A Facile, One-Step Synthesis of Silicon/Silicon Carbide/Carbon Nanotube Nanocomposite as a Cycling-Stable Anode for Lithium Ion Batteries
Abstract
1. Introduction
2. Experimental
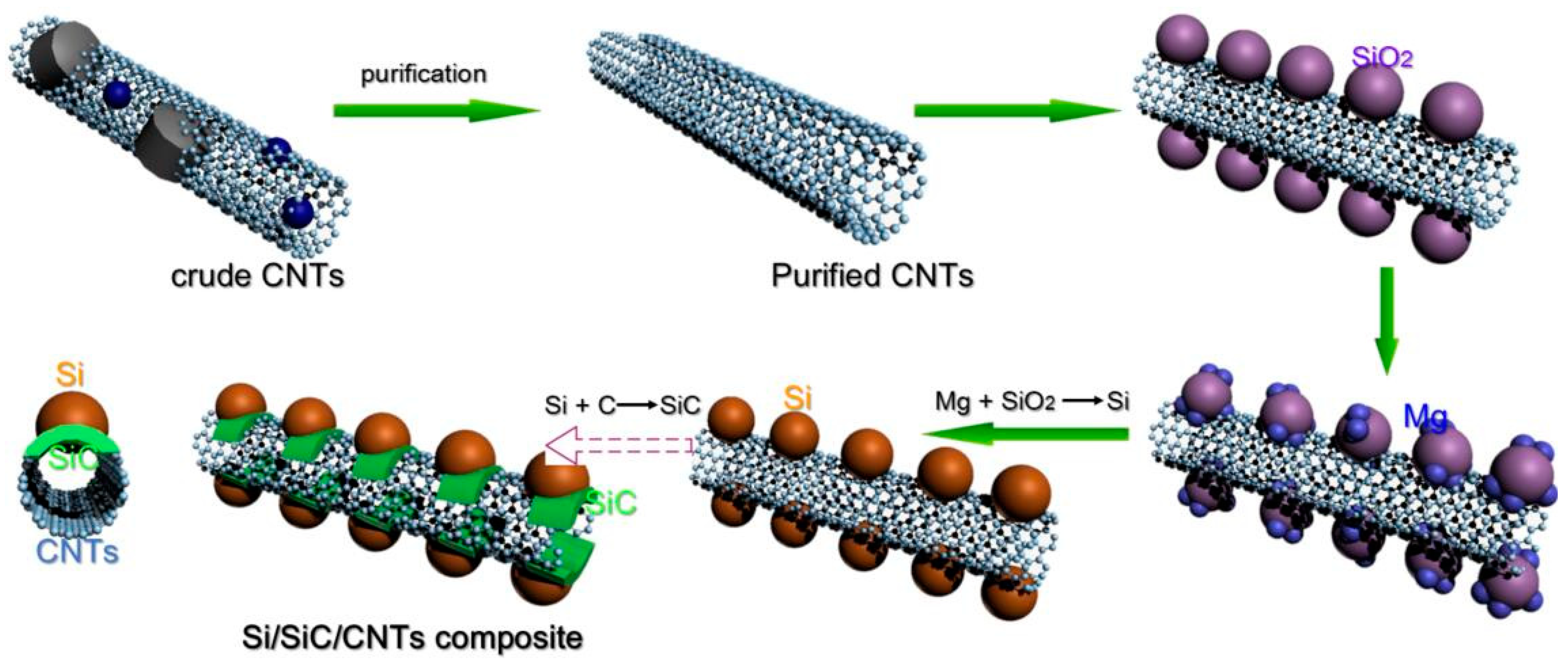
2.1. Preparation of Si/SiC/CNT Nanocomposite
2.2. Characterization
2.3. Electrochemical Measurements
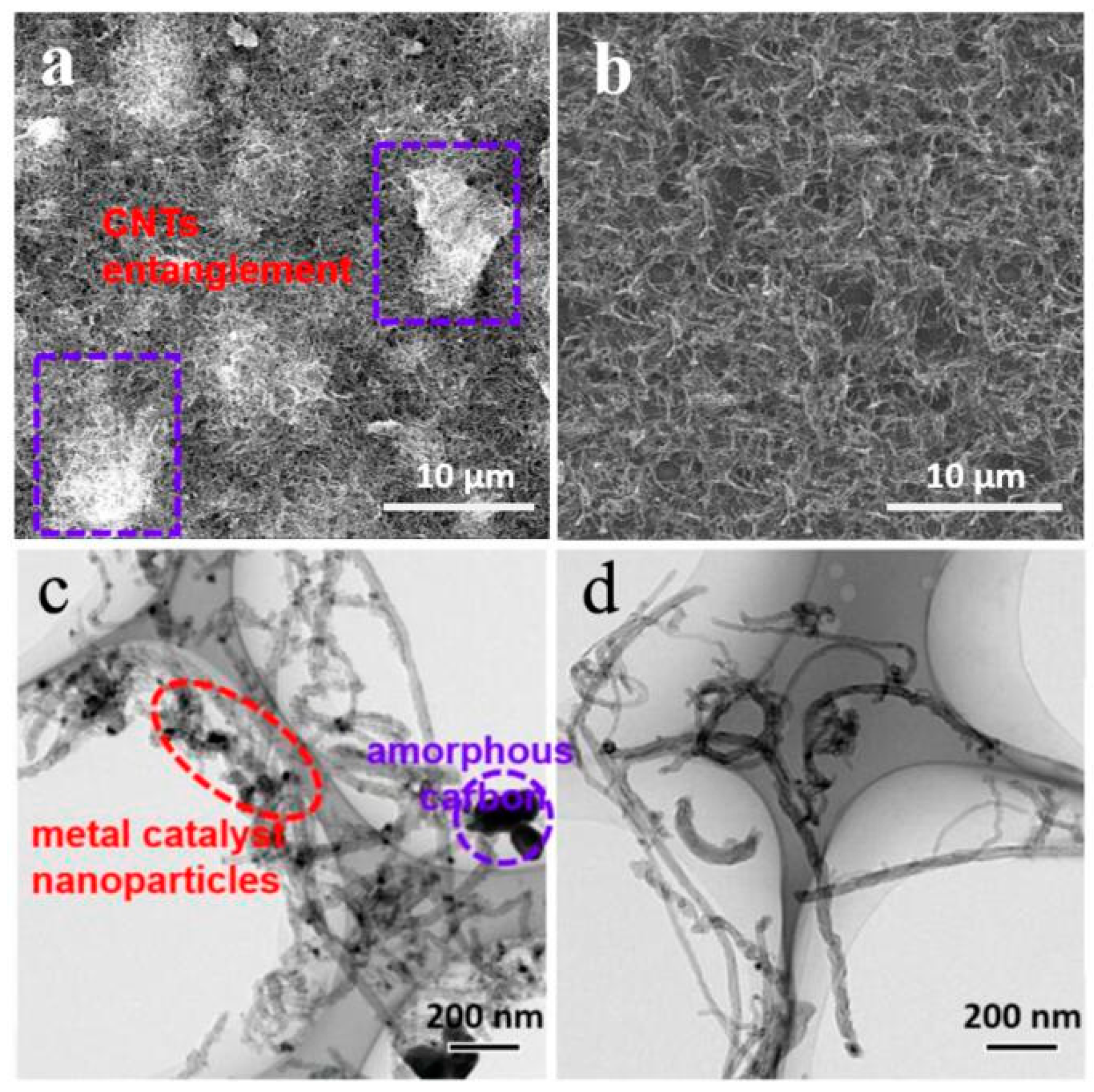
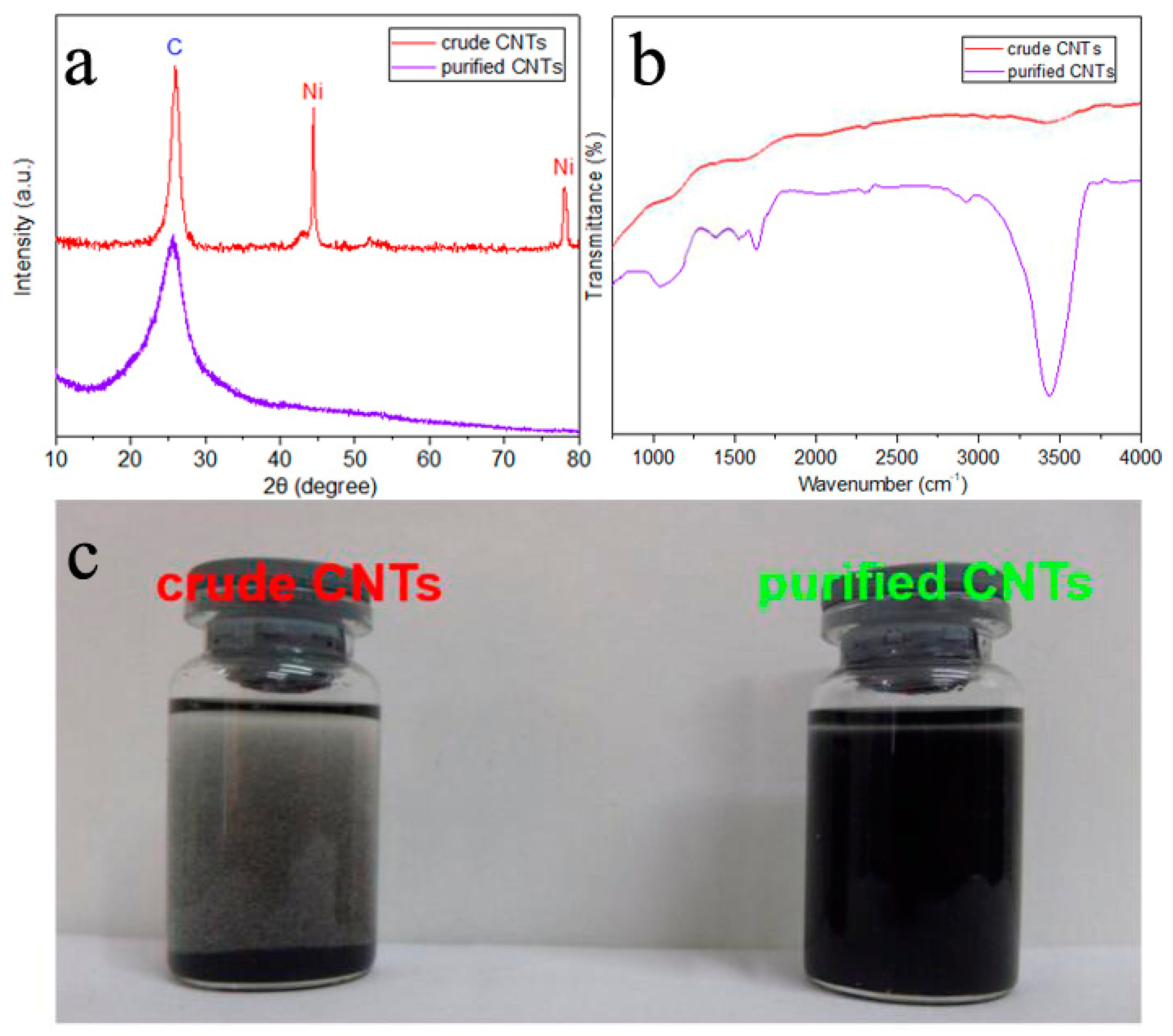
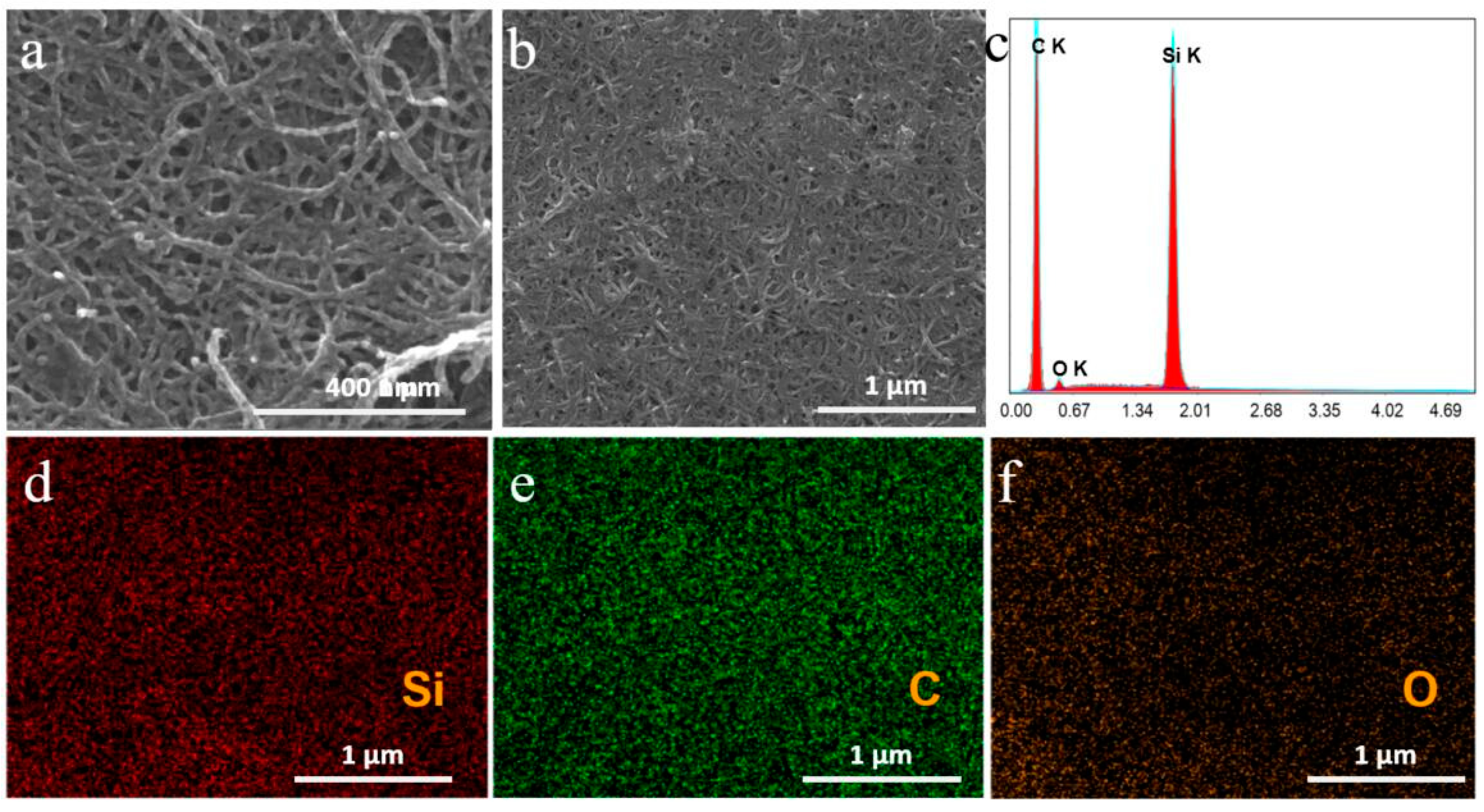
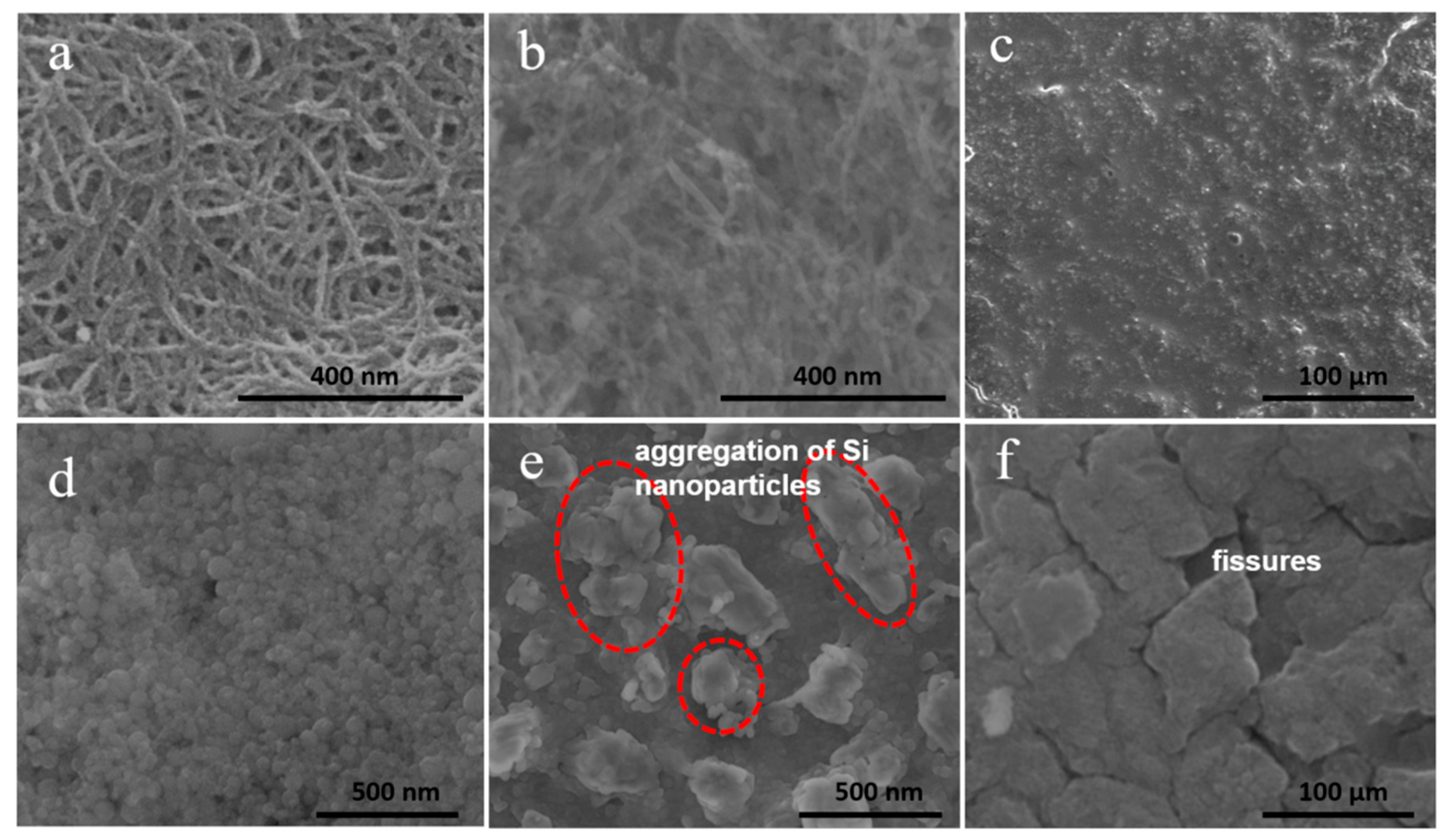
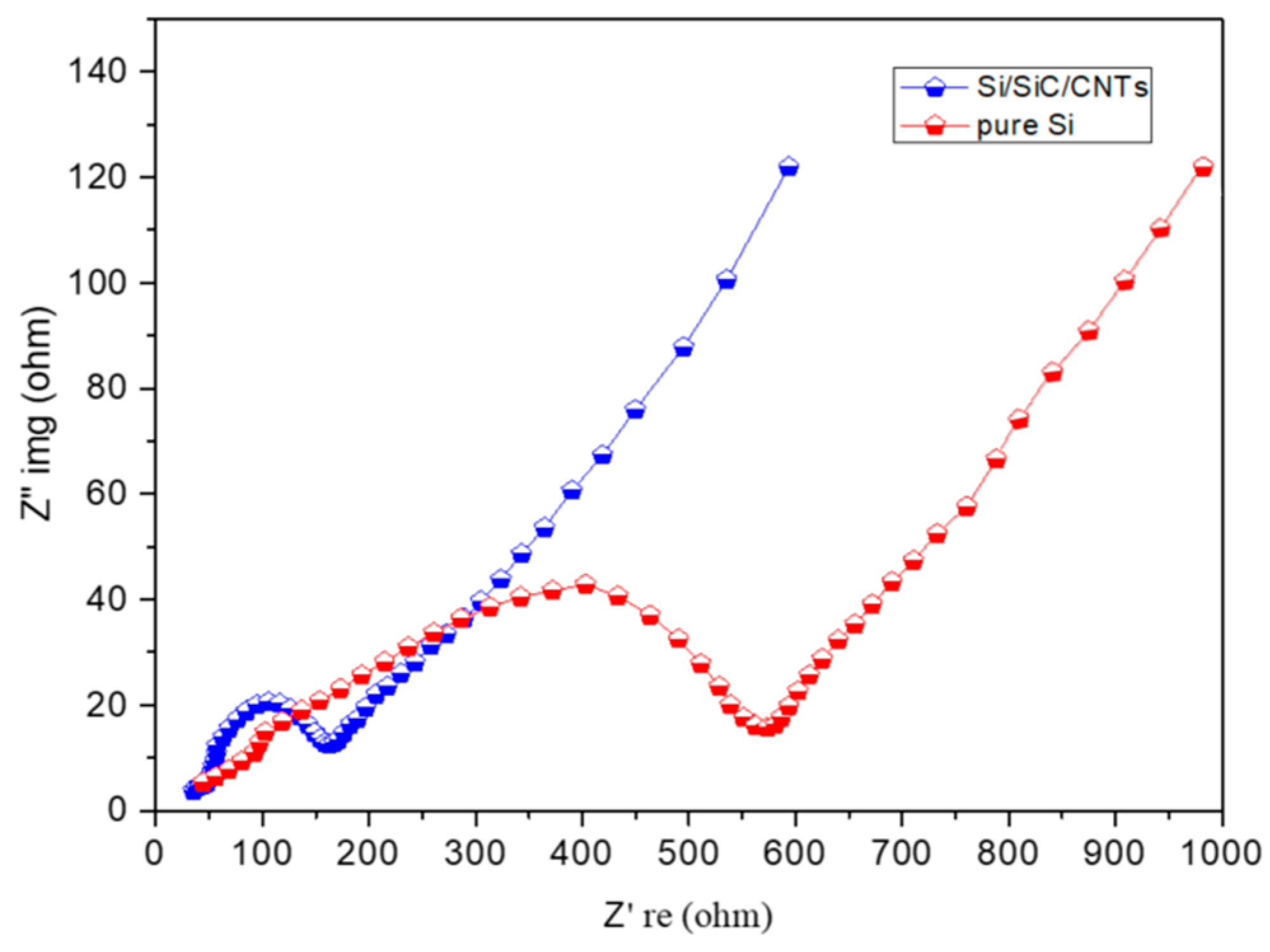
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Chen, X.; Shi, T.; Zhong, K.; Wu, G.; Lu, Y. Capacitive behavior of MoS2 decorated with FeS2@carbon nanospheres. Chem. Eng. J. 2020, 379, 122240. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Feng, Y.; Zhang, T.; Chen, Q.; Chi, Q.; Liu, L.; Li, G.; Cui, Y.; Wang, X.; et al. Excellent energy storage performance and thermal property of polymer-based composite induced by multifunctional one-dimensional nanofibers oriented in-plane direction. Nano Energy 2019, 56, 138–150. [Google Scholar] [CrossRef]
- Zhang, C.; Song, H.; Liu, C.; Liu, Y.; Zhang, C.; Nan, X.; Cao, G. Fast and reversible li ion insertion in carbon-encapsulated Li3VO4 as anode for lithium-ion battery. Adv. Funct. Mater. 2015, 25, 3497–3504. [Google Scholar] [CrossRef]
