Effect of Heterointerface on NO2 Sensing Properties of In-Situ Formed TiO2 QDs-Decorated NiO Nanosheets
Abstract
1. Introduction
2. Experimental
2.1. Sources of TiO2 Nanoparticles of Different Sizes
2.2. Synthesis of the NiO Nanosheets
2.3. Synthesis of TiO2 Nanoparticle-Decorated NiO Nanosheets
2.4. Characterization
2.5. Fabrication of Gas Sensors
2.6. Gas Sensing Performance Testing
3. Results and Discussion
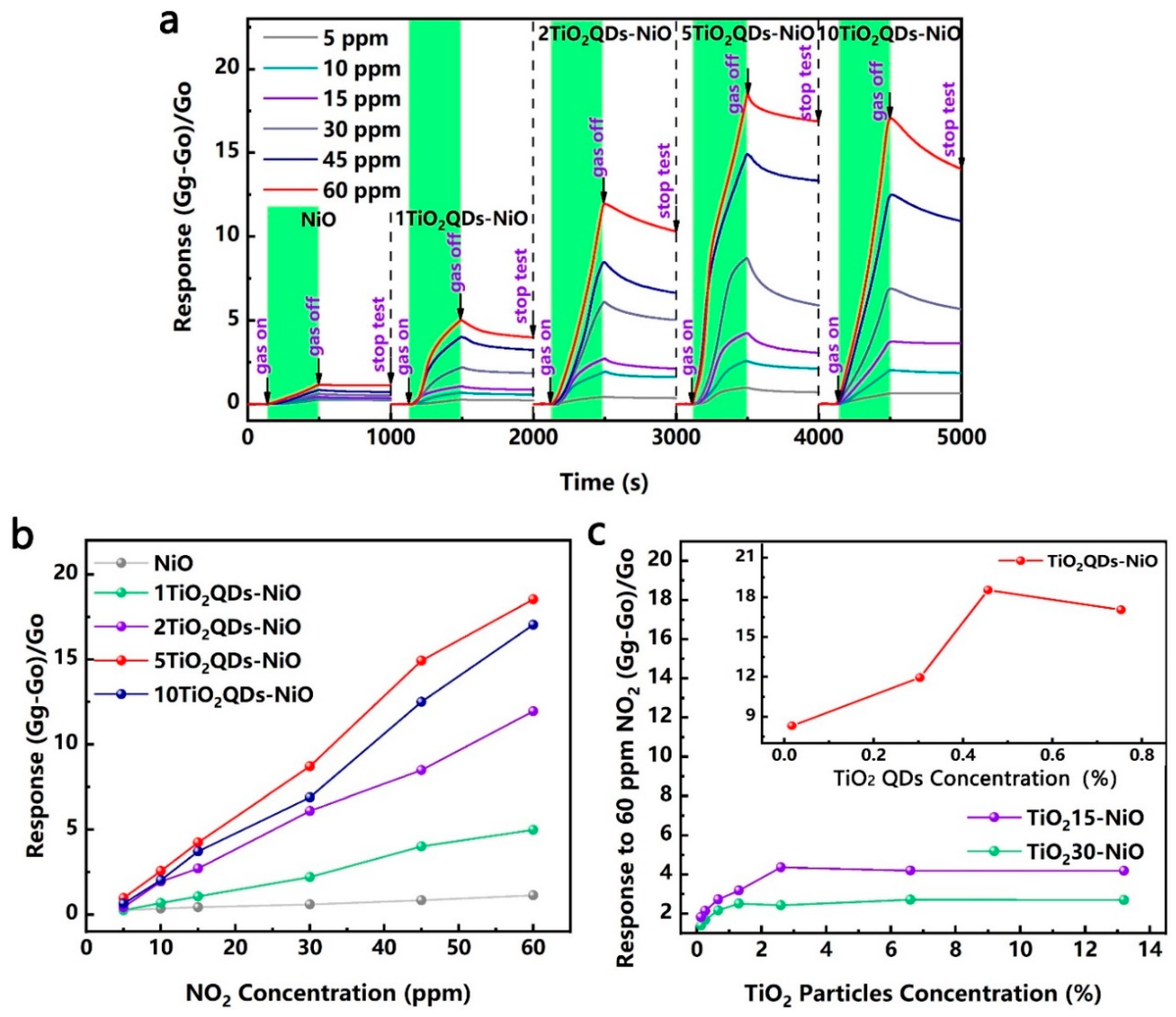
3.1. Gas Sensing Performances
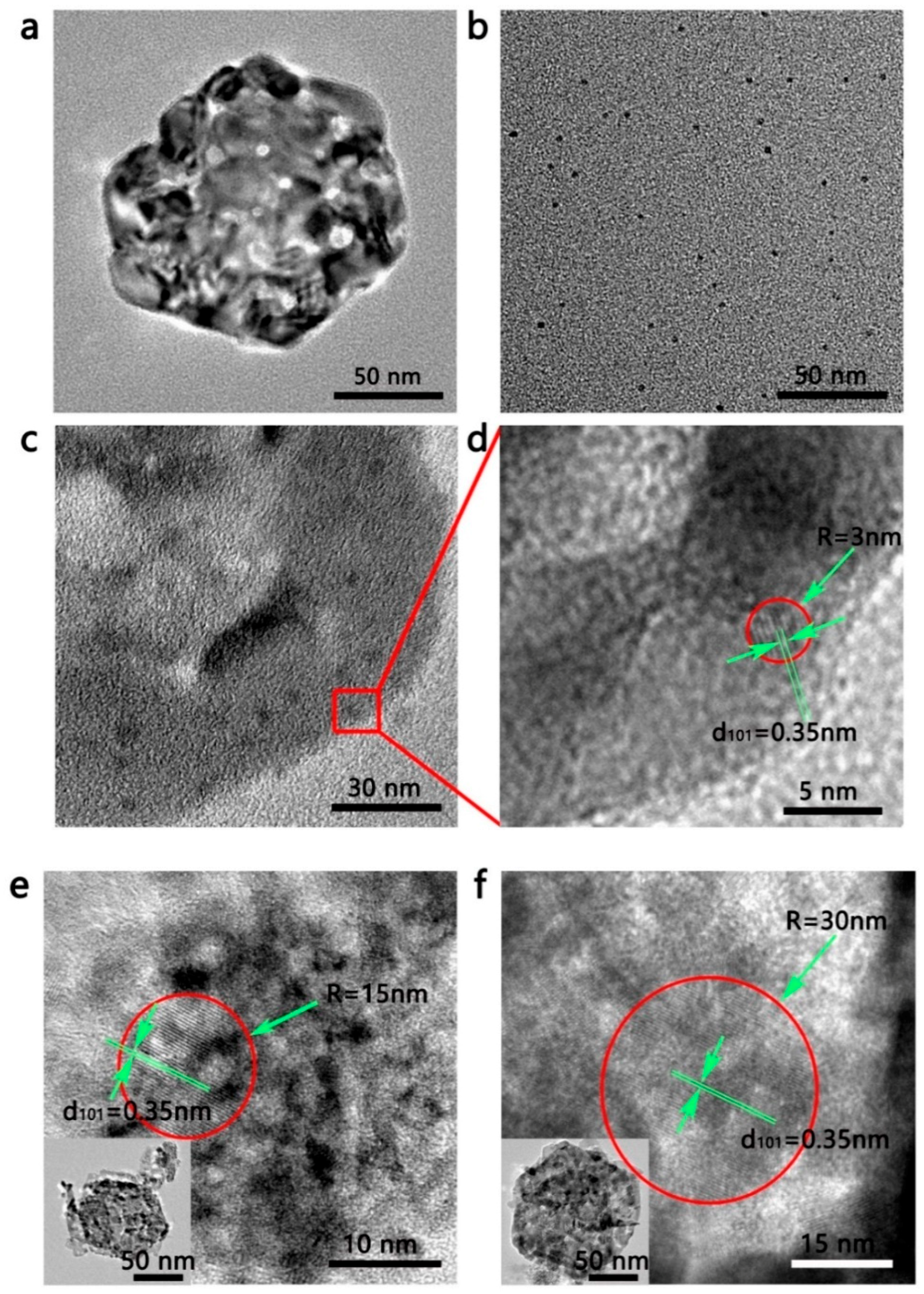
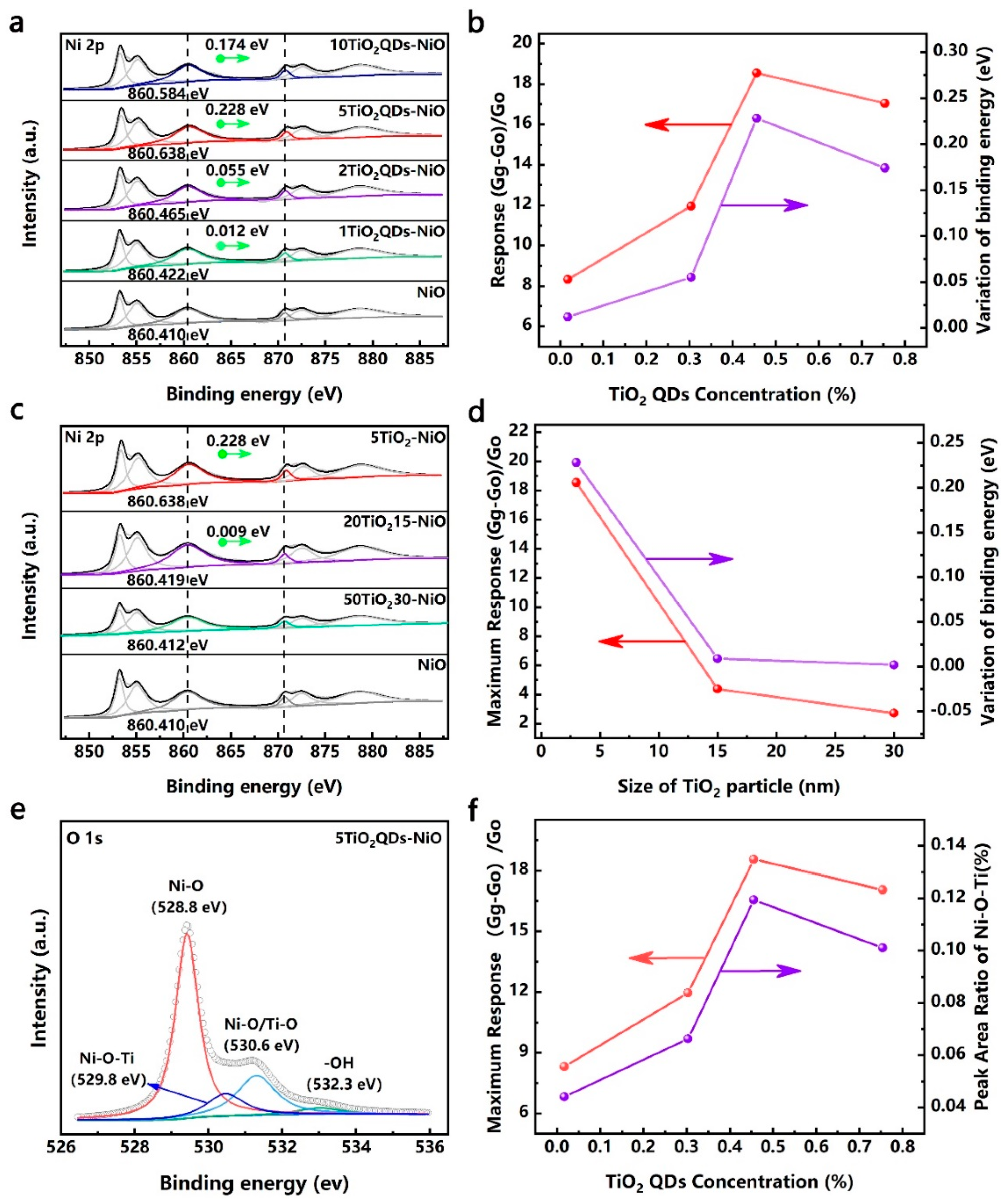
3.2. Structure Characterization
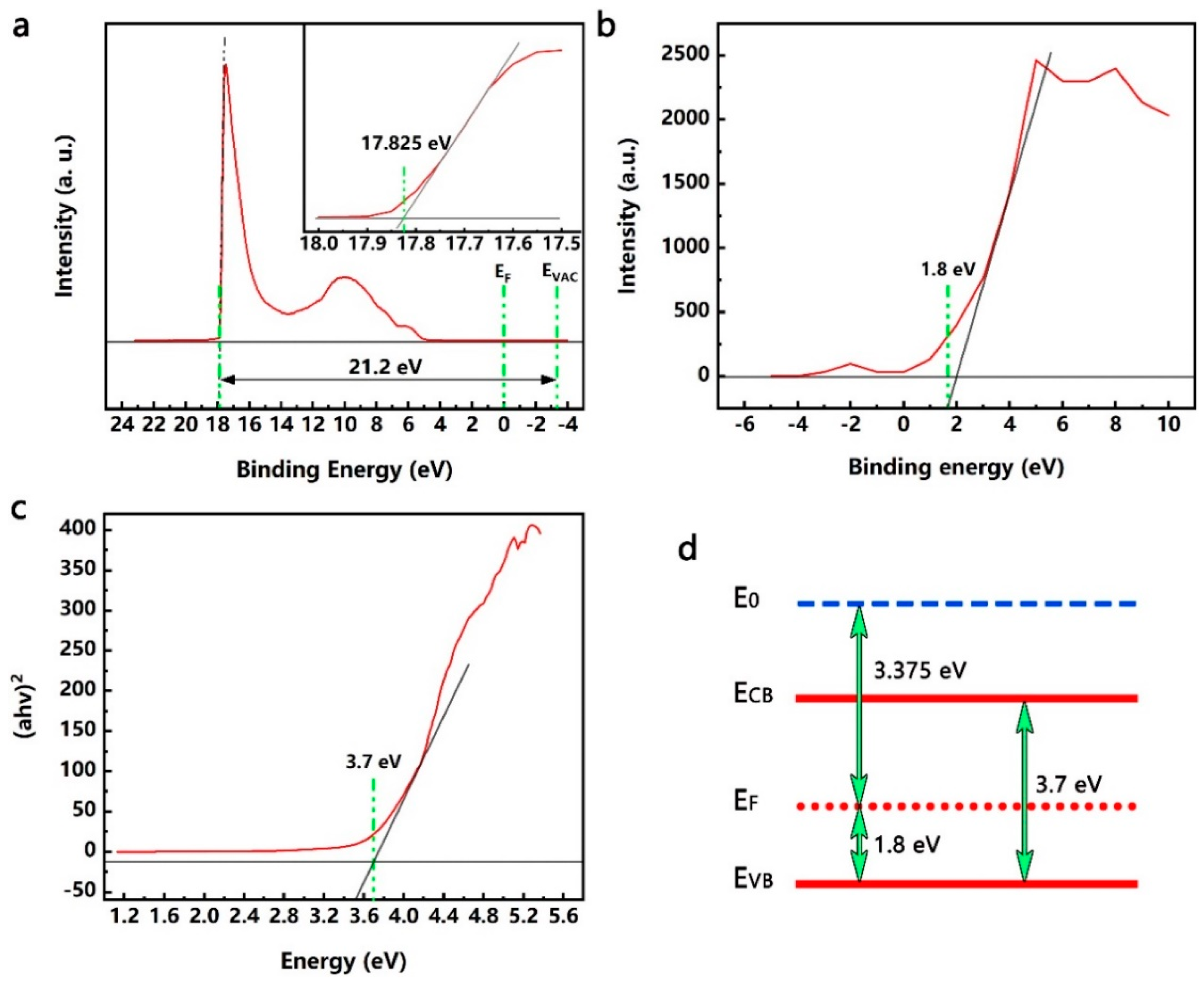
3.3. Energy Band Structure of the TiO2 QDs
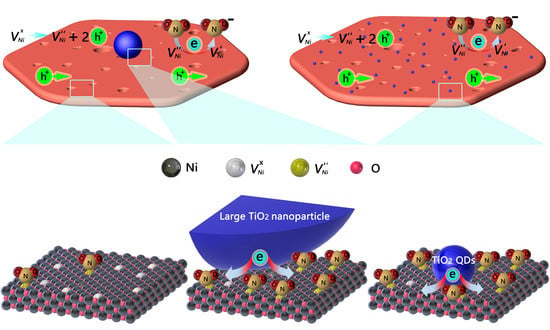
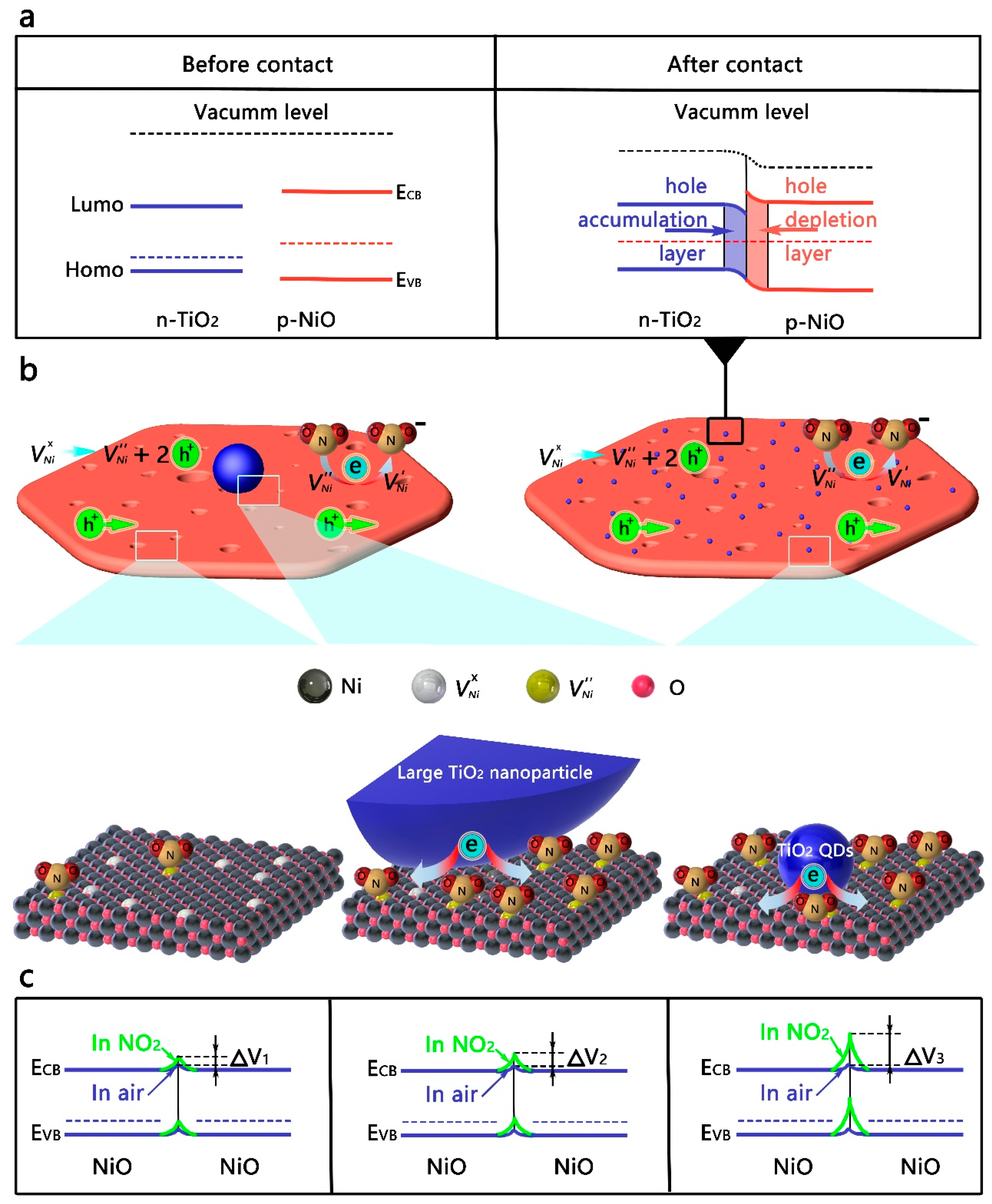
3.4. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, F.Y.; Que, W.X.; He, Y.C.; Yuan, Y.; Yin, X.T.; Wang, G.F. Enhanced Photocatalytic Activity of ZnO Microspheres via Hybridization with CuInSe2 and CuInS2 Nanocrystals. Acs Appl. Mater. Interfaces 2012, 4, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.Y.; Da, P.M.; Wang, J.; Wang, W.Y.; Chen, Z.H.; Xiu, F.X.; Zheng, G.F.; Wang, Z.S. Enhancing Perovskite Solar Cell Performance by Interface Engineering Using CH3NH3PbBr0.9I2.1 Quantum Dots. J. Am. Chem. Soc. 2016, 138, 8581–8587. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; An, W.J.; Springer, J.W.; Modesto-Lopez, L.B.; Gullapalli, S.; Holten, D.; Wong, M.S.; Biswas, P. Linker-free quantum dot sensitized TiO2 photoelectrochemical cells. Int. J. Hydrogen Energy 2012, 37, 6422–6430. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.J.; Wang, S.B.; Huang, Z.F.; Yu, A.; Wang, L.; Zhang, X.W. Quantum dot self-decorated TiO2 nanosheets. Chem. Commun. 2013, 49, 6593–6595. [Google Scholar] [CrossRef]
