Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
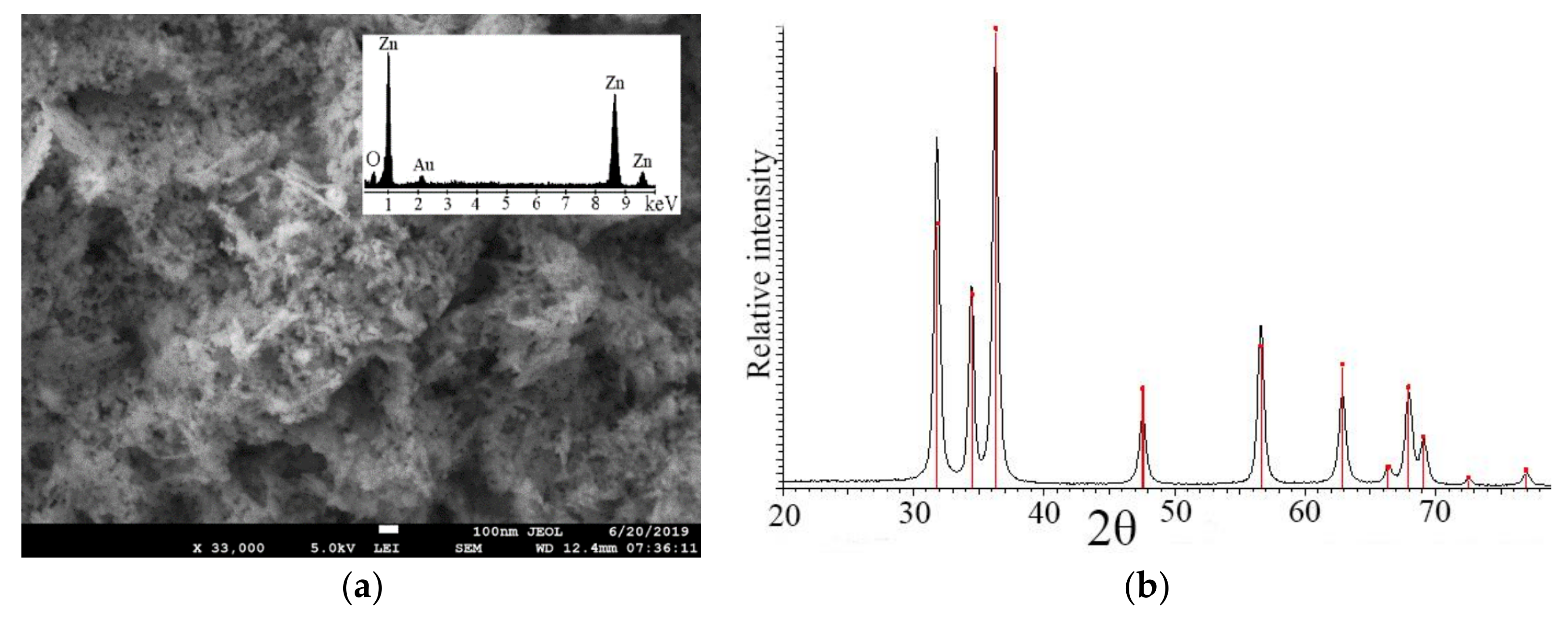
2.1. Characterization of ZnO Nanoparticles
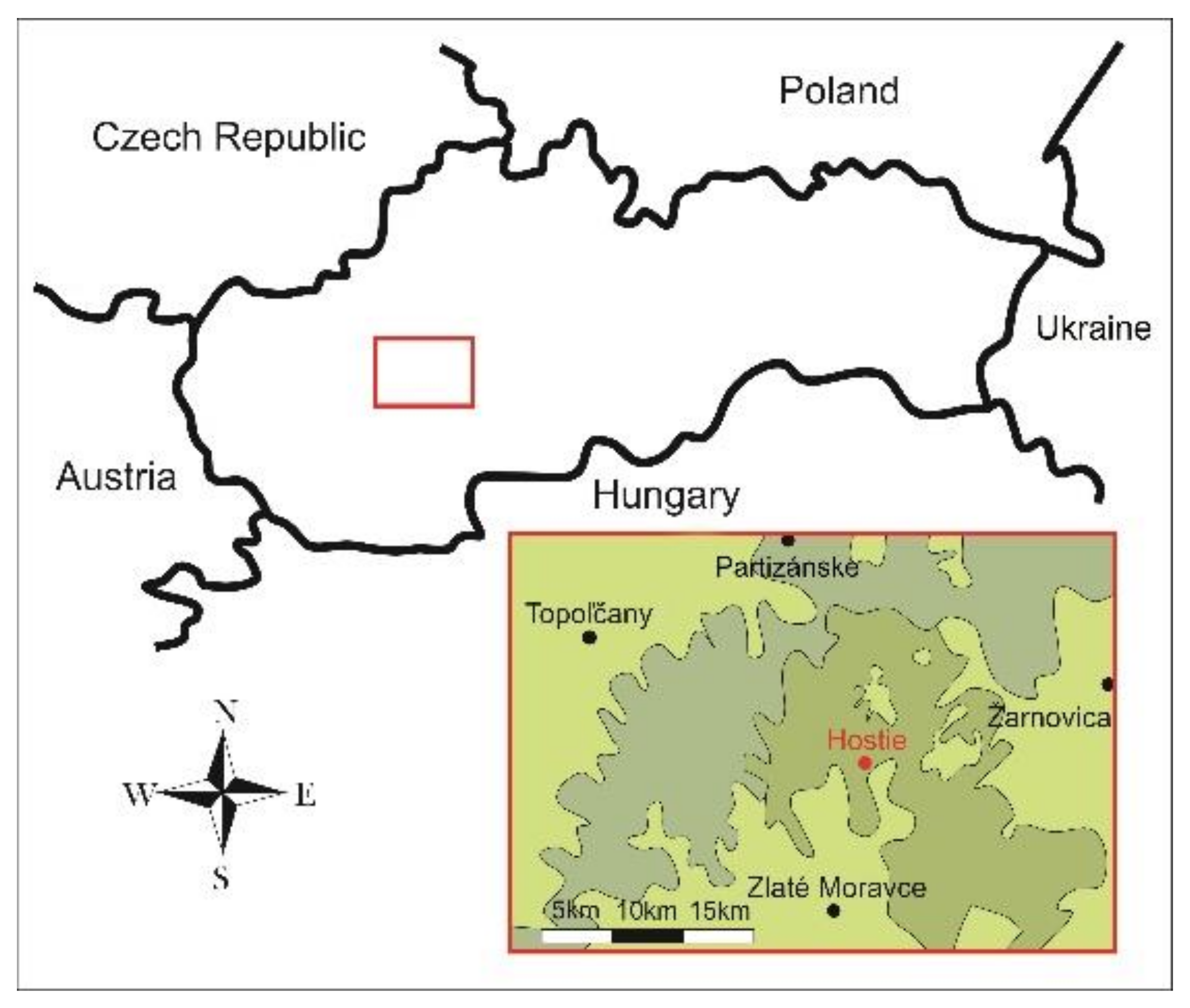
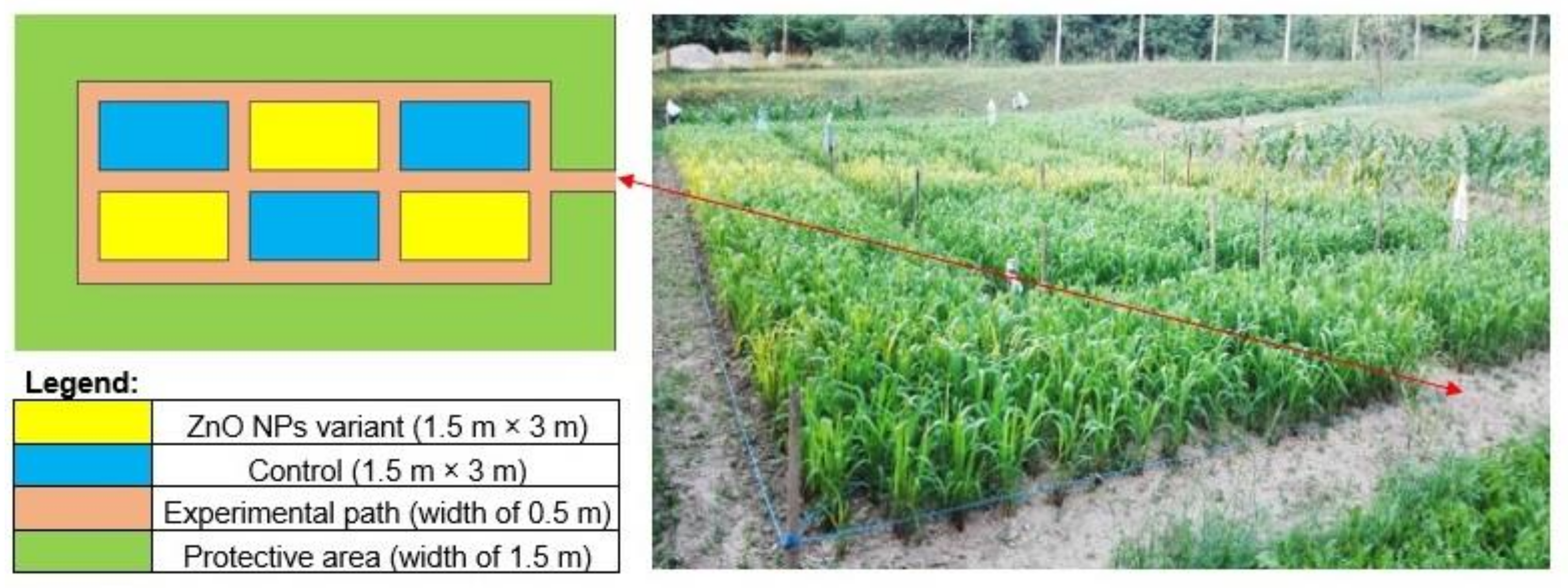
2.2. Site Description and Field Experiments
2.3. Analysis of Foxtail Millet Quantitative and Nutritional Parameters
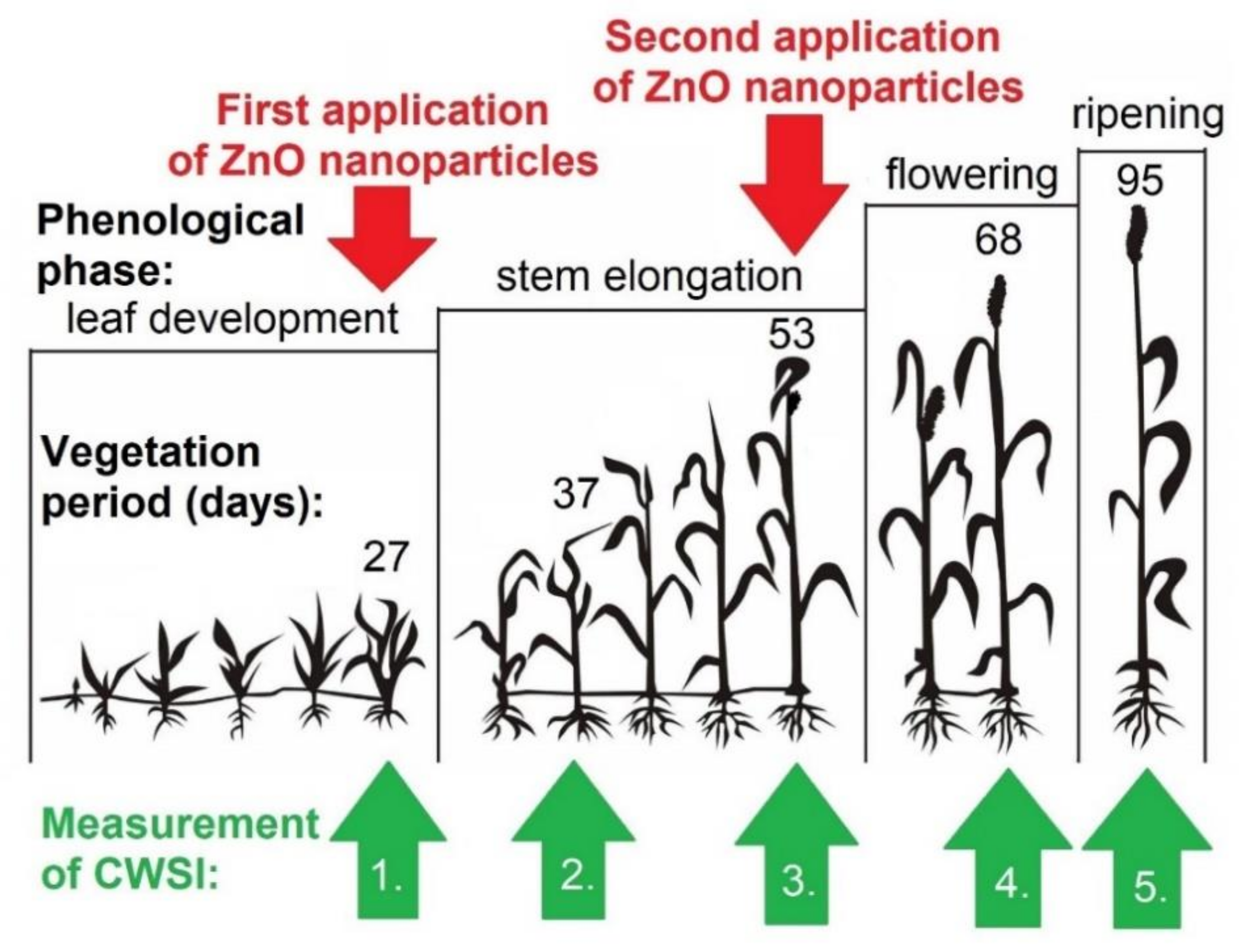
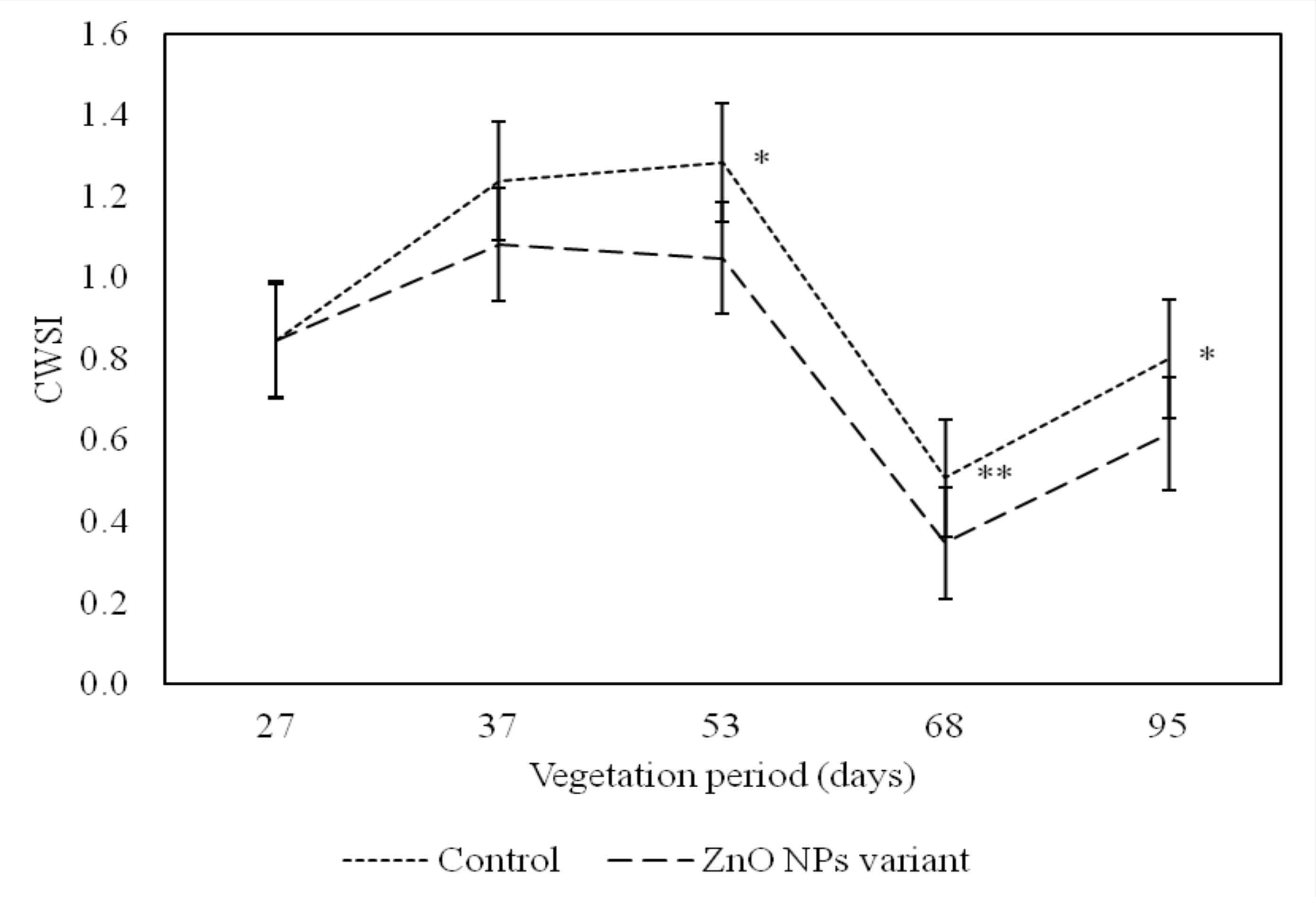
2.4. Observation of the Foxtail Millet Crop Water Stress Index Physiological Parameter
2.5. Statistical Analysis
3. Results and Discussion
3.1. Zinc Oxide Nanoparticle Characterization
3.2. Effects of Zinc Oxide Nanoparticles on Foxtail Millet’s Quantitative, Nutritional, and Physiological Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schils, R.; Olesen, J.E.; Kersebaum, K.-C.; Rijk, B.; Oberforster, M.; Kalyada, V.; Khitrykau, M.; Gobin, A.; Kirchev, H.; Manolova, V.; et al. Cereal yield gaps across Europe. Eur.J. Agron. 2018, 101, 109–120. [Google Scholar] [CrossRef]
- You, L.; Wood-Sichra, U.; Fritz, S.; Guo, Z.; See, L.; Koo, J. Spatial Production Allocation Model (SPAM). 2014. Available online: http://mapspam (accessed on 21 April 2015).
- Chandra, D.; Chandra, S.; Sharma, A.K. Review of Finger millet (Eleusine coracana (L.) Gaertn): A power house of health benefiting nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Cheng, L.; Zhang, Y.; Hou, S.; Sun, Z.; Li, H.; Han, Y. Carotenoid composition and expression of biosynthetic genes in yellow and white foxtail millet [Setaria italica (L.) Beauv]. J. Cereal Sci. 2019, 85, 84–90. [Google Scholar] [CrossRef]
