Redox Control of IL-6-Mediated Dental Pulp Stem-Cell Differentiation on Alginate/Hydroxyapatite Biocomposites for Bone Ingrowth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Alginate/HAp Composites (Alg/HAp Scaffolds)
2.2. Cell Culture of DPSCs on Alg/HAp Scaffolds
2.3. Alg/HAp Scaffold Preparation for Cell Culture and Cell Seeding
2.4. Protein Extraction and Quantification
2.5. Immunoblot Analysis
2.6. Cytokine Assays
2.7. Statistical Analysis
3. Results
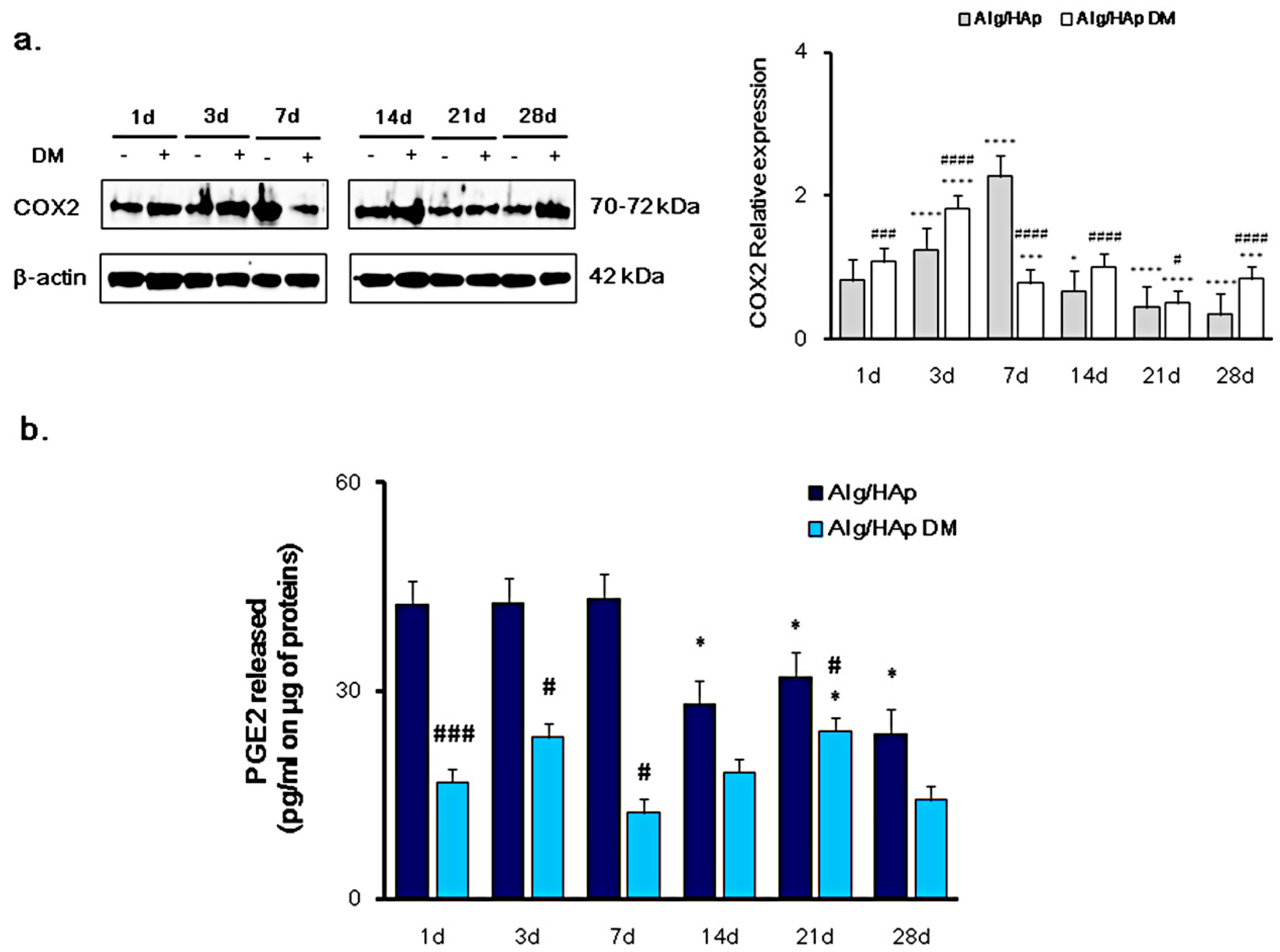
3.1. Cyclooxygenase-2 and PGE2 Modulation in DPSC Growth onto Alg/HAp Scaffolds
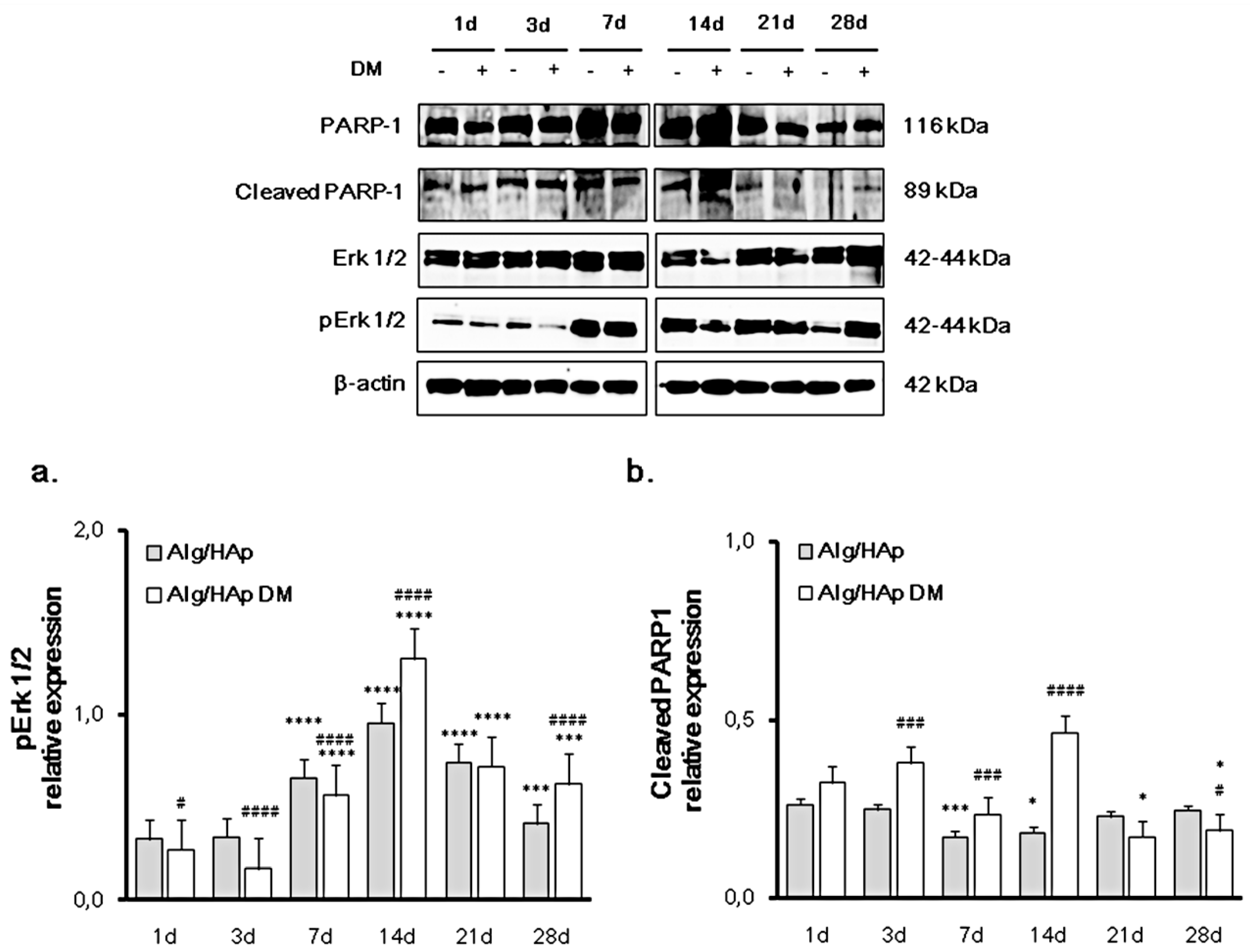
3.2. Erk 1/2 Phosphorylation and PARP-1 Cleavage in the DPSC/Scaffold Model
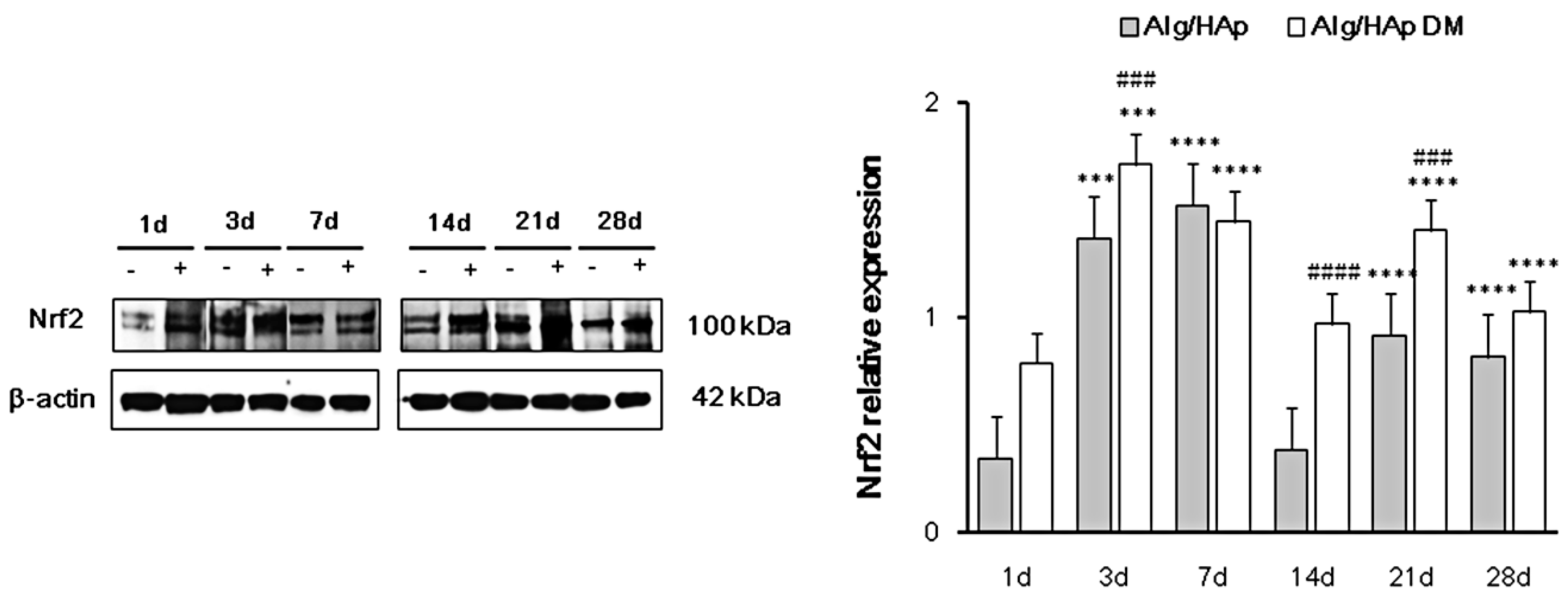
3.3. Nrf2 Early Activation in DPSCs in the Presence of Alg/HAp Scaffolds
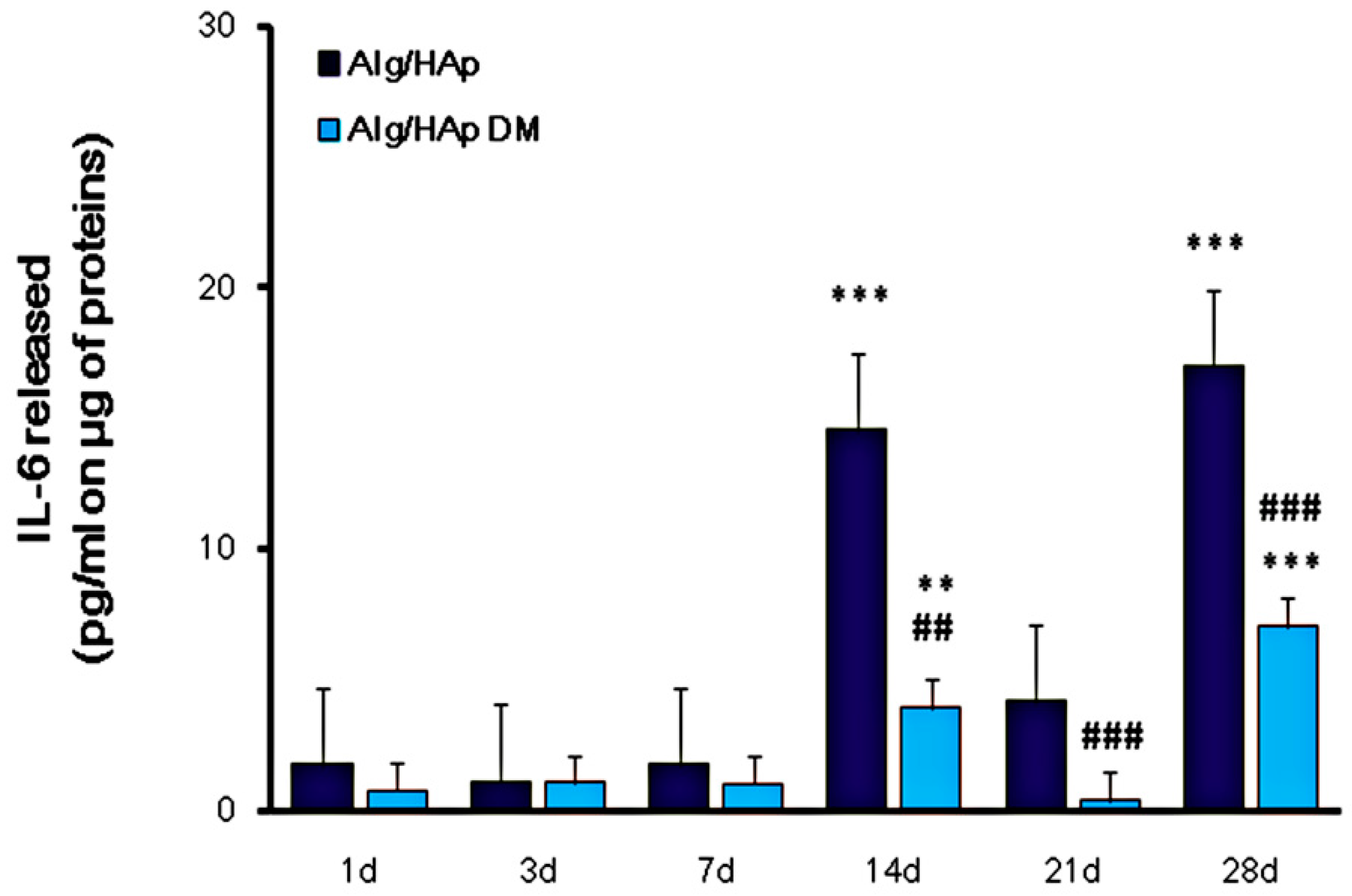
3.4. Influence of Alg/HAp Scaffolds on IL-6 Released from DPSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deshpande, A.S.; Fang, P.A.; Zhang, X.; Jayaraman, T.; Sfeir, C.; Beniash, E. Primary structure and phosphorylation of dentin matrix protein 1 (DMP1) and dentin phosphophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules 2011, 12, 2933–2945. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. (Elite Ed.) 2011, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [PubMed]