- Danish, R.; Ahmed, F.; Koo, B.H. Rapid synthesis of high surface area anatase Titanium Oxide quantum dots. Ceram. Int. 2014, 40, 12675–12680. [Google Scholar] [CrossRef]
- Frasco, M.F.; Chaniotakis, N. Semiconductor Quantum Dots in Chemical Sensors and Biosensors. Sensors 2009, 9, 7266–7286. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, L.L.; Wang, H.R.; Xiong, M.Y.; Yang, T.Q.; Zakharova, G.S. Highly sensitive and selective ammonia gas sensors based on PbS quantum dots/TiO2 nanotube arrays at room temperature. Sens. Actuators B-Chem. 2016, 236, 529–536. [Google Scholar] [CrossRef]
- Song, Z.L.; Wei, Z.R.; Wang, B.; Luo, Z.; Xu, S.M.; Zhang, W.K.; Yu, H.X.; Li, M.; Huang, Z.; Zang, J.F.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Cмирнoв, B.M.; Pумянцева, M.H.; Гаськoв, A.M. Light activated sub-ppm NO2 detection by hybrid ZnO/QD nanomaterials vs charge localization in core-shell QD. ACS Sens. 2018, 6, 1–14. [Google Scholar]
- Wei, Z.; Xi-Wen, H.; Yang, C.; Wen-You, L.; Yu-Kui, Z. Composite of CdTe quantum dots and molecularly imprinted polymer as a sensing material for cytochrome c. Biosens. Bioelectron. 2011, 26, 2553–2558. [Google Scholar]
- Peng, W.; Jinyi, Z.; Shanlin, W.; Airu, Z.; Xiandeng, H. Sensing during in situ growth of Mn-doped ZnS QDs: A phosphorescent sensor for detection of H2S in biological samples. Chem. Eur. J. 2014, 20, 952–956. [Google Scholar]