- Liu, J.; Tang, X.; Zhang, Y.; Zhao, W. Determination of the volatile composition in brown millet, milled millet and millet bran by gas chromatography/mass spectrometry. Molecules 2012, 17, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Belay, G.; De Wet, J. Plant Resources of Tropical Africa 1: Cereals and Pulses; PROTA Foundation: Wageningen, The Netherlands, 2006. [Google Scholar]
- Rasnake, M.; Lacefield, G.; Miksch, D.; Bitzer, M. Producing Summer Annual Grasses for Emergency or Supplemental Forage; AGR–88; University of Kentucky: Lexington, KY, USA, 1998. [Google Scholar]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- López-Vargas, E.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Bellesi, F.J.; Arata, A.F.; Martínez, M.; Arrigoni, A.C.; Stenglein, S.A.; Dinolfo, M.I. Degradation of gluten proteins by Fusarium species and their impact on the grain quality of bread wheat. J. Stored Prod. Res. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Wagner, G.; Korenkov, V.; Judy, J.; Bertsch, P. Nanoparticles composed of Zn and ZnO inhibit Peronospora tabacina spore germination in vitro and P. tabacina infectivity on tobacco leaves. Nanomaterials 2016, 6, 50. [Google Scholar] [CrossRef]
- Gkanatsiou, C.; Ntalli, N.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Essential metal-based nanoparticles (copper/iron nps) as potent nematicidal agents against Meloidogyne spp. J. Nanotechnol. Res. 2019, 2, 043–057. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Urbina, J.; Cruz-Alonso, A.; Santander-González, M.; Méndez-Albores, A.; Vázquez-Durán, A. Nanoscale zinc oxide particles for improving the physiological and sanitary quality of a Mexican landrace of red maize. Nanomaterials 2018, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): Investigating a wide range of concentrations. Toxicol. Environ. Chem. 2014, 96, 861–868. [Google Scholar] [CrossRef]
- Schmidt, M.; Horstmann, S.; De Colli, L.; Danaher, M.; Speer, K.; Zannini, E.; Arendt, E.K. Impact of fungal contamination of wheat on grain quality criteria. J. Cereal Sci. 2016, 69, 95–103. [Google Scholar] [CrossRef]
- Matzen, N.; Ravn Jørgensen, J.; Holst, N.; Nistrup Jørgensen, L. Grain quality in wheat—Impact of disease management. Eur. J. Agron. 2019, 103, 152–164. [Google Scholar] [CrossRef]
- Nandhini, M.; Rajini, S.B.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Shetty, H.S.; Prakash, H.S. Biofabricated zinc oxide nanoparticles as an eco-friendly alternative for growth promotion and management of downy mildew of pearl millet. Crop Prot. 2019, 121, 103–112. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, H.-M.; Li, Y.-W.; Huang, X.-P.; Wu, X.-L.; Zhai, T.; Yuan, Y.; Cai, Q.-Y.; Mo, C.-H. Effects of the size and morphology of zinc oxide nanoparticles on the germination of Chinese cabbage seeds. Environ. Sci. Pollut. Res. 2015, 22, 10452–10462. [Google Scholar] [CrossRef]
- Čurlík, J.; Šefčík, P. Geochemical atlas of the Slovak Republic. Part V Soils. In Slovak-Ministry for the Environ. MŽP; Soil Science and Conservation Research Institute (SSCRI): Bratislava, Slovakia, 1999; p. 99. [Google Scholar]
