Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution
Abstract
1. Introduction
2. Experimental Methodology
2.1. Synthesis of Silver Nanoparticles (Ag NPs)
2.2. X-ray Diffraction
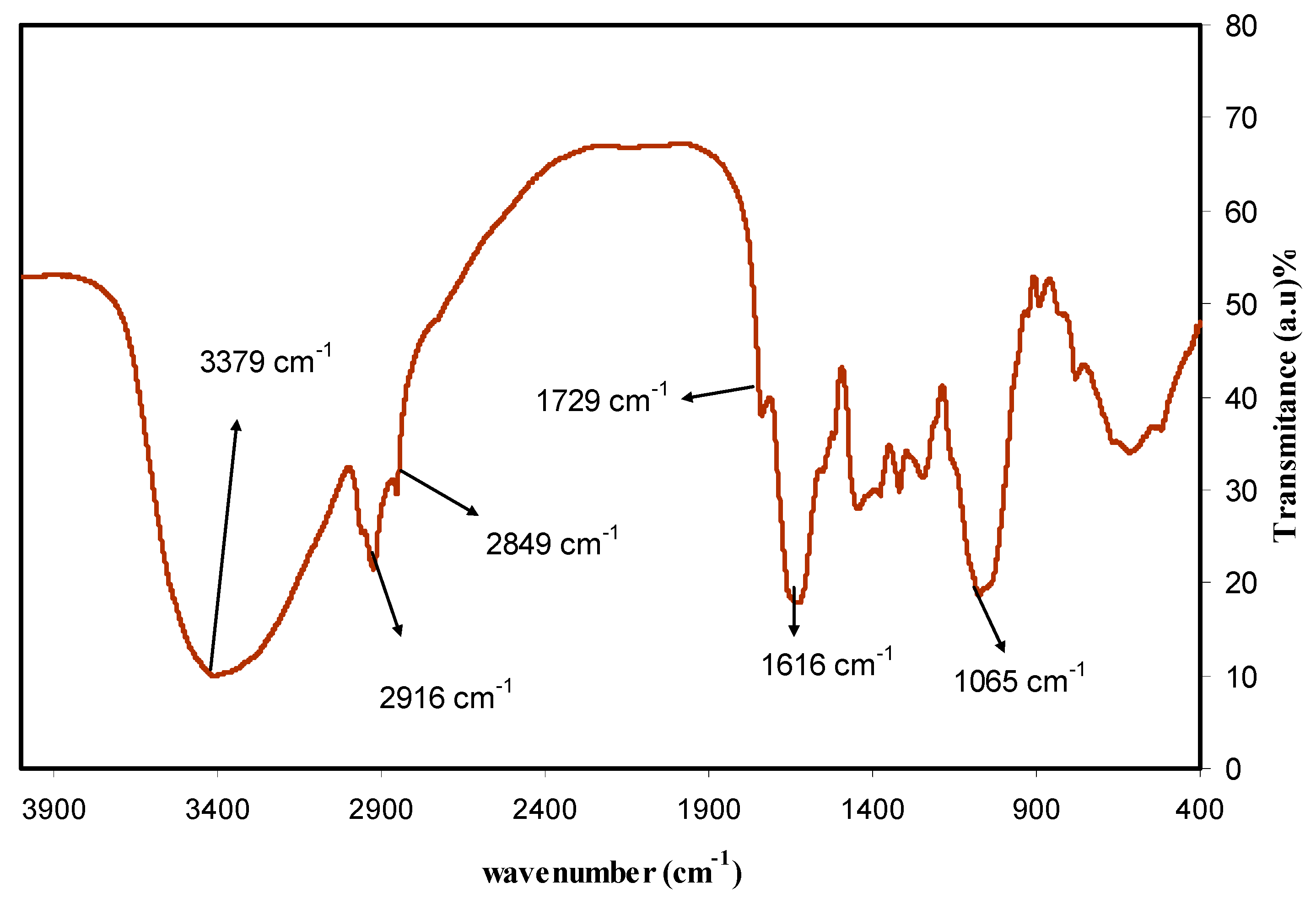