- Xia, Y.S.; Zhu, C.Q. Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 2008, 75, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.; Yao, X.; Pan, W.; Lei, Z.; Yanxin, L.; Limin, T. Quantum-dot-doped polymer nanofibers for optical sensing. Adv. Mater. 2011, 23, 3770–3774. [Google Scholar]
- Waghuley, S.A. LPG Sensing Application Of Graphene/CeO2 Quantum Dots Composite. In Proceedings of the Aip Proceeding, Bikaner, India; 2013; 1536, pp. 1258–1259. [Google Scholar]
- Zhang, B.; Wang, F.; Zhu, C.; Li, Q.; Song, J.; Zheng, M.; Ma, L.; Shen, W. A facile self-assembly synthesis of hexagonal ZnO nanosheet films and their photoelectrochemical properties. Nano-Micro. Lett. 2016, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, D.; Tian, S.; Qin, Z.; Zeng, D.; Xie, C. CuInS 2 QDs decorated ring-like NiO for significantly enhanced room-temperature NO 2 sensing performances via effective interfacial charge transfer. Sens.Actuators B Chem. 2017, 256, 1001–1010. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.; Han, K.; Lv, B.; Bao, K.; Wu, X.; Gao, X.; Cen, K. Smog chamber study on aging of combustion soot in isoprene/SO2/NOx system: Changes of mass, size, effective density, morphology and mixing state. Atmos. Res. 2017, 184, 139–148. [Google Scholar] [CrossRef]
- Likens, G.E.; Butler, T.J. Acid Rain: Causes, Consequences, and Recovery in Terrestrial, Aquatic, and Human Systems. Reference Module Earth Syst. Environ. Sci. 2017, 5, 23–31. [Google Scholar]
- Zhang, M.; Su, H.C.; Rheem, Y.; Hangarter, C.M.; Myung, N.V. A Rapid Room-Temperature NO2 Sensor Based on Tellurium-SWNT Hybrid Nanostructures. J. Phys. Chem. C 2012, 37, 20067–20074. [Google Scholar] [CrossRef]
- Alving, K.; Weitzberg, E.; Lundberg, J.M. Increased amount of nitric oxide in exhaled air of asthmatics. Eur. Respir. J. 1993, 6, 1368–1370. [Google Scholar]
