Synthesis and Antibacterial Properties of Novel ZnMn2O4–Chitosan Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitin Refinement
2.2. Mn3O4–CS Synthesis
2.3. ZnMn2O4–CS Synthesis
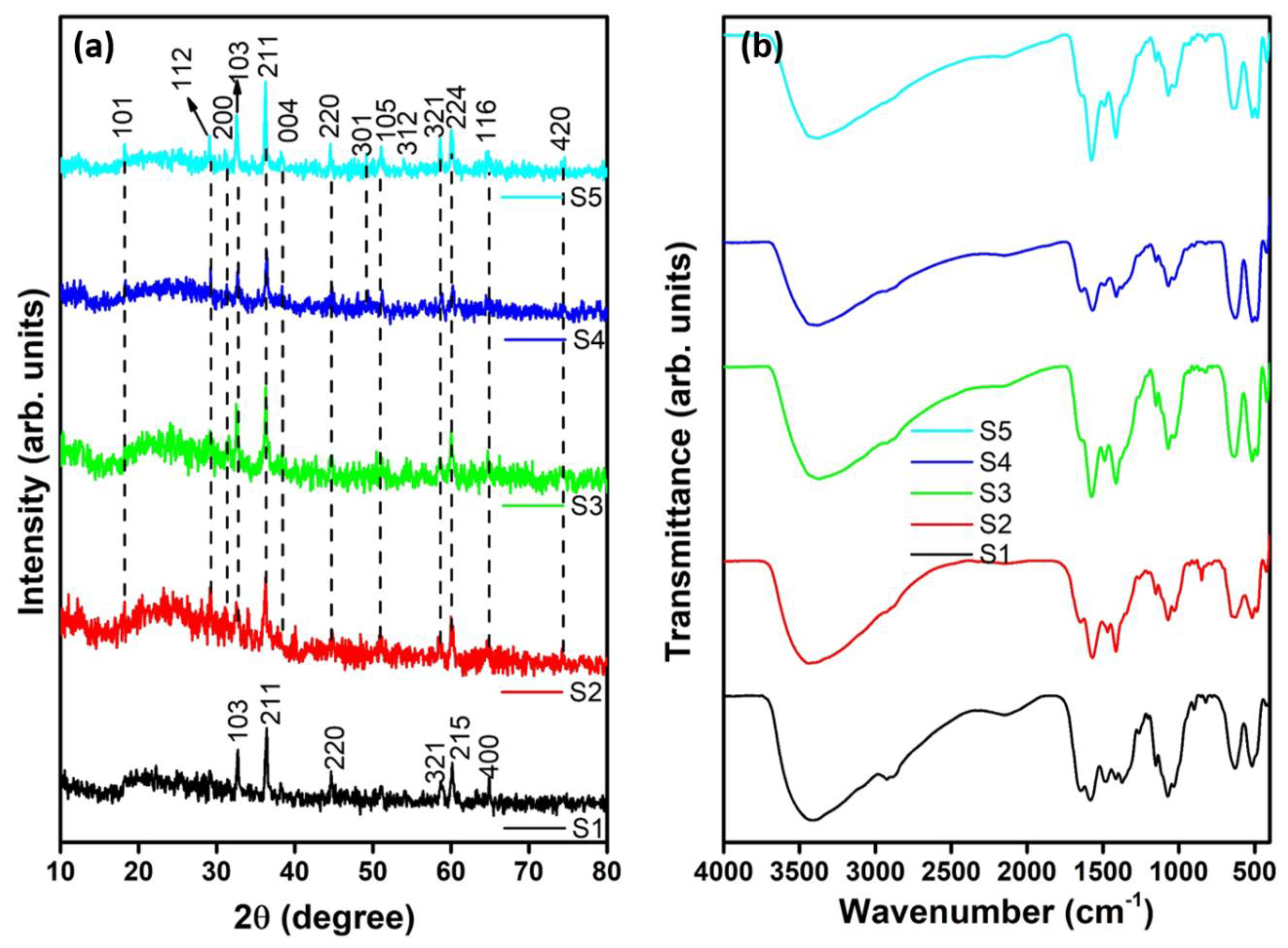
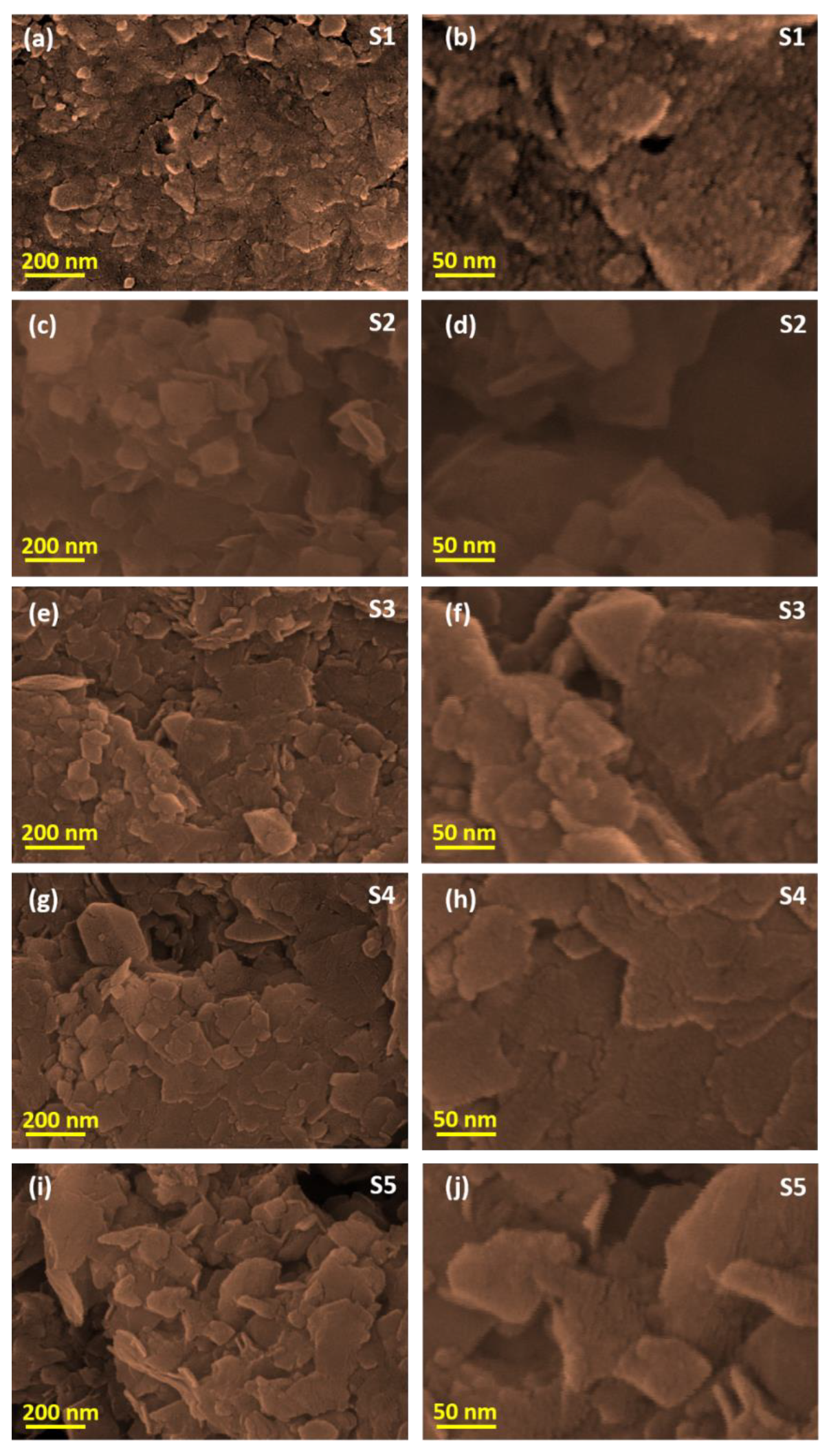
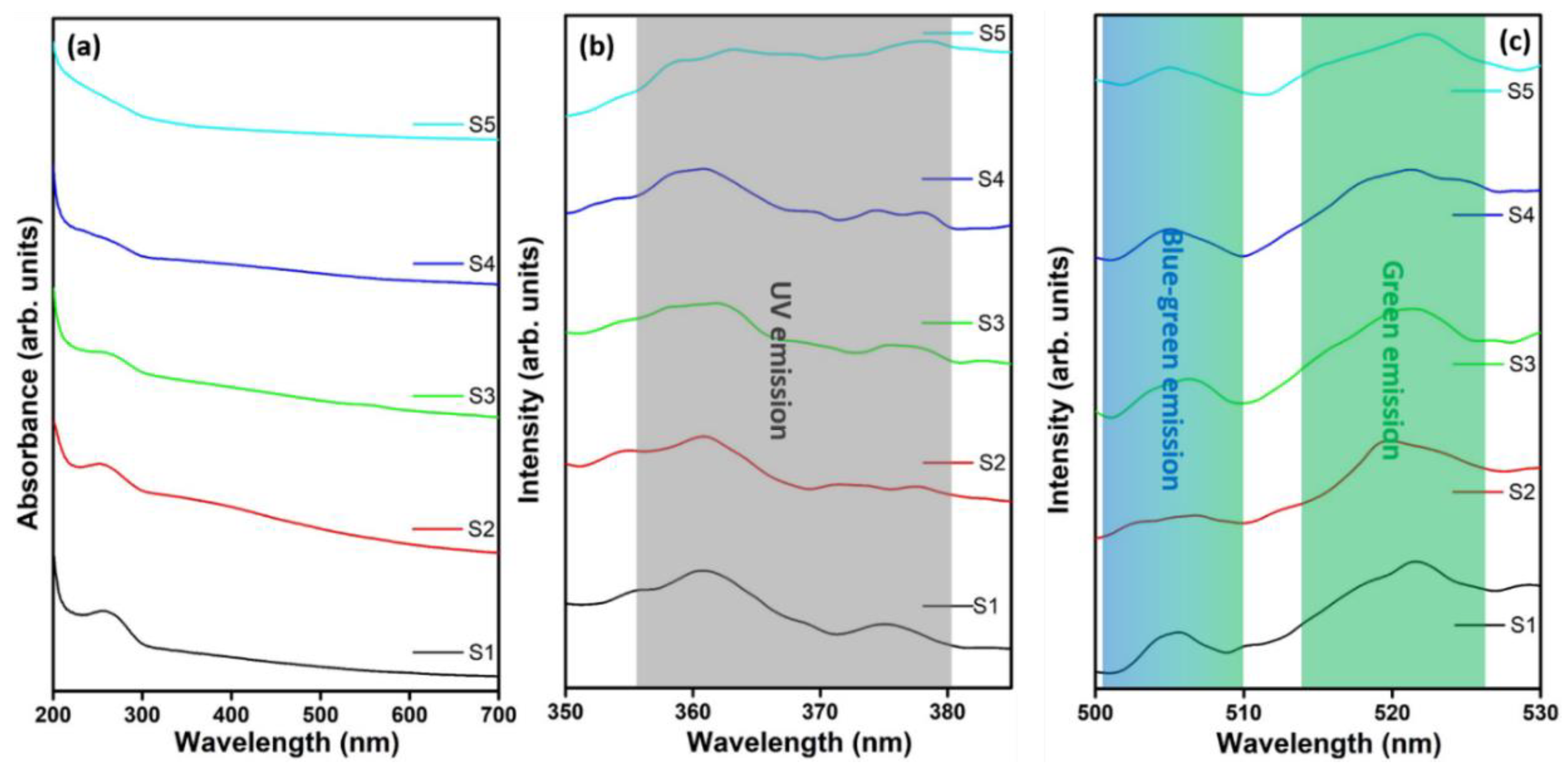
2.4. Characterization
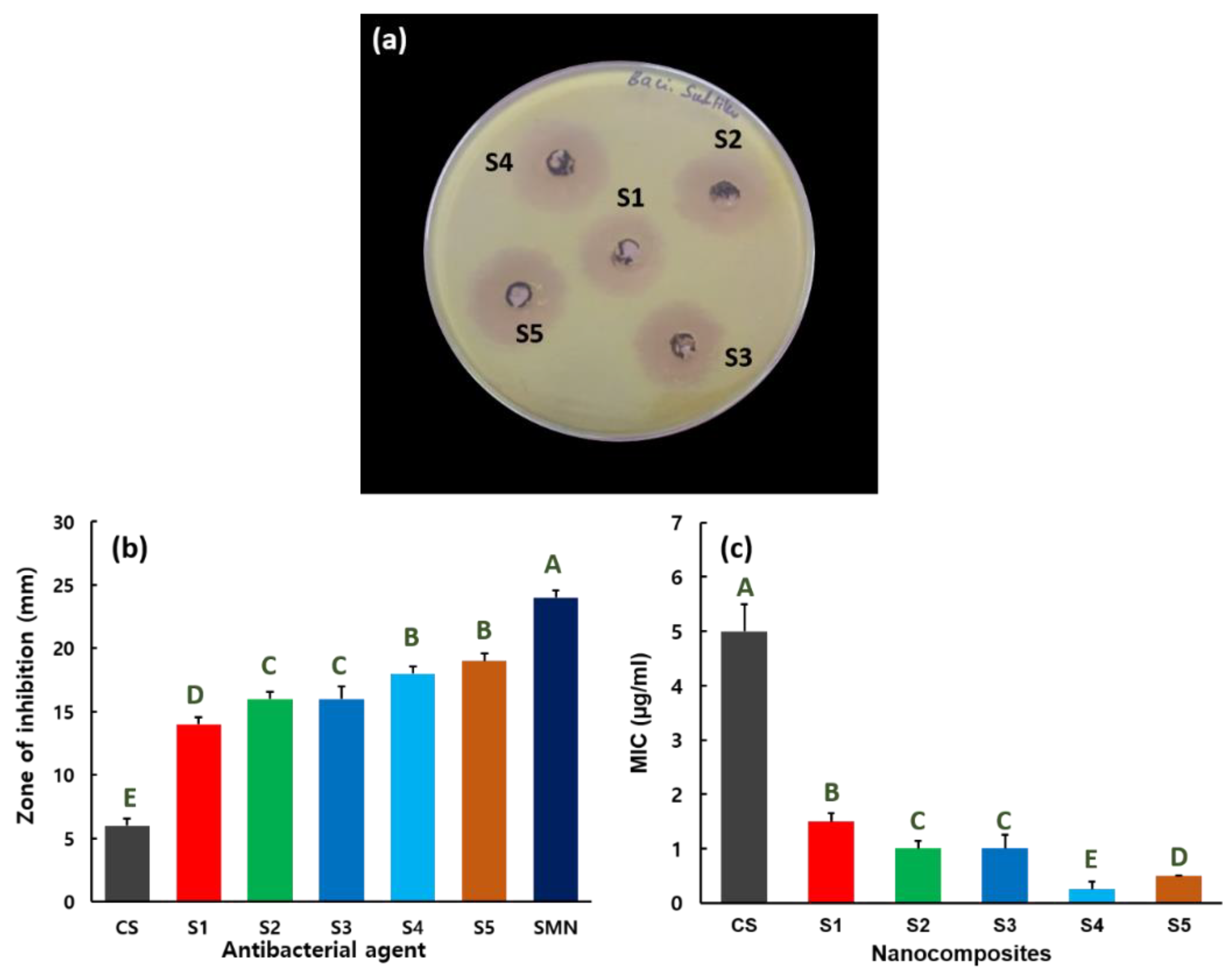
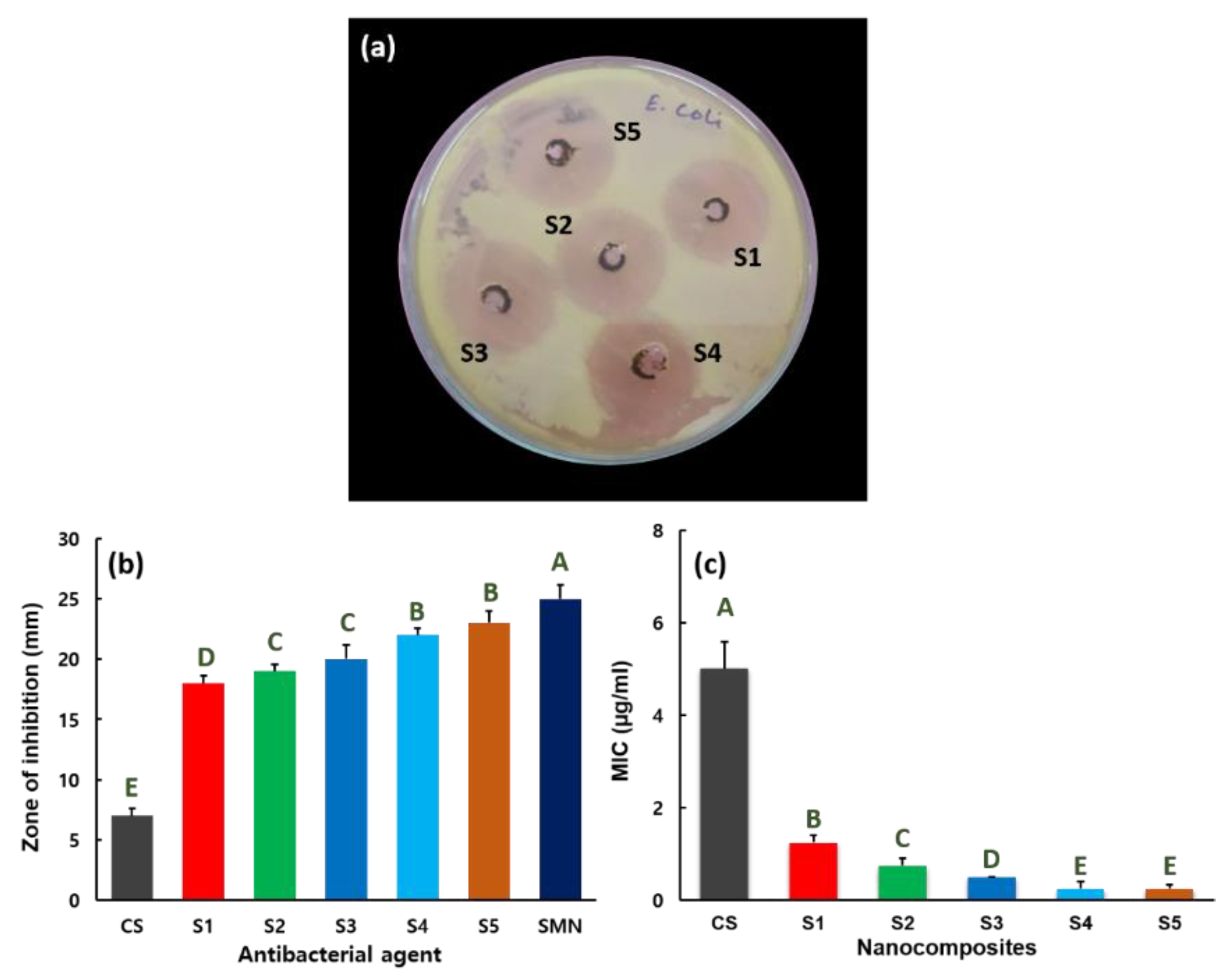
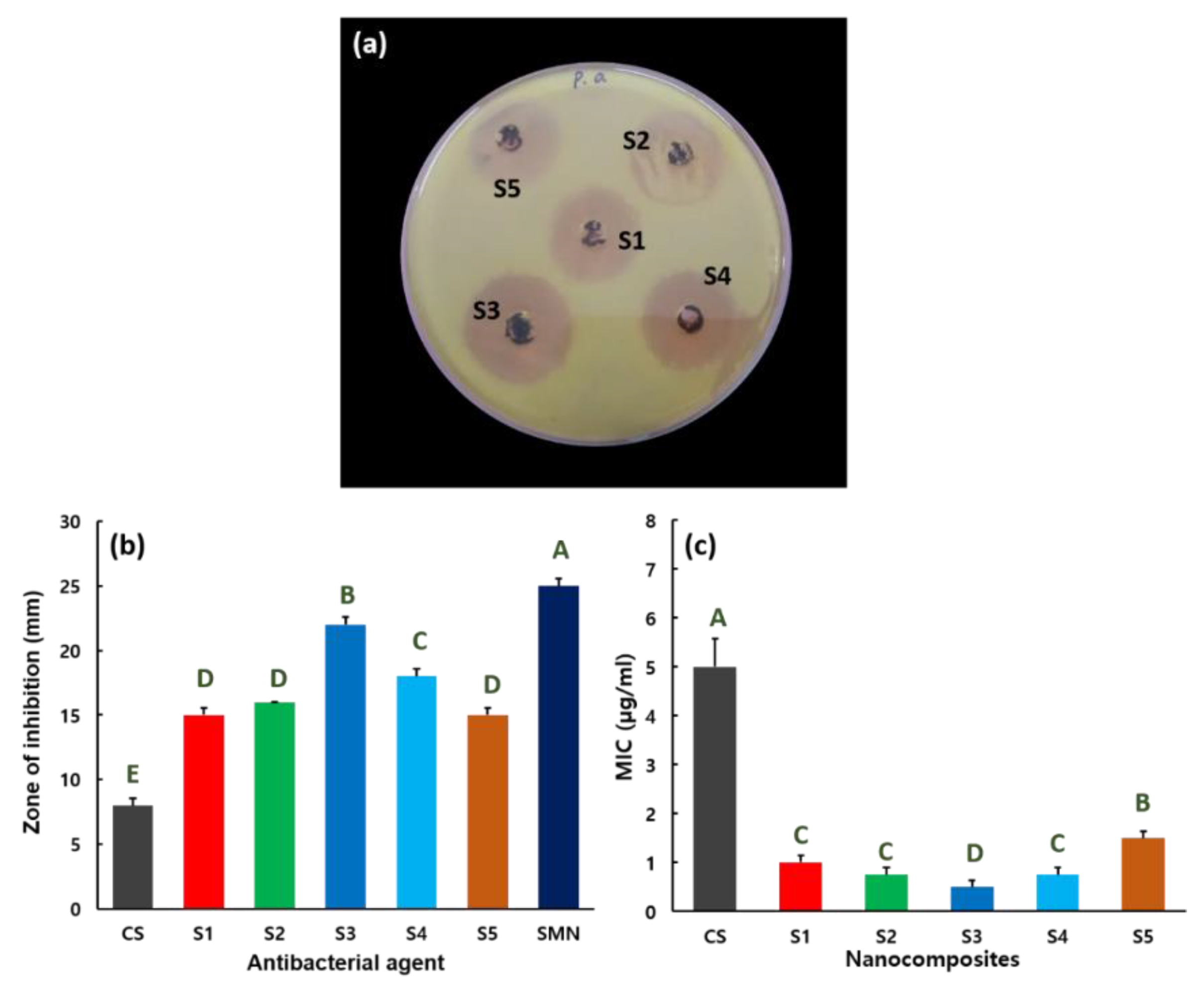
2.5. Antibacterial Activity
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Perl, T.M.; Cullen, J.J.; Wenzel, R.P.; Zimmerman, M.B.; Pfaller, M.A.; Sheppard, D.; Twombley, J.; French, P.P.; Herwaldt, L.A. Intranasal mupirocin to prevent postoperative staphylococcus aureus infections. N. Engl. J. Med. 2002, 346, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Chatterjee, A.; Modarai, M.; Naylor, N.R.; Boyd, S.E.; Atun, R.; Barlow, J.; Holmes, A.H.; Johnson, A.; Robotham, J.V. Quantifying drivers of antibiotic resistance in humans: A systematic review. Lancet Infect. Dis. 2018, 18, e368–e378. [Google Scholar] [CrossRef]
- Packirisamy, R.G.; Govindasamy, C.; Sanmugam, A.; Venkatesan, S.; Kim, H.-S.; Vikraman, D. Synthesis of novel sn1-xznxo-chitosan nanocomposites: Structural, morphological and luminescence properties and investigation of antibacterial properties. Int. J. Biol. Macromol. 2019, 138, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.; Zhao, X.; Gong, N.; Chen, J.; Li, X.; Gan, Y.; Wang, Y.; Zhang, Z.; Zhang, Y.; Guo, W.; et al. Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection. Nat. Commun. 2019, 10, 4336. [Google Scholar] [CrossRef]