- Tian, B.; Luan, S.; Zhang, L.; Liu, Y.; Zhang, L.; Li, H. Penalties in yield and yield associated traits caused by stem lodging at different developmental stages in summer and spring foxtail millet cultivars. Field Crops Res. 2018, 217, 104–112. [Google Scholar] [CrossRef]
- Duflo, E.; Banerjee, A. Handbook of Field Experiments, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Chisi, M.; Peterson, G. Breeding and Agronomy. In Sorghum and Millets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–50. [Google Scholar]
- Choudhary, M.; Rana, K.S.; Bana, R.S.; Ghasal, P.C.; Choudhary, G.L.; Jakhar, P.; Verma, R.K. Energy budgeting and carbon footprint of pearl millet—Mustard cropping system under conventional and conservation agriculture in rainfed semi-arid agro-ecosystem. Energy 2017, 141, 1052–1058. [Google Scholar] [CrossRef]
- Hrivňáková, K.; Makovníková, J.; Barančíková, G.; Bezák, P.; Bezáková, Z.; Dodok, R.; Grečo, V.; Chlpík, J.; Kobza, J.; Lištjak, M.; et al. The Uniform Methods of Soil Analysis; VÚPOP: Bratislava, Slovakia, 2011; p. 136. [Google Scholar]
- Šinkovičová, M.; Igaz, D.; Kondrlová, E.; Jarošová, M. Soil particle size analysis by laser diffractometry: Result comparison with pipette method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 072025. [Google Scholar] [CrossRef]
- Kondrlova, E.; Igaz, D.; Horak, J. Effect of calculation models on particle size distribution estimated by laser diffraction. J. Ege Univ. Fac. Agric. Spec. Issue 2015, 21–27. [Google Scholar]