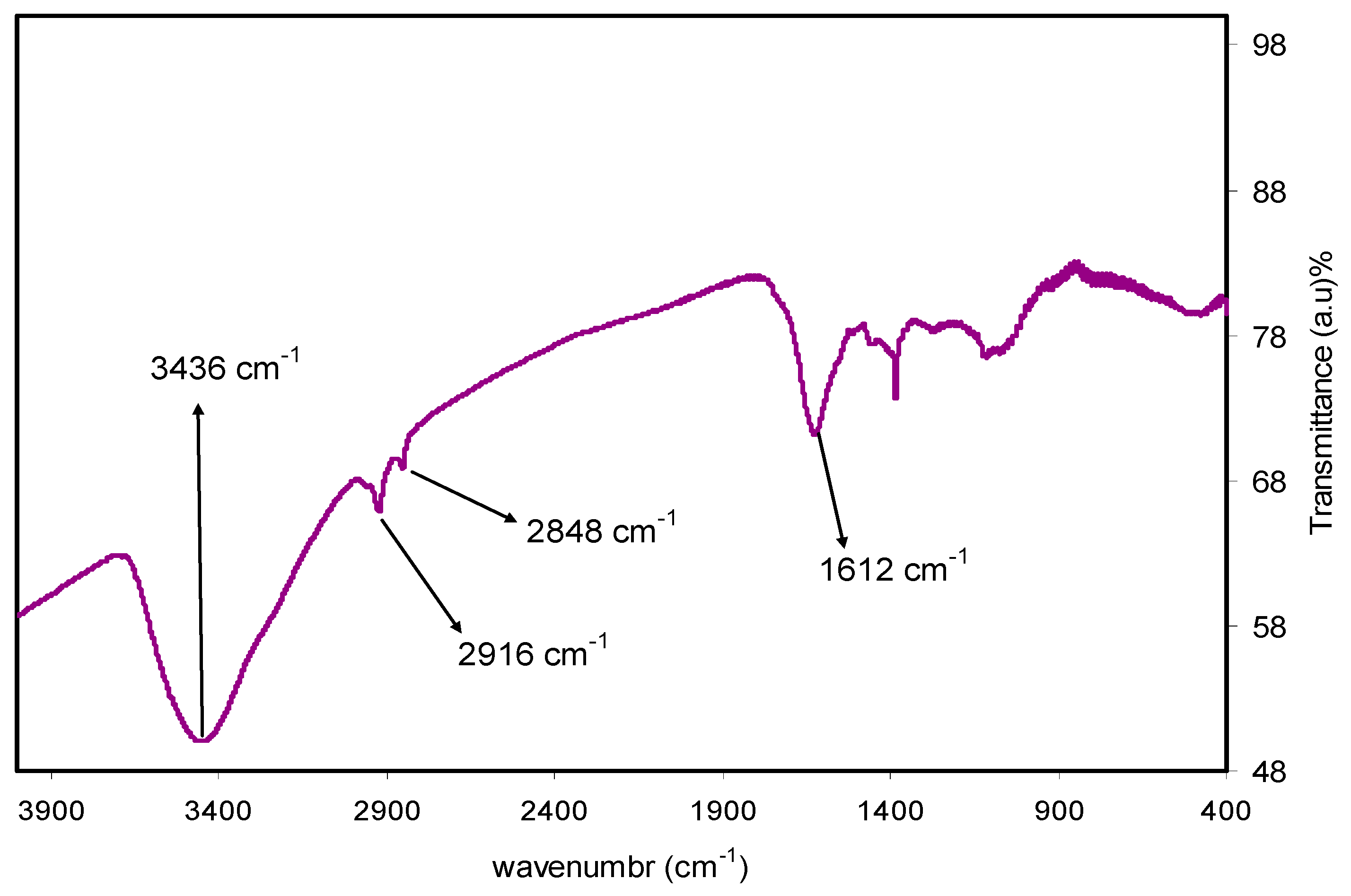
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Ultraviolet–Visible (UV-Vis) Measurement