- Clardy, J.; Fischbach, M.A.; Currie, C.R. The natural history of antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef]
- Waksman, S.A.; Tishler, M. The chemical nature of actinomycin, an anti-microbial substance produced by actinomyces antibioticus. J. Biol. Chem. 1942, 142, 519–528. [Google Scholar]
- Oloke, J.K. Activity pattern of natural and synthetic antibacterial agents among hospital isolates. Microbios 2000, 102, 175–181. [Google Scholar]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1. [Google Scholar] [CrossRef]
- Sugden, R.; Kelly, R.; Davies, S. Combatting antimicrobial resistance globally. Nat. Microbiol. 2016, 1, 16187. [Google Scholar] [CrossRef]
- Aminov, R. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent advances and challenges on applications of nanotechnology in food packaging. A literature review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Karimi, N. Ultrasound assisted-phytofabricated fe3o4 nps with antioxidant properties and antibacterial effects on growth, biofilm formation, and spreading ability of multidrug resistant bacteria. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2405–2423. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar]
- Cavalieri, F.; Tortora, M.; Stringaro, A.; Colone, M.; Baldassarri, L. Nanomedicines for antimicrobial interventions. J. Hosp. Infect. 2014, 88, 183–190. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Houghton, F. Antimicrobial resistance (amr) and the united nations (un). J. Infect. Public Health 2017, 10, 139–140. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on escherichia coli and staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. Schiff base rare earth metal complexes: Studies on functional, optical and thermal properties and assessment of antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 403–410. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. Zno nanofluids—A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater. Sci. Eng. C 2015, 53, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Andiappan, K.; Sanmugam, A.; Deivanayagam, E.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. In vitro cytotoxicity activity of novel schiff base ligand–lanthanide complexes. Sci. Rep. 2018, 8, 3054. [Google Scholar] [CrossRef]
- Bera, R.K.; Mandal, S.M.; Raj, C.R. Antimicrobial activity of fluorescent ag nanoparticles. Lett. Appl. Microbiol. 2014, 58, 520–526. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef]
- Nešović, K.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Perić-Grujić, A.; Rhee, K.Y.; Mišković-Stanković, V. Silver/poly(vinyl alcohol)/chitosan/graphene hydrogels—Synthesis, biological and physicochemical properties and silver release kinetics. Compos. Part. B: Eng. 2018, 154, 175–185. [Google Scholar]
