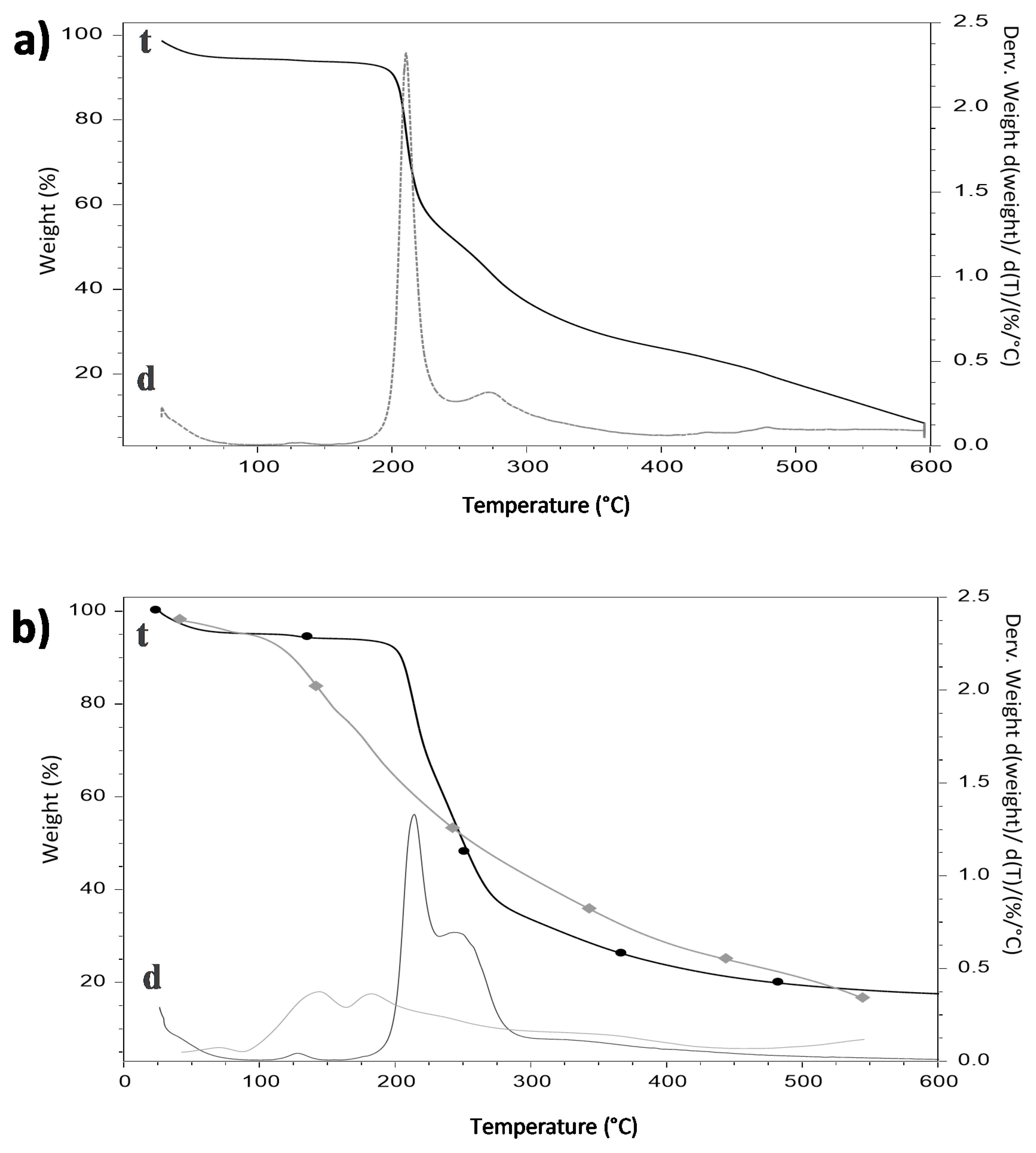
2.6. Thermogravimetric Analysis (TGA)
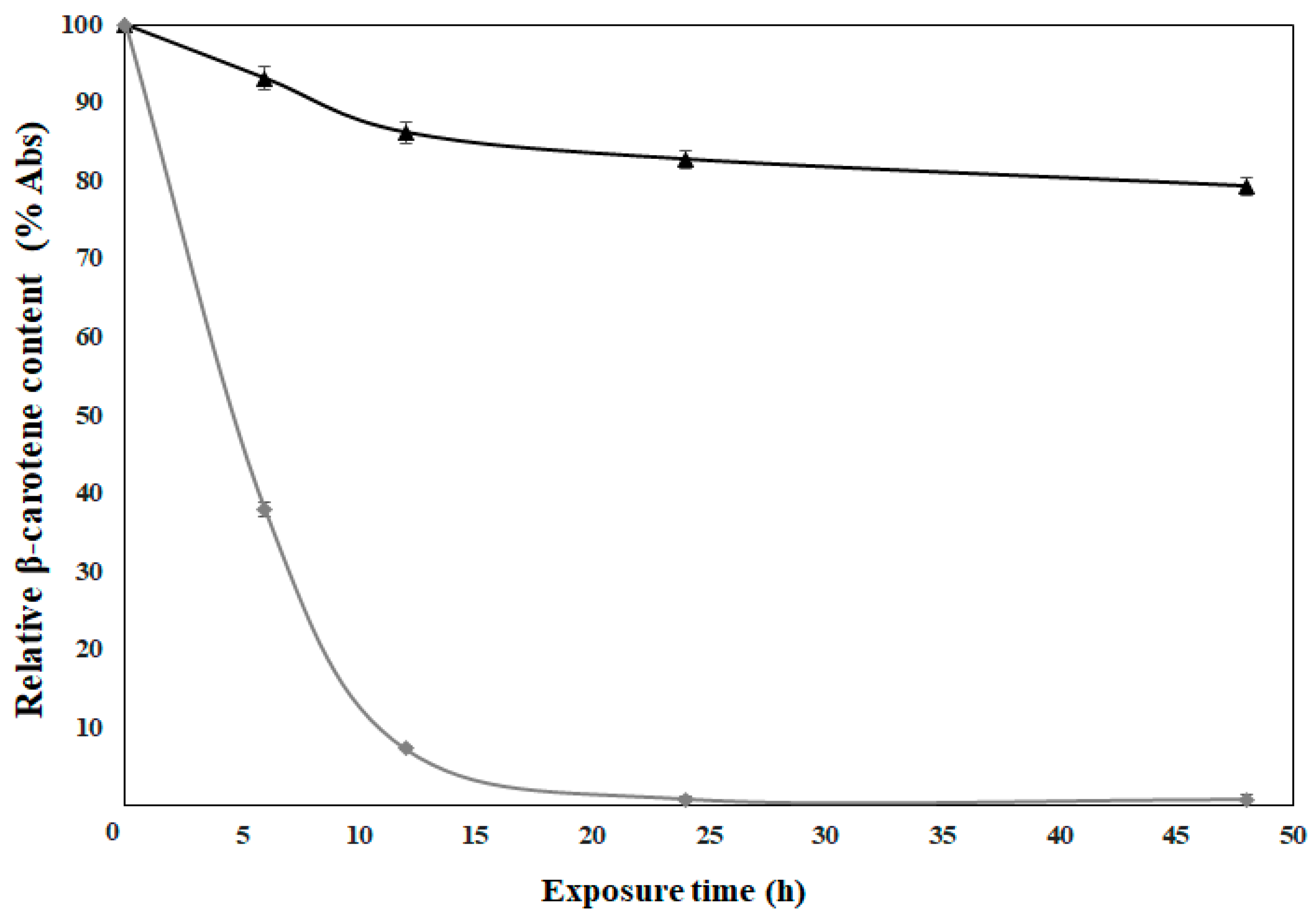
2.7. UV Photostability
2.8. Statistic Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Polymer Solutions
3.2. Micro-Nanofiber Formation Process
3.3. Morphology Analysis
3.4. Loading and Encapsulation Efficiency (LE and EE)
3.5. Thermogravimetric Analysis
3.6. Photostability Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Apolinário, A.C.; De Lima Damasceno, B.P.G.; De Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; Da Silva, J.A. Inulin-Type Fructans: A Review on Different Aspects of Biochemical and Pharmaceutical Technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by Spray Drying of Bioactive Compounds from Cactus Pear (Opuntia Ficus-Indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Bostyn, S.; Flores-Montaño, J.L.; Camacho-Ruiz, R.M. Size-Exclusion Chromatography (HPLC-SEC) Technique Optimization by Simplex Method to Estimate Molecular Weight Distribution of Agave Fructans. Food Chem. 2017, 237, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Waleckx, E.; Gschaedler, A.; Colonna-Ceccaldi, B.; Monsan, P. Hydrolysis of Fructans from Agave Tequilana Weber Var. Azul during the Cooking Step in a Traditional Tequila Elaboration Process. Food Chem. 2008, 108, 40–48. [Google Scholar] [CrossRef]
- García Gamboa, R.; Ortiz Basurto, R.I.; Calderón Santoyo, M.; Bravo Madrigal, J.; Ruiz Álvarez, B.E.; González Avila, M. In Vitro Evaluation of Prebiotic Activity, Pathogen Inhibition and Enzymatic Metabolism of Intestinal Bacteria in the Presence of Fructans Extracted from Agave: A Comparison Based on Polymerization Degree. LWT Food Sci. Technol. 2018, 92, 380–387. [Google Scholar] [CrossRef]
- Farías-Cervantes, V.S.; Chávez-Rodríguez, A.; Delgado-Licon, E.; Aguilar, J.; Medrano-Roldan, H.; Andrade-González, I. Effect of Spray Drying of Agave Fructans, Nopal Mucilage and Aloe Vera Juice. J. Food Process. Preserv. 2017, 41, e13027. [Google Scholar] [CrossRef]
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Diaz, G. Molecular Structures of Fructans from Agave Tequilana Weber Var. Azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef]