- Li, H.; Henkelman, G. Dehydrogenation selectivity of ethanol on close-packed transition metal surfaces: A computational study of monometallic, Pd/Au, and Rh/Au catalysts. J. Phys. Chem. C 2017, 121, 27504–27510. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Pei, Y.; Li, S.; Cao, X.; Massé, R.C.; Cao, G. Novel carbon-encapsulated porous SnO2 anode for lithium-ion batteries with much improved cyclic stability. Small 2016, 12, 1945–1955. [Google Scholar] [CrossRef]
- Wu, G.; Jia, Z.; Cheng, Y.; Zhang, H.; Zhou, X.; Wu, H. Easy synthesis of multi-shelled ZnO hollow spheres and their conversion into hedgehog-like ZnO hollow spheres with superior rate performance for lithium ion batteries. Appl. Surf. Sci. 2019, 464, 472–478. [Google Scholar] [CrossRef]
- Liu, T.; Chu, Q.; Yan, C.; Zhang, S.; Lin, Z.; Lu, J. Interweaving 3D network binder for high-areal-capacity Si anode through combined hard and soft polymers. Adv. Energy Mater. 2019, 9, 1802645. [Google Scholar] [CrossRef]
- Hu, L.; Luo, B.; Wu, C.; Hu, P.; Wang, L.; Zhang, H. Yolk-shell Si/C composites with multiple Si nanoparticles encapsulated into double carbon shells as lithium-ion battery anodes. J. Energy Chem. 2019, 32, 124–130. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Huang, B.; Dai, Y. Achieving high energy density for lithium-ion battery anodes by Si/C nanostructure design. J. Mater. Chem. A 2019, 7, 2165–2171. [Google Scholar] [CrossRef]
- Kasukabe, T.; Nishihara, H.; Iwamura, S.; Kyotani, T. Remarkable performance improvement of inexpensive ball-milled Si nanoparticles by carbon-coating for Li-ion batteries. J. Power Sources 2016, 319, 99–103. [Google Scholar] [CrossRef]
- Chen, S.; Shen, L.; van Aken, P.A.; Maier, J.; Yu, Y. Dual-functionalized double carbon shells coated silicon nanoparticles for high performance lithium-ion batteries. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, J.; Zhou, X.; Zhang, M.; Ren, Y.; Nie, Y. Silicon nanoparticles embedded in a porous carbon matrix as a high-performance anode for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 11381–11387. [Google Scholar] [CrossRef]
- Tesfaye, A.T.; Gonzalez-Rodriguez, R.; Coffer, J.L.; Djenizian, T. Self-supported silicon nanotube arrays as an anode electrode for li-ion batteries. ECS Trans. 2017, 77, 349–350. [Google Scholar] [CrossRef]