- Kováčik, P.; Wiśniowska-Kielian, B.; Smoleń, S. Effect of application of Mg-tytanit stimulator on winter wheat yielding and quantitative parameters of wheat straw and grain. J. Elem. 2018, 23, 697–708. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Burghardt, M.; Schreiber, L.; Riederer, M. Enhancement of the diffusion of active ingredients in barley leaf cuticular wax by monodisperse alcohol ethoxylates. J. Agric. Food Chem. 1998, 46, 1593–1602. [Google Scholar] [CrossRef]
- Räsch, A.; Hunsche, M.; Mail, M.; Burkhardt, J.; Noga, G.; Pariyar, S. Agricultural adjuvants may impair leaf transpiration and photosynthetic activity. Plant Physiol. Biochem. 2018, 132, 229–237. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1997. [Google Scholar]
- Shahidi, F. Extraction and measurement of total lipids. Curr. Protoc. Food Anal. Chem. 2003, 7. [Google Scholar] [CrossRef]
- Dvořáček, V.; Bradová, J.; Sedláček, T.; Šárka, E. Relationships among Mixolab rheological properties of isolated starch and white flour and quality of baking products using different wheat cultivars. J. Cereal Sci. 2019, 89, 102801. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef]
- Kovár, M.; Černý, I.; Ernst, D. Analysis of relations between crop temperature indices and yield of different sunflower hybrids foliar treated by biopreparations. Agriculture (Polnohospodárstvo) 2016, 62, 28–40. [Google Scholar] [CrossRef]
- Kirnak, H.; Irik, H.A.; Unlukara, A. Potential use of crop water stress index (CWSI) in irrigation scheduling of drip-irrigated seed pumpkin plants with different irrigation levels. Sci. Hortic. 2019, 256, 108608. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Archana, B.; Manjunath, K.; Nagaraju, G.; Chandra Sekhar, K.B.; Kottam, N. Enhanced photocatalytic hydrogen generation and photostability of ZnO nanoparticles obtained via green synthesis. Int. J. Hydrogen Energy 2017, 42, 5125–5131. [Google Scholar] [CrossRef]