3. Results and Discussion
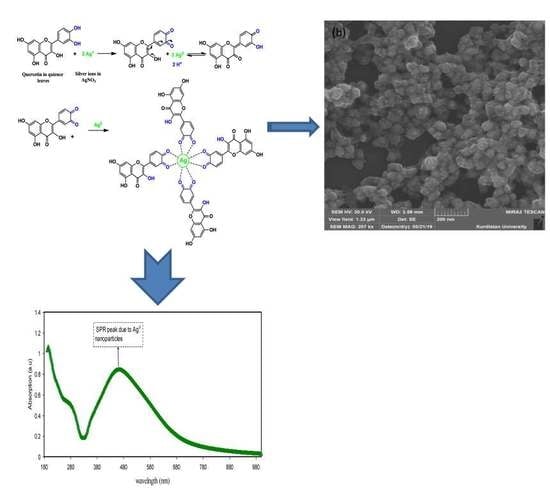
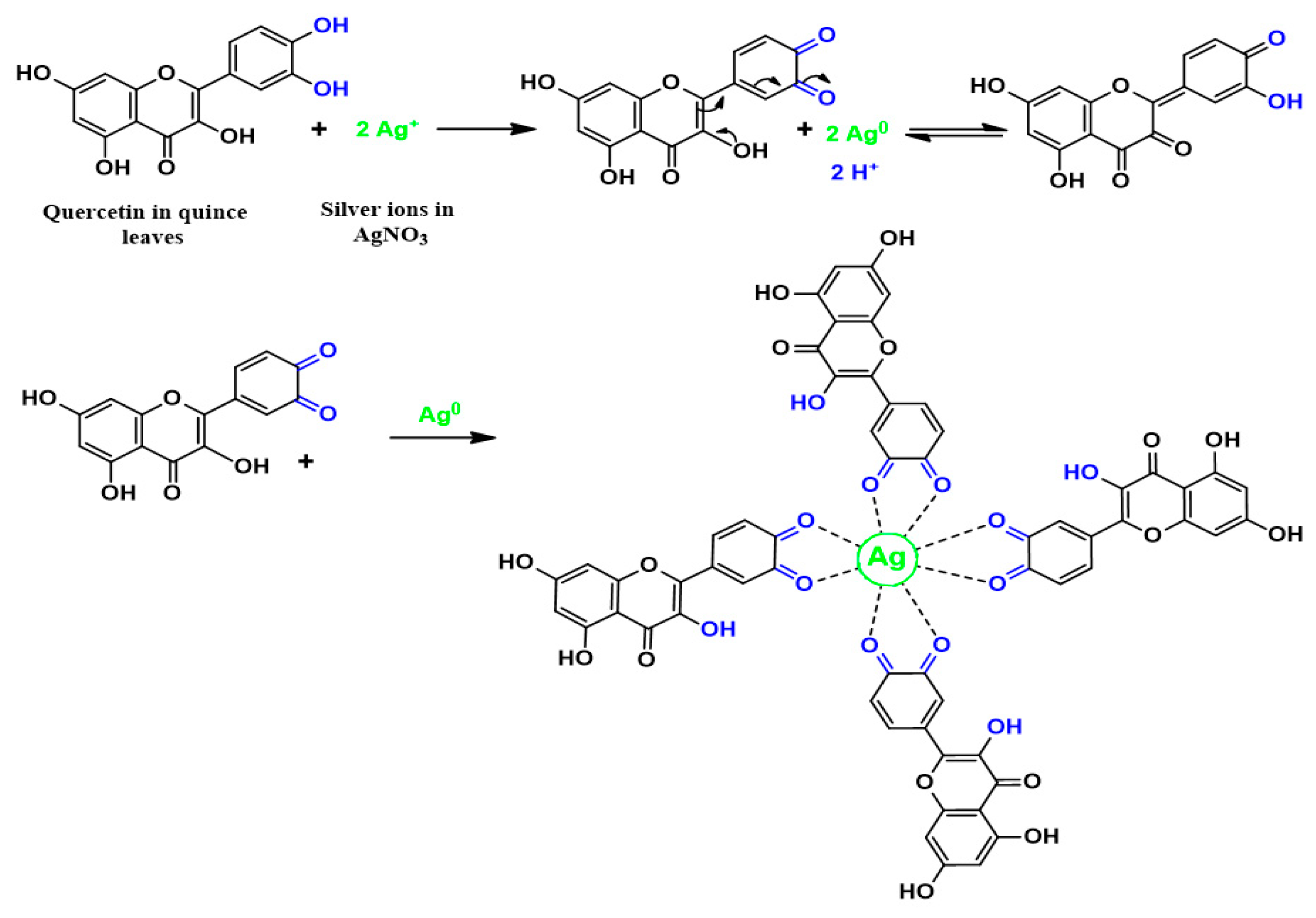