- Kurniasih, M.; Purwati; Cahyati, T.; Dewi, R.S. Carboxymethyl chitosan as an antifungal agent on gauze. Int. J. Biol. Macromol. 2018, 119, 166–171. [Google Scholar] [CrossRef]
- Sanmugam, A.; Vikraman, D.; Park, H.J.; Kim, H.S. One-pot facile methodology to synthesize chitosan-zno-graphene oxide hybrid composites for better dye adsorption and antibacterial activity. Nanomaterials 2017, 7, 363. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nmgo) against pathogenic bacteria, yeasts, and biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, T.A.; Sunzel, B.; Holm, S.; Elmros, T.; Hallmans, G.; Sjöberg, S. Antibacterial effect of zinc oxide in vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325. [Google Scholar] [PubMed]
- Krishnan, B.; Mahalingam, S. Synthesis and characterization of mn3o4/bc nanocomposite and its antimicrobial activity. J. Inorg. Organomet. Polym. Mater. 2017, 27, 275–284. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Selvanathan, G. Ionic liquid-mediated: Enhanced surface morphology of silver/manganese oxide/bentonite nanocomposite for improved biological activities. J. Mol. Liq. 2018, 249, 1020–1032. [Google Scholar]
- Chowdhury, A.-N.; Azam, M.S.; Aktaruzzaman, M.; Rahim, A. Oxidative and antibacterial activity of mn3o4. J. Hazard. Mater. 2009, 172, 1229–1235. [Google Scholar] [CrossRef]
- Bomila, R.; Srinivasan, S.; Venkatesan, A.; Bharath, B.; Perinbam, K. Structural, optical and antibacterial activity studies of ce-doped zno nanoparticles prepared by wet-chemical method. Mater. Res. Innov. 2018, 22, 379–386. [Google Scholar] [CrossRef]