- Amghar-Maach, S.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Regeneration of periodontal bone defects with dental pulp stem cells grafting: Systematic Review. J. Clin. Exp. Dent. 2019, 11, e373–e381. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Monroy-García, A.; Ledesma-Martínez, E.; Mendoza-Núñez, V.M. Mesenchymal Stem Cells of Dental Origin for Inducing Tissue Regeneration in Periodontitis: A Mini-Review. Int. J. Mol. Sci. 2018, 19, 944. [Google Scholar] [CrossRef]
- Kondo, M.; Yamaoka, K.; Sakata, K.; Sonomoto, K.; Lin, L.; Nakano, K.; Tanaka, Y. Contribution of the interleukin-6/STAT-3 signaling pathway to chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheumatol. 2015, 67, 1250–1260. [Google Scholar] [CrossRef]
- Xie, Z.; Tang, S.; Ye, G.; Wang, P.; Li, J.; Liu, W.; Li, M.; Wang, S.; Wu, X.; Cen, S.; et al. Interleukin-6/interleukin-6 receptor complex promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 13. [Google Scholar] [CrossRef]
- Caetano-Lopes, J.; Lopes, A.; Rodrigues, A.; Fernandes, D.; Perpetuo, I.P.; Monjardino, T.; Lucas, R.; Monteiro, J.; Konttinen, Y.T.; Canhao, H.; et al. Upregulation of inflammatory genes and downregulation of sclerostin gene expression are key elements in the early phase of fragility fracture healing. PLoS ONE 2011, 6, e16947. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef]
- Grasman, J.M.; Zayas, M.J.; Page, R.L.; Pins, G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015, 25, 2–15. [Google Scholar] [CrossRef]