- Da Fonseca Contado, E.W.N.; de Rezende Queiroz, E.; Rocha, D.A.; Fraguas, R.M.; Simao, A.A.; Botelho, L.N.S.; de Fatima Abreu, A.; de Abreu, M.A.B.C.M.P. Extraction, Quantification and Degree of Polymerization of Yacon (Smallanthus Sonchifolia) Fructans. Afr. J. Biotechnol. 2015, 14, 1783–1789. [Google Scholar] [CrossRef]
- Carranza, C.O.; Fernandez, A.Á.; Bustillo Armendáriz, G.R.; López-Munguía, A. Processing of Fructans and Oligosaccharides from Agave Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Toriz, G.; Delgado, E.; Zúñiga, V. A Proposed Chemical Structure for Fructans from Blue Agave Plant (Tequilana Weber Var. Azul). Rev. Electrónica Y Tecnológica e-Gnosis 2007, 5, 1. [Google Scholar]
- Ortiz-Basurto, R.I.; Rubio-Ibarra, M.E.; Ragazzo-Sanchez, J.A.; Beristain, C.I.; Jiménez-Fernández, M. Microencapsulation of Eugenia Uniflora L. Juice by Spray Drying Using Fructans with Different Degrees of Polymerisation. Carbohydr. Polym. 2017, 175, 603–609. [Google Scholar] [CrossRef]
- Farías Cervantes, V.S.; Delgado Lincon, E.; Solís Soto, A.; Medrano Roldan, H.; Andrade González, I. Effect of Spray Drying Temperature and Agave Fructans Concentration as Carrier Agent on the Quality Properties of Blackberry Powder. Int. J. Food Eng. 2016, 12, 451–459. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A Novel Nano-Encapsulation Approach for Bioactive Compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Le Corre-Bordes, D.; Hofman, K.; Hall, B. Guide to Electrospinning Denatured Whole Chain Collagen from Hoki Fish Using Benign Solvents. Int. J. Biol. Macromol. 2018, 112, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, B.; Tucker, N. Fundamentals of Electrospinning as a Novel Delivery Vehicle for Bioactive Compounds in Food Nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Ramos-Hernández, J.; Ragazzo-Sánchez, J.; Calderón-Santoyo, M.; Ortiz-Basurto, R.; Prieto, C.; Lagaron, J. Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials 2018, 8, 868. [Google Scholar] [CrossRef]