- Smith, A.D.; Cowan, J.O.; Brassett, K.P.; Taylor, G.P.H.D.R. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N. Engl. J. Med. 2005, 352, 2163–2173. [Google Scholar] [CrossRef]
- Mondal, S.P.; Dutta, P.K.; Hunter, G.W.; Ward, B.J.; Dweik, D.L.R.A. Development of high sensitivity potentiometric NOx sensor and its application to breath analysis. Sens. Actuators B-Chem. 2011, 158, 292–298. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Zhao, H.; An, L.; Zhang, L.; Shi, R.; Wang, L.; Chen, L.B.Y. Synthesis and enhanced gas-sensing properties of ultralong NiO nanowires assembled with NiO nanocrystals. Sens. Actuators B-Chem. 2011, 156, 251–262. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B-Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Wang, B.C.; Nisar, J.; Ahuja, R. Molecular Simulation for Gas Adsorption at NiO (100) Surface. ACS Appl. Mater. Interfaces 2012, 4, 5691–5697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, D.W.; Zhu, Q.; Wu, J.J.; Xu, K.; Liao, T.G.; Zhang, G.Z.; Xie, C.S. Effect of Grain-Boundaries in NiO Nanosheet Layers Room-Temperature Sensing Mechanism under NO2. J. Phys. Chem. C 2015, 119, 17930–17939. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.W.; Zhu, Q.; Wu, J.J.; Xie, Q.W.H.C.S. Effect of Nickel Vacancies on the Room-Temperature NO2 Sensing Properties of Mesoporous NiO Nanosheets. J. Phys. Chem. C 2016, 120, 3936–3945. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Kalathil, S.; Khan, M.M.; Ansari, S.A.; Lee, J.; Cho, M.H. Band gap narrowing of titanium dioxide (TiO2) nanocrystals by electrochemically active biofilms and their visible light activity. Nanoscale 2013, 5, 6323–6326. [Google Scholar] [CrossRef]