- Sharma, N.; Jandaik, S.; Kumar, S.; Chitkara, M.; Sandhu, I.S. Synthesis, characterisation and antimicrobial activity of manganese- and iron-doped zinc oxide nanoparticles. J. Exp. Nanosci. 2016, 11, 54–71. [Google Scholar] [CrossRef]
- Sanmugam, A.; Vikraman, D.; Karuppasamy, K.; Lee, J.Y.; Kim, H.-S. Evaluation of the corrosion resistance properties of electroplated chitosan-zn1−xcuxo composite thin films. Nanomaterials 2017, 7, 432. [Google Scholar] [CrossRef]
- Sundar, K.; Harikarthick, V.; Karthika, V.S.; Ravindran, A. Preparation of chitosan-graphene oxide nanocomposite and evaluation of its antimicrobial activity. J. Bionanosci. 2014, 8, 207–212. [Google Scholar] [CrossRef]
- Hu, H.; Xin, J.H.; Hu, H.; Chan, A.; He, L. Glutaraldehyde–chitosan and poly (vinyl alcohol) blends, and fluorescence of their nano-silica composite films. Carbohydr. Polym. 2013, 91, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Konwar, A.; Kalita, S.; Kotoky, J.; Chowdhury, D. Chitosan–iron oxide coated graphene oxide nanocomposite hydrogel: A robust and soft antimicrobial biofilm. ACS Appl. Mater. Interfaces 2016, 8, 20625–20634. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Feizy, J.; Mehrjo, F.; Farrokhnia, M. Detection and quantification of food colorant adulteration in saffron sample using chemometric analysis of ft-ir spectra. RSC Adv. 2016, 6, 23085–23093. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chun, C.S.; Chandrasekaran, M. Preparation and in vitro characterization of chitosan nanoparticles and their broad-spectrum antifungal action compared to antibacterial activities against phytopathogens of tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef]
- Wang, S.-M.; Huang, Q.-Z.; Wang, Q.-S. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr. Res. 2005, 340, 1143–1147. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An xps, ft-ir and sem study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.; Liu, Y.; Tang, S.; Han, X.; Chen, M. Controlled synthesis of mn3o4 nanocrystallites and mnooh nanorods by a solvothermal method. J. Cryst. Growth 2004, 263, 394–399. [Google Scholar] [CrossRef]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Lokhande, C.D. Conversion of chemically prepared interlocked cubelike mn3o4 to birnessite mno2 using electrochemical cycling. J. Electrochem. Soc. 2010, 157, A812–A817. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Tripathy, S.; Chandra, S.; Roy, S.; Sahu, S.K. A zno decorated chitosan–graphene oxide nanocomposite shows significantly enhanced antimicrobial activity with ros generation. RSC Adv. 2015, 5, 49420–49428. [Google Scholar] [CrossRef]
- Chithra, M.J.; Sathya, M.; Pushpanathan, K. Effect of ph on crystal size and photoluminescence property of zno nanoparticles prepared by chemical precipitation method. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 394–404. [Google Scholar] [CrossRef]