3.1. FTIR Analysis and Mechanism of Ag+1Ion Reduction
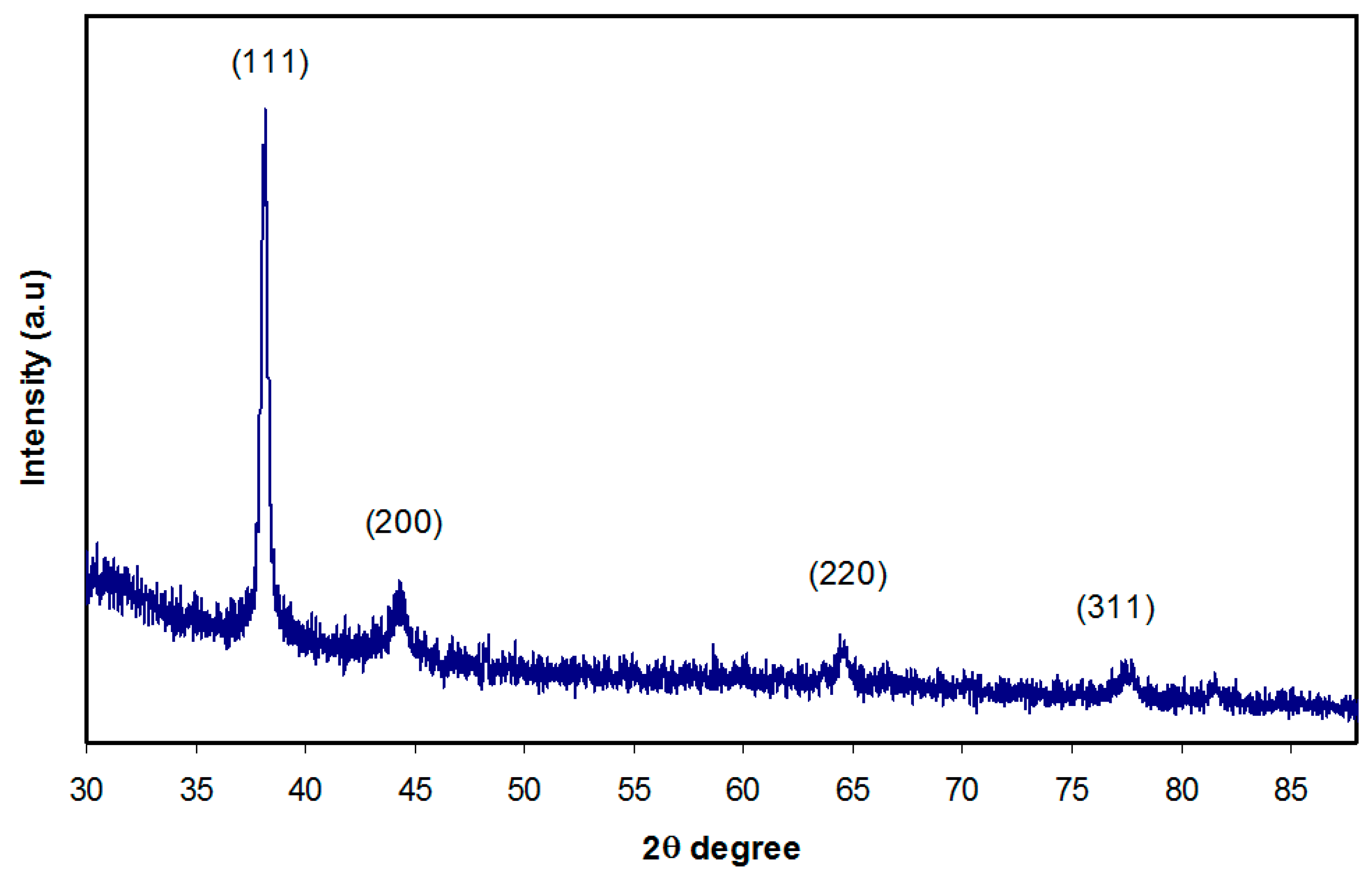
3.2. XRD Analysis