- Ruiz, A.M.; Sakai, G.; Cornet, A.; Shimanoe, K.; Morante, J.R.; Yamazoe, N. Cr-doped TiO 2 gas sensor for exhaust NO 2 monitoring. Sens. Actuators B Chem. 2003, 93, 509–518. [Google Scholar] [CrossRef]
- Yamada, Y.; Seno, Y.; Masuoka, Y.; Nakamura, T.; Yamashita, K. NO 2 sensing characteristics of Nb doped TiO 2 thin films and their electronic properties. Sens. Actuators B 2000, 66, 164–166. [Google Scholar] [CrossRef]
- Calderon-Moreno, J.M.; Preda, S.; Predoana, L.; Zaharescu, M.; Anastasescu, M.; Nicolescu, M.; Stoica, M.; Stroescu, H.; Gartner, M.; Buiu, O.; et al. Effect of polyethylene glycol on porous transparent TiO2 films prepared by sol-gel method. Ceram. Int. 2014, 40, 2209–2220. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, D.W.; Zhao, S.Q.; Wu, J.J.; Xu, K.; Zhu, Q.; Zhang, G.Z.; Xie, C.S. Room temperature NO2 sensing: what advantage does the rGO-NiO nanocomposite have over pristine NiO? Phys. Chem. Chem. Phys. 2015, 17, 14903–14911. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.B.; Tai, H.L.; Xie, T.; Yuan, Z.; Liu, C.H.; Jiang, Y.D. Room temperature formaldehyde sensor with enhanced performance based on reduced graphene oxide/titanium dioxide. Sens. Actuators B-Chem. 2016, 223, 149–156. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.J.; Wang, X.X.; Zeng, D.W.; Xie, C.S. Enhancing room-temperature NO2 sensing properties via forming heterojunction for NiO-rGO composited with SnO2 nanoplates. Sens. Actuators B-Chem. 2017, 243, 1010–1019. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Li, T.; Xie, C.; Zeng, D. Highly sensitive and ultralow detection limit of room-temperature NO2 sensors using in-situ growth of PPy on mesoporous NiO nanosheets. Org. Electron. 2019, 105504, (Recently published article, no page number and volume number yet.). [Google Scholar] [CrossRef]