- Fernandez, A.; Torres-Giner, S.; Lagaron, J.M. Novel Route to Stabilization of Bioactive Antioxidants by Encapsulation in Electrospun Fibers of Zein Prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; McClements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-Carotene in Wheat Gluten Nanoparticle-Xanthan Gum-Stabilized Pickering Emulsions: Enhancement of Carotenoid Stability and Bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Quintanar-Guerrero, D.; Flores-Minutti, J.J.; Gutiérrez-Cortez, E.; Zambrano-Zaragoza, M.L. Nanocapsules of β-Carotene: Thermal Degradation Kinetics in a Scraped Surface Heat Exchanger (SSHE). LWT Food Sci. Technol. 2015, 60, 124–130. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Electrospinning of Whey and Soy Protein Mixed with Maltodextrin—Influence of Protein Type and Ratio on the Production and Morphology of Fibers. Food Hydrocoll. 2019, 93, 206–214. [Google Scholar] [CrossRef]
- Hulsey, S.; Absar, S.; Choi, H. Comparative Study of Polymer Dissolution Techniques for Electrospinning. Procedia Manuf. 2017, 10, 652–661. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of Polysaccharides for Regenerative Medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable Polymers for Electrospinning: Towards Biomedical Applications. Mater. Sci. Eng. C 2015, 45, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.Y.; Yoon, G.J.; Lee, S.W.; Kim, H.W. Effect of Humidity and Benign Solvent Composition on Electrospinning of Collagen Nanofibrous Sheets. Mater. Lett. 2016, 181, 136–139. [Google Scholar] [CrossRef]
- Cai, X.; Du, X.; Cui, D.; Wang, X.; Yang, Z.; Zhu, G. Improvement of Stability of Blueberry Anthocyanins by Carboxymethyl Starch/Xanthan Gum Combinations Microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Ge, W.; Li, D.; Chen, M.; Wang, X.; Liu, S.; Sun, R. Characterization and Antioxidant Activity of β-Carotene Loaded Chitosan-Graft-Poly (Lactide) Nanomicelles. Carbohydr. Polym. 2015, 117, 169–176. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Whey Protein Capsules Obtained through Electrospraying for the Encapsulation of Bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Balaguer, M.P.; Gavara, R.; Hernandez-Munoz, P. Formation of Zein Nanoparticles by Electrohydrodynamic Atomization: Effect of the Main Processing Variables and Suitability for Encapsulating the Food Coloring and Active Ingredient Curcumin. Food Hydrocoll. 2012, 28, 82–91. [Google Scholar] [CrossRef]