- Drissi, S.; Houssa, A.A.; Bamouh, A.; Benbella, M. Corn silage (Zea mays L.) response to zinc foliar spray concentration when grown on sandy soil. J. Agric. Sci. 2015, 7, 68–79. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant Nutr. 2016, 39, 172–180. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.W.; Bolan, N.; Kim, K.-H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Sorghum and Millets in Human Nutrition; (Food and Nutrition Series); FAO: Rome, Italy, 1995. [Google Scholar]
- Mitchell, M.; Pritchard, J.; Okada, S.; Larroque, O.; Yulia, D.; Pettolino, F.; Szydlowski, N.; Singh, S.; Liu, Q.; Ral, J.-P. Oil accumulation in transgenic potato tubers alters starch quality and nutritional profile. Front. Plant Sci. 2017, 8, 554. [Google Scholar] [CrossRef]
- Raigond, P.; Raigond, B.; Kaundal, B.; Singh, B.; Joshi, A.; Dutt, S. Effect of zinc nanoparticles on antioxidative system of potato plants. J. Environ. Biol. 2017, 38, 435–439. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.M.; Ju, W.; Wang, H.; Qiu, F.; Yang, F.; Fan, W.; Huang, Q.; Wang, Y.-P.; Feng, Y.; et al. Improving the ability of the photochemical reflectance index to track canopy light use efficiency through differentiating sunlit and shaded leaves. Remote Sens. Environ. 2017, 194, 1–15. [Google Scholar] [CrossRef]