- Chun, W.J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Song, H.P.; Zheng, G.L.; Yang, A.L.; Guo, Y.; Wei, H.Y.; Li, C.M.; Yang, S.Y.; Liu, X.L.; Zhu, Q.S.; Wang, Z.G. The growth of ZnO on buffer layers and the valence band offset determined by X-ray photoemission spectroscopy. Solid State Commun. 2010, 150, 1991–1994. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Malagu, C.; Morandi, S.; Perez, N.; Mandayo, G.G.; Castano, E. Properties of NiO sputtered thin films and modeling of their sensing mechanism under formaldehyde atmospheres. Acta Mater. 2013, 61, 1146–1153. [Google Scholar] [CrossRef]
- Hou, G.Y.; Xie, Y.Y.; Wu, L.K.; Cao, H.Z.; Tang, Y.P.; Zheng, G.Q. Electrocatalytic performance of Ni-Ti-O nanotube arrays/NiTi alloy electrode annealed under H 2 atmosphere for electro-oxidation of methanol. Int. J. Hydrogen Energy 2016, 41, 9295–9302. [Google Scholar] [CrossRef]
- Liang, J.; Tan, H.; Xiao, C.; Zhou, G.; Guo, S.; Ding, S. Hydroxyl-riched halloysite clay nanotubes serving as substrate of NiO nanosheets for high-performance supercapacitor. J. Power Sources 2015, 285, 210–216. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, R.F.; Liu, S.H.; Song, J.; Chen, J.S.; Dong, B.; Song, H.W. NiO@ZnO Heterostructured Nanotubes: Coelectrospinning Fabrication, Characterization, and Highly Enhanced Gas Sensing Properties. Inorg. Chem. 2012, 51, 7733–7740. [Google Scholar] [CrossRef] [PubMed]