- Peinado, I.; Mason, M.; Romano, A.; Biasioli, F.; Scampicchio, M. Stability of β-Carotene in Polyethylene Oxide Electrospun Nanofibers. Appl. Surf. Sci. 2016, 370, 111–116. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Urias-Silvas, J.E. Thermal Properties of Agave Fructans (Agave Tequilana Weber Var. Azul). Carbohydr. Polym. 2012, 87, 2671–2676. [Google Scholar] [CrossRef]
- De Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid Encapsulation Structures Based on β-Carotene-Loaded Nanoliposomes within Electrospun Fibers. Colloids Surf. B Biointerfaces 2015, 134, 475–482. [Google Scholar] [CrossRef] [PubMed]
| Parameter | 70% HDPAF without β-Carotene | 70% HDPAF with β-Carotene |
|---|---|---|
| Viscosity 1 (Pa·s) | 3.69 ± 0.05 a | 3.36 ± 0.03 a |
| Surface tension (mN/m) | 30.1 ± 0.1 b | 29.6 ± 0.2 b |
| Electrical conductivity (mS/cm) | 5.54 ± 0.01 c | 7.30 ± 0.03 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Salas, C.N.; Prieto, C.; Calderón-Santoyo, M.; Lagarón, J.M.; Ragazzo-Sánchez, J.A. Micro- and Nanostructures of Agave Fructans to Stabilize Compounds of High Biological Value via Electrohydrodynamic Processing. Nanomaterials 2019, 9, 1659. https://doi.org/10.3390/nano9121659
Cruz-Salas CN, Prieto C, Calderón-Santoyo M, Lagarón JM, Ragazzo-Sánchez JA. Micro- and Nanostructures of Agave Fructans to Stabilize Compounds of High Biological Value via Electrohydrodynamic Processing. Nanomaterials. 2019; 9(12):1659. https://doi.org/10.3390/nano9121659
Chicago/Turabian StyleCruz-Salas, Carla N., Cristina Prieto, Montserrat Calderón-Santoyo, José M. Lagarón, and Juan A. Ragazzo-Sánchez. 2019. "Micro- and Nanostructures of Agave Fructans to Stabilize Compounds of High Biological Value via Electrohydrodynamic Processing" Nanomaterials 9, no. 12: 1659. https://doi.org/10.3390/nano9121659
APA StyleCruz-Salas, C. N., Prieto, C., Calderón-Santoyo, M., Lagarón, J. M., & Ragazzo-Sánchez, J. A. (2019). Micro- and Nanostructures of Agave Fructans to Stabilize Compounds of High Biological Value via Electrohydrodynamic Processing. Nanomaterials, 9(12), 1659. https://doi.org/10.3390/nano9121659