- Taghvaeian, S.; Comas, L.; DeJonge, K.C.; Trout, T.J. Conventional and simplified canopy temperature indices predict water stress in sunflower. Agric. Water Manag. 2014, 144, 69–80. [Google Scholar] [CrossRef]
| pH | Carbonates Content (%) | Grain-Size Distribution (mm) % | Content of Nutrients (mg.kg−1) | ||||
|---|---|---|---|---|---|---|---|
| 7.01 | 3.55 ± 0.06 | up to 0.002 | 25.66 | Nin | P | K | Zn available |
| 0.002–0.05 | 63.04 | 23.2 | 82.5 | 200 | 4.24 ± 0.24 | ||
| 0.05–2000 | 11.3 | ||||||
| silt loam | |||||||
| Measurement Number | Dates | Report |
|---|---|---|
| 1. | 27 May 2018 | Measurement before first ZnO NPs application |
| 2. | 6 June 2018 | Measurement at two weeks after first ZnO NPs application |
| 3. | 22 June 2018 | Measurement before the second ZnO NPs application |
| 4. | 7 July 2018 | Measurement at two weeks after second ZnO NPs application |
| 5. | 3 August 2018 | Measurement before harvest |
| Crystal Symmetry | Hexagonal |
|---|---|
| a-axes | 3.25077 ± 0.00008 Å |
| c-axe | 5.2097 ± 0.0002 Å |
| α | 90° |
| γ | 120° |
| Space group | P63m |
| Unit cell volume | 47.58 Å3 (Calculated from Unit Cell) |
| Lvol-IB | 17.3 ± 0.1 nanometer (Calculated from X-ray diffraction data) |
| ZnO NPs Foliarly Applied Variant | Control Variant (without ZnO NPs Application) | |
|---|---|---|
| Quantitative parameters | ||
| Plant high (mm) | 1089 ± 121 | 1031 ± 192 |
| Seed head length (mm) | 79.72 ± 8 | 76.94 ± 17 |
| Weigh of dry seed head (g) | 2.54 ± 0.24 | 2.91 ± 0.33 |
| Weight of thousand grains (TGW) (g) | 5.19 ± 0.51 | 5.37± 0.89 |
| Grain yield (g) | 1244 ± 199 | 1304 ± 157 |
| Nutritional parameters | ||