- Cui, L.F.; Hu, L.; Choi, J.W.; Cui, Y. Light-weight free-standing carbon nanotube-silicon films for anodes of lithium ion batteries. ACS Nano 2010, 4, 3671–3678. [Google Scholar] [CrossRef]
- Miao, F.; Miao, R.; Wu, W.; Cong, W.; Zang, Y.; Tao, B. A stable hybrid anode of graphene/silicon nanowires array for high performance lithium-ion battery. Mater. Lett. 2018, 228, 262–265. [Google Scholar] [CrossRef]
- Kim, H.; Huang, X.; Wen, Z.; Cui, S.; Guo, X.; Chen, J. Novel hybrid Si film/carbon nanofibers as anode materials in lithium-ion batteries. J. Mater. Chem. A 2015, 3, 1947–1952. [Google Scholar] [CrossRef]
- Yang, T.; Tian, X.; Li, X.; Wang, K.; Liu, Z.; Guo, Q.; Song, Y. Double core–shell Si@C@SiO2 for anode material of lithium-ion batteries with excellent cycling stability. Chem. A Eur. J. 2017, 23, 2165–2170. [Google Scholar] [CrossRef]
- Jing, S.; Jiang, H.; Hu, Y.; Shen, J.; Li, C. Face-to-face contact and open-void coinvolved Si/C nanohybrids lithium-ion battery anodes with extremely long cycle life. Adv. Funct. Mater. 2015, 25, 5395–5401. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Fu, L.; Meng, J.; Yu, N.; Wang, J.; Wu, Y. Si/C composites as negative electrode for high energy lithium ion batteries. Chin. J. Chem. 2017, 35, 21–29. [Google Scholar] [CrossRef]