- Bandara, J.; Divarathne, C.M.; Nanayakkara, S.D. Fabrication of n-p junction electrodes made of n-type SnO2 and p-type NiO for control of charge recombination in dye-sensitized solar cells. Solar Energy Mater. Solar Cells 2005, 85, 141–142. [Google Scholar] [CrossRef]
- Bandara, J.; Yasomanee, J.P. p-type oxide semiconductors as hole collectors in dye-sensitized solid-state solar cells. Semicond. Sci. Technol. 2007, 22, 20–24. [Google Scholar] [CrossRef]
- Bernede, J.C.; Houari, S.; Nguyen, D.; Jouan, P.Y.; Khelil, A.; Mokrani, A.; Cattin, L.; Predeep, P. XPS study of the band alignment at ITO/oxide (n-type MoO3 or p-type NiO) interface. Phys. Status Solidi Appl. Mater. Sci. 2012, 209, 1291–1297. [Google Scholar] [CrossRef]
- Bonomo, M.; Marrani, A.G.; Novelli, V.; Awais, M.; Dowling, D.P.; Vos, J.G.; Dini, D. Surface properties of nanostructured NiO undergoing electrochemical oxidation in 3-methoxy-propionitrile. Appl. Surf. Sci. 2017, 403, 441–447. [Google Scholar] [CrossRef]
- Rodriguez, J.L.; Valenzuela, M.A.; Poznyak, T.; Lartundo, L.; Chairez, I. Reactivity of NiO for 2,4-D degradation with ozone: XPS studies. J. Hazard. Mater. 2013, 262, 472–481. [Google Scholar] [CrossRef]
- Li, Z.W. Supersensitive and superselective formaldehyde gas sensor based on NiO nanowires. Vacuum 2017, 143, 50–53. [Google Scholar] [CrossRef]
- Yin, X.T.; Liu, J.; Ma, J.Q.; Zhang, C.X.; Chen, P.; Que, M.D.; Yang, Y.W.; Que, W.X.; Niu, C.M.; Shao, J.Y. Solvothermal derived crystalline NiOx nanoparticles for high performance perovskite solar cells. J. Power Sources 2016, 329, 398–405. [Google Scholar] [CrossRef]