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D Printing of Bioceramic Scaffolds—Barriers to the Clinical Translation: From Promise to Reality, and Future Perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/Hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Gallorini, M.; Di Nisio, C.; Marsich, E.; Di Pietro, R.; Schweikl, H.; Cataldi, A. Alginate/Hydroxyapatite-Based Nanocomposite Scaffolds for Bone Tissue Engineering Improve Dental Pulp Biomineralization and Differentiation. Stem Cells Int. 2018, 2018, 9643721. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Berardi, A.C.; Gissi, C.; Cataldi, A.; Osti, L. Nrf2-mediated cytoprotective effect of four different hyaluronic acids by molecular weight in human tenocytes. J. Drug Target. 2019, 1–13. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Kandasamy, M.; Mak, K.K.; Devadoss, T.; Thanikachalam, P.V.; Sakirolla, R.; Choudhury, H.; Pichika, M.R. Construction of a novel quinoxaline as a new class of Nrf2 activator. BMC Chem. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Haversath, M.; Catelas, I.; Li, X.; Tassemeier, T.; Jäger, M. PGE₂ and BMP-2 in bone and cartilage metabolism: 2 intertwining pathways. Can. J. Physiol. Pharmacol. 2012, 90, 1434–1445. [Google Scholar] [CrossRef]
- De Colli, M.; Radunovic, M.; Zizzari, V.L.; di Giacomo, V.; Di Nisio, C.; Piattelli, A.; Calvo Guirado, J.L.; Zavan, B.; Cataldi, A.; Zara, S. Osteoblastic differentiating potential of dental pulp stem cells in vitro cultured on a chemically modified microrough titanium surface. Dent. Mater. J. 2018, 37, 197–205. [Google Scholar] [CrossRef]