- Palomino, J.; Varshney, D.; Weiner, B.R.; Morell, G. Study of the structural changes undergone by hybrid nanostructured Si-CNTs employed as an anode material in a rechargeable lithium-ion battery. J. Phys. Chem. C 2015, 119, 21125–21134. [Google Scholar] [CrossRef]
- Chen, Y.; Du, N.; Zhang, H.; Yang, D. Facile synthesis of uniform MWCNT@Si nanocomposites as high-performance anode materials for lithium-ion batteries. J. Alloy. Compd. 2015, 622, 966–972. [Google Scholar] [CrossRef]
- Bai, X.; Yu, Y.; Kung, H.H.; Wang, B.; Jiang, J. Si@ SiOx/graphene hydrogel composite anode for lithium-ion battery. J. Power Sources 2016, 306, 42–48. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Li, B.; Zhang, Y.; Xie, C.; Zhang, J.; Chen, G.; Niu, C. Interfacial engineering of Si/multi-walled carbon nanotube nanocomposites towards enhanced lithium storage performance. Carbon 2016, 107, 600–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Li, Y.; Li, B.; Li, Z.; Niu, C. Preparation of nanographite sheets supported Si nanoparticles by in situ reduction of fumed SiO2 with magnesium for lithium ion battery. J. Power Sources 2015, 281, 425–431. [Google Scholar] [CrossRef]