- Kar, P.; Sardar, S.; Ghosh, S.; Parida, M.R.; Liu, B.; Mohammed, O.F.; Lemmens, P.; Pal, S.K. Nano surface engineering of mn2o3 for potential light-harvesting application. J. Mater. Chem. C 2015, 3, 8200–8211. [Google Scholar] [CrossRef]
- Yousefi, R.; Zak, A.K.; Jamali-Sheini, F. Growth, x-ray peak broadening studies, and optical properties of mg-doped zno nanoparticles. Mater. Sci. Semicond. Process. 2013, 16, 771–777. [Google Scholar] [CrossRef]
- Mohammad Toufiq, A.; Wang, F.; Javed, Q.-U.-A.; Li, Q.; Li, Y. Hydrothermal synthesis of cu0.45mn0.55o2 nanowhiskers: Structural characterizations and optical properties. Mater. Lett. 2014, 118, 34–38. [Google Scholar] [CrossRef]
- Rani, B.J.; Ravina, M.; Ravi, G.; Ravichandran, S.; Ganesh, V.; Yuvakkumar, R. Synthesis and characterization of hausmannite (mn3o4) nanostructures. Surf. Interfaces 2018, 11, 28–36. [Google Scholar] [CrossRef]
- Thunus, L.; Lejeune, R. Overview of transition metal and lanthanide complexes as diagnostic tools. Coord. Chem. Rev. 1999, 184, 125–155. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on e. Coli and s. Aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Jeon, S.J.; Oh, M.; Yeo, W.-S.; Galvão, K.N.; Jeong, K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef]
- Felicio, R.; Cavalheiro, E.; Dockal, E. Preparation, characterization and thermogravimetric studies of [n, n′-cis-1, 2-cyclohexylene bis (salicylideneaminato)] cobalt (ii) and [n, n′-(±)-trans-1, 2-cyclo-hexylene bis (salicylideneaminato)] cobalt (ii). Polyhedron 2001, 20, 261–268. [Google Scholar] [CrossRef]