- Angelova Volponi, A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth Repair and Regeneration. Curr. Oral Health Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental Implant Complications. Semin. Ultrasound CT MR 2015, 36, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Gallorini, M.; Di Giulio, M.; Guarnieri, S.; Mariggiò, M.A.; Traini, T.; Di Pietro, R.; Cellini, L.; Marsich, E.; Sancilio, S. Adhesion of human gingival fibroblasts/Streptococcus mitis Co-Culture on the nanocomposite system Chitlac-nAg. J. Mater. Sci. Mater. Med. 2016, 27, 88. [Google Scholar] [CrossRef] [PubMed]
- Radunovic, M.; De Colli, M.; De Marco, P.; Di Nisio, C.; Fontana, A.; Piattelli, A.; Cataldi, A.; Zara, S. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J. Biomed. Mater. Res. A 2017, 105, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Rapino, M.; Di Valerio, V.; Zara, S.; Gallorini, M.; Marconi, G.D.; Sancilio, S.; Marsich, E.; Ghinassi, B.; di Giacomo, V.; Cataldi, A. Chitlac-coated Thermosets Enhance Osteogenesis and Angiogenesis in a Co-culture of Dental Pulp Stem Cells and Endothelial Cells. Nanomaterials 2019, 9, 928. [Google Scholar] [CrossRef]
- Hossein-Khannazer, N.; Hashemi, S.M.; Namaki, S.; Ghanbarian, H.; Sattari, M.; Khojasteh, A. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019, 216, 111–118. [Google Scholar] [CrossRef]