- Chen, C.M.; Dai, Y.M.; Huang, J.G.; Jehng, J.M. Intermetallic catalyst for carbon nanotubes (CNTs) growth by thermal chemical vapor deposition method. Carbon 2006, 44, 1808–1820. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Wang, J.; Gu, L.; Maier, J.; Yu, Y. Three-dimensionally interconnected nickel–antimony intermetallic hollow nanospheres as anode material for high-rate sodium-ion batteries. Nano Energy 2015, 16, 389–398. [Google Scholar] [CrossRef]
- Xiao, W.; Jin, X.; Chen, G.Z. Up-scalable and controllable electrolytic production of photo-responsive nanostructured silicon. J. Mater. Chem. A 2013, 1, 10243–10250. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Some considerations related to the use of the scherrer equation in powder X-ray diffraction as applied to heterogeneous catalysts. Catal. Struct. React. 2016, 2, 33–37. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Meng, J.; Li, Y. Enhancing the activity of a SiC-TiO2 composite catalyst for photo-stimulated catalytic water splitting. Int. J. Hydrog. Energy 2013, 38, 3898–3904. [Google Scholar] [CrossRef]
- Zeng, X.B.; Liao, X.B.; Wang, B.; Dai, S.T.; Xu, Y.Y.; Xiang, X.B.; Hu, Z.H.; Diao, H.W.; Kong, G.L. Optical properties of boron-doped Si nanowires. J. Cryst. Growth 2004, 265, 94–98. [Google Scholar] [CrossRef]