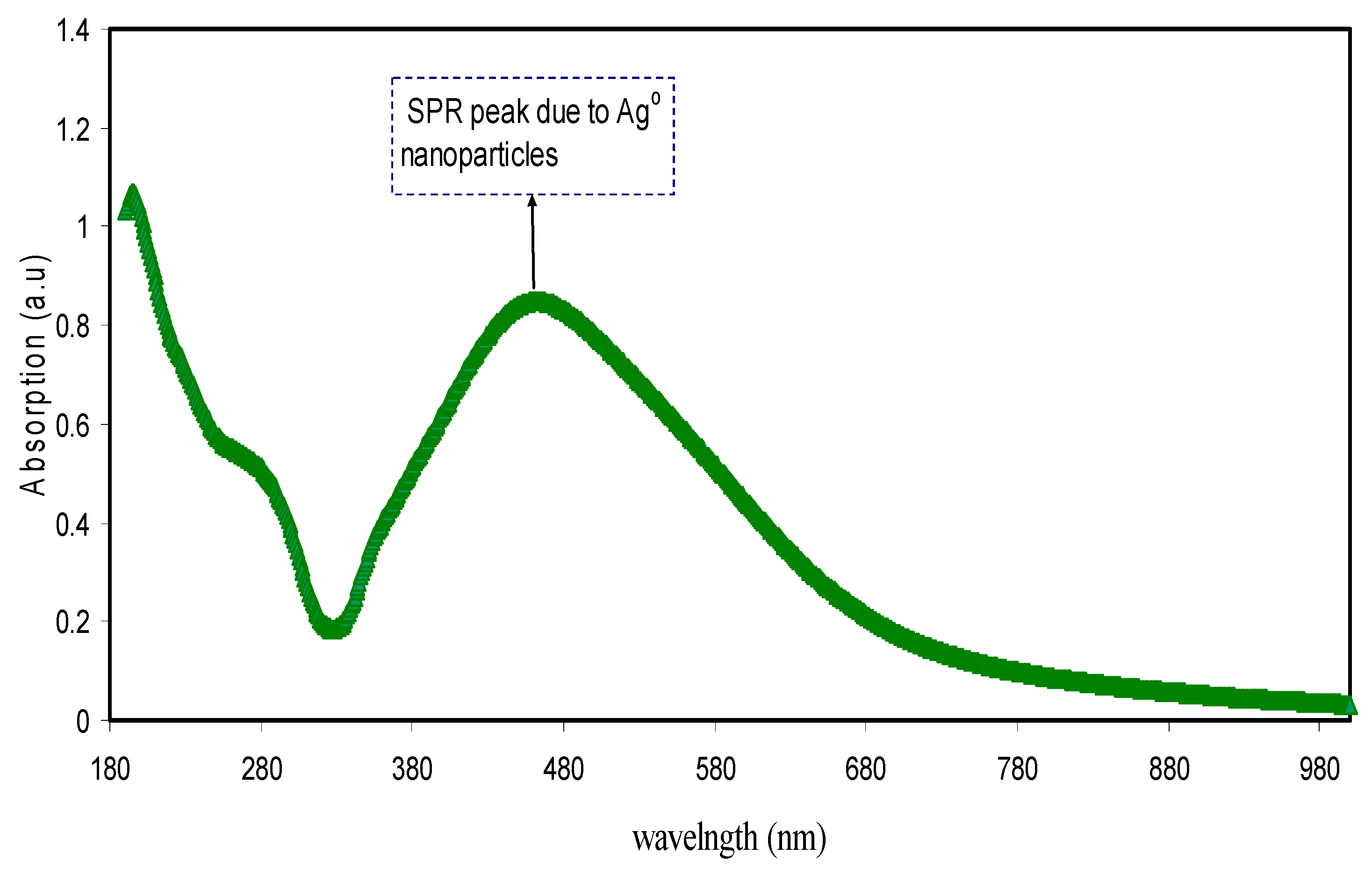
3.3. UV–Visible Absorption Study
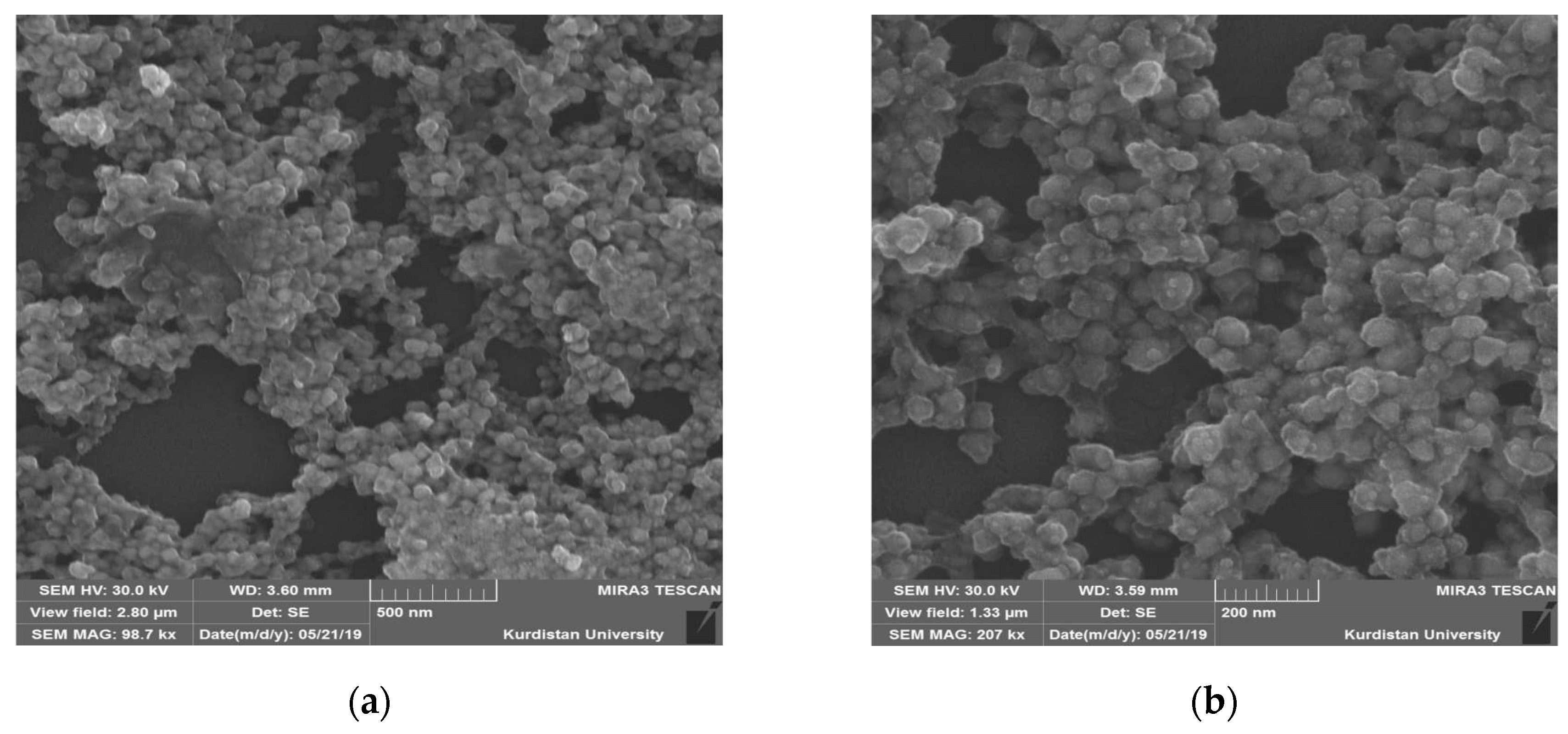