| Nanocomposites | Source | Label | ||
|---|---|---|---|---|
| Chitin (g) | MnCl2 (w/v) % | ZnCl2 (w/v) % | ||
| Mn3O4–CS | 0.25 | 0.5 | 0 | S1 |
| ZnMn2O4–CS | 0.25 | 0.5 | 0.5 | S2 |
| ZnMn2O4–CS | 0.25 | 1.0 | 1.0 | S3 |
| ZnMn2O4–CS | 0.25 | 1.5 | 1.5 | S4 |
| ZnMn2O4–CS | 0.25 | 2.0 | 2.0 | S5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Packirisamy, R.G.; Govindasamy, C.; Sanmugam, A.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. Synthesis and Antibacterial Properties of Novel ZnMn2O4–Chitosan Nanocomposites. Nanomaterials 2019, 9, 1589. https://doi.org/10.3390/nano9111589
Packirisamy RG, Govindasamy C, Sanmugam A, Karuppasamy K, Kim H-S, Vikraman D. Synthesis and Antibacterial Properties of Novel ZnMn2O4–Chitosan Nanocomposites. Nanomaterials. 2019; 9(11):1589. https://doi.org/10.3390/nano9111589
Chicago/Turabian StylePackirisamy, Rajiv Gandhi, Chandramohan Govindasamy, Anandhavelu Sanmugam, K. Karuppasamy, Hyun-Seok Kim, and Dhanasekaran Vikraman. 2019. "Synthesis and Antibacterial Properties of Novel ZnMn2O4–Chitosan Nanocomposites" Nanomaterials 9, no. 11: 1589. https://doi.org/10.3390/nano9111589
APA StylePackirisamy, R. G., Govindasamy, C., Sanmugam, A., Karuppasamy, K., Kim, H.-S., & Vikraman, D. (2019). Synthesis and Antibacterial Properties of Novel ZnMn2O4–Chitosan Nanocomposites. Nanomaterials, 9(11), 1589. https://doi.org/10.3390/nano9111589