- Chandran, S.V.; Vairamani, M.; Selvamurugan, N. Osteostimulatory effect of biocomposite scaffold containing phytomolecule diosmin by Integrin/FAK/ERK signaling pathway in mouse mesenchymal stem cells. Sci. Rep. 2019, 9, 11900. [Google Scholar] [CrossRef]
- Cohen-Armon, M.; Yeheskel, A.; Pascal, J.M. Signal-induced PARP1-Erk synergism mediates IEG expression. Signal Transduct. Target Ther. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, X.; Zhu, Y.; Shu, T.; Han, X. Identification of PARP-1 as one of the transcription factors binding to the repressor element in the promoter region of COX-2. Arch. Biochem. Biophys. 2011, 505, 123–129. [Google Scholar] [CrossRef]
- Schweikl, H.; Gallorini, M.; Pöschl, G.; Urmann, V.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W. Functions of transcription factors NF-κB and Nrf2 in the inhibition of LPS-stimulated cytokine release by the resin monomer HEMA. Dent. Mater. 2018, 34, 1661–1678. [Google Scholar] [CrossRef]
- Gómez-Puerto, M.C.; Verhagen, L.P.; Braat, A.K.; Lam, E.W.; Coffer, P.J.; Lorenowicz, M.J. Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation. Autophagy 2016, 12, 1804–1816. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Huang, Y.; Zhang, Y.; Liu, W.; Wang, G.; Zhang, Q.; Wang, G.; Yang, Y.; Li, H.; et al. NRF2 overexpression in mesenchymal stem cells induces stem-cell marker expression and enhances osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2017, 491, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Wozney, J.M. Biological mediators for periodontal regeneration. Periodontology 2000, 19, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Zara, S.; De Colli, M.; di Giacomo, V.; Zizzari, V.L.; Di Nisio, C.; Di Tore, U.; Salini, V.; Gallorini, M.; Tetè, S.; Cataldi, A. Zoledronic acid at subtoxic dose extends osteoblastic stage span of primary human osteoblasts. Clin. Oral Investig. 2015, 19, 601–611. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancilio, S.; Marsich, E.; Schweikl, H.; Cataldi, A.; Gallorini, M. Redox Control of IL-6-Mediated Dental Pulp Stem-Cell Differentiation on Alginate/Hydroxyapatite Biocomposites for Bone Ingrowth. Nanomaterials 2019, 9, 1656. https://doi.org/10.3390/nano9121656
Sancilio S, Marsich E, Schweikl H, Cataldi A, Gallorini M. Redox Control of IL-6-Mediated Dental Pulp Stem-Cell Differentiation on Alginate/Hydroxyapatite Biocomposites for Bone Ingrowth. Nanomaterials. 2019; 9(12):1656. https://doi.org/10.3390/nano9121656
Chicago/Turabian StyleSancilio, Silvia, Eleonora Marsich, Helmut Schweikl, Amelia Cataldi, and Marialucia Gallorini. 2019. "Redox Control of IL-6-Mediated Dental Pulp Stem-Cell Differentiation on Alginate/Hydroxyapatite Biocomposites for Bone Ingrowth" Nanomaterials 9, no. 12: 1656. https://doi.org/10.3390/nano9121656
APA StyleSancilio, S., Marsich, E., Schweikl, H., Cataldi, A., & Gallorini, M. (2019). Redox Control of IL-6-Mediated Dental Pulp Stem-Cell Differentiation on Alginate/Hydroxyapatite Biocomposites for Bone Ingrowth. Nanomaterials, 9(12), 1656. https://doi.org/10.3390/nano9121656