| Content of total nitrogen Ntot (mg.kg−1) | 17611 ± 38 ** | 17302 ± 11 ** |
| Content of oil (%) | 12.5 ± 0.29 ** | 9.33 ± 0.003 ** |
| Content of starch (%) | 48.23 ± 0.002 ** | 48.9 ± 0.002 ** |
| Dry mass (%) | 89.51 ± 0.02 * | 89.42 ± 0.04 * |
| Total proteins (%) | 11.77 ± 1.06 | 10.79 ± 0.05 |
| Physiological parameter | ||
| CWSI 1 | 0.7875 ± 0.042 ** | 0.9345 ± 0.031 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolenčík, M.; Ernst, D.; Komár, M.; Urík, M.; Šebesta, M.; Dobročka, E.; Černý, I.; Illa, R.; Kanike, R.; Qian, Y.; et al. Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomaterials 2019, 9, 1559. https://doi.org/10.3390/nano9111559
Kolenčík M, Ernst D, Komár M, Urík M, Šebesta M, Dobročka E, Černý I, Illa R, Kanike R, Qian Y, et al. Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomaterials. 2019; 9(11):1559. https://doi.org/10.3390/nano9111559
Chicago/Turabian StyleKolenčík, Marek, Dávid Ernst, Matej Komár, Martin Urík, Martin Šebesta, Edmud Dobročka, Ivan Černý, Ramakanth Illa, Raghavendra Kanike, Yu Qian, and et al. 2019. "Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions" Nanomaterials 9, no. 11: 1559. https://doi.org/10.3390/nano9111559
APA StyleKolenčík, M., Ernst, D., Komár, M., Urík, M., Šebesta, M., Dobročka, E., Černý, I., Illa, R., Kanike, R., Qian, Y., Feng, H., Orlová, D., & Kratošová, G. (2019). Effect of Foliar Spray Application of Zinc Oxide Nanoparticles on Quantitative, Nutritional, and Physiological Parameters of Foxtail Millet (Setaria italica L.) under Field Conditions. Nanomaterials, 9(11), 1559. https://doi.org/10.3390/nano9111559