- Nakashima, S.I.; Harima, H. Raman investigation of SiC polytypes. Phys. Status Solidi A 1997, 162, 39–64. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, J.Y.; Shim, H.T.; Lee, J.K.; Park, J.K. SEI layer formation on amorphous Si thin electrode during precycling. J. Electrochem. Soc. 2007, 154, A515–A519. [Google Scholar] [CrossRef]
- Ma, D.; Cao, Z.; Hu, A. Si-based anode materials for Li-ion batteries: A mini review. Nano Micro Lett. 2014, 6, 347–358. [Google Scholar] [CrossRef]
- Xie, J.; Tong, L.; Su, L.; Xu, Y.; Wang, L.; Wang, Y. Core-shell yolk-shell Si@C@Void@C nanohybrids as advanced lithium ion battery anodes with good electronic conductivity and corrosion resistance. J. Power Sources 2017, 342, 529–536. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Du, C.; Ren, Y.; Mu, T.; Zuo, P.; Yin, G.; Ma, Y.; Cheng, X.; Gao, Y. Polyaniline-encapsulated silicon on three-dimensional carbon nanotubes foam with enhanced electrochemical performance for lithium-ion batteries. J. Power Sources 2018, 381, 156–163. [Google Scholar] [CrossRef]
- Tao, H.; Xiong, L.; Zhu, S.; Yang, X.; Zhang, L. Flexible binder-free reduced graphene oxide wrapped Si/carbon fibers paper anode for high-performance lithium ion batteries. Int. J. Hydrog. Energy 2016, 41, 21268–21277. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hu, K.; Zhou, Y.; Xia, Y.; Yu, N.; Wu, G.; Zhu, Y.; Wu, Y.; Huang, H. A Facile, One-Step Synthesis of Silicon/Silicon Carbide/Carbon Nanotube Nanocomposite as a Cycling-Stable Anode for Lithium Ion Batteries. Nanomaterials 2019, 9, 1624. https://doi.org/10.3390/nano9111624
Zhang Y, Hu K, Zhou Y, Xia Y, Yu N, Wu G, Zhu Y, Wu Y, Huang H. A Facile, One-Step Synthesis of Silicon/Silicon Carbide/Carbon Nanotube Nanocomposite as a Cycling-Stable Anode for Lithium Ion Batteries. Nanomaterials. 2019; 9(11):1624. https://doi.org/10.3390/nano9111624
Chicago/Turabian StyleZhang, Yi, Kai Hu, Yunlei Zhou, Yingbin Xia, Nengfei Yu, Guanglei Wu, Yusong Zhu, Yuping Wu, and Haibo Huang. 2019. "A Facile, One-Step Synthesis of Silicon/Silicon Carbide/Carbon Nanotube Nanocomposite as a Cycling-Stable Anode for Lithium Ion Batteries" Nanomaterials 9, no. 11: 1624. https://doi.org/10.3390/nano9111624
APA StyleZhang, Y., Hu, K., Zhou, Y., Xia, Y., Yu, N., Wu, G., Zhu, Y., Wu, Y., & Huang, H. (2019). A Facile, One-Step Synthesis of Silicon/Silicon Carbide/Carbon Nanotube Nanocomposite as a Cycling-Stable Anode for Lithium Ion Batteries. Nanomaterials, 9(11), 1624. https://doi.org/10.3390/nano9111624