- Qin, Z.; Ouyang, C.; Zhang, J.; Wan, L.; Wang, S.; Xie, C.; Zeng, D. 2D WS2 nanosheets with TiO2 quantum dots decoration for high-performance ammonia gas sensing at room temperature. Sens. Actuators B-Chem. 2017, 253, 1034–1042. [Google Scholar] [CrossRef]
- Wang, S.B.; Wang, F.F.; Su, Z.M.; Wang, X.N.; Han, Y.C.; Zhang, L.; Xiang, J.; Du, W.; Tang, N. Controllable Fabrication of Heterogeneous p-TiO2 QDs@g-C3N4 p-n Junction for Efficient Photocatalysis. Catalysts 2019, 9, 439. [Google Scholar] [CrossRef]
- Wang, X.; Xia, R.; Muhire, E.; Jiang, S.B.; Huo, X.J.; Gao, M.Z. Highly enhanced photocatalytic performance of TiO2 nanosheets through constructing TiO2/TiO2 quantum dots homojunction. Appl. Surf. Sci. 2018, 459, 9–15. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, J.; Wang, X.; Xie, C.; Shi, S.; Zeng, D. Effect of Heterointerface on NO2 Sensing Properties of In-Situ Formed TiO2 QDs-Decorated NiO Nanosheets. Nanomaterials 2019, 9, 1628. https://doi.org/10.3390/nano9111628
Wu C, Zhang J, Wang X, Xie C, Shi S, Zeng D. Effect of Heterointerface on NO2 Sensing Properties of In-Situ Formed TiO2 QDs-Decorated NiO Nanosheets. Nanomaterials. 2019; 9(11):1628. https://doi.org/10.3390/nano9111628
Chicago/Turabian StyleWu, Congyi, Jian Zhang, Xiaoxia Wang, Changsheng Xie, Songxin Shi, and Dawen Zeng. 2019. "Effect of Heterointerface on NO2 Sensing Properties of In-Situ Formed TiO2 QDs-Decorated NiO Nanosheets" Nanomaterials 9, no. 11: 1628. https://doi.org/10.3390/nano9111628
APA StyleWu, C., Zhang, J., Wang, X., Xie, C., Shi, S., & Zeng, D. (2019). Effect of Heterointerface on NO2 Sensing Properties of In-Situ Formed TiO2 QDs-Decorated NiO Nanosheets. Nanomaterials, 9(11), 1628. https://doi.org/10.3390/nano9111628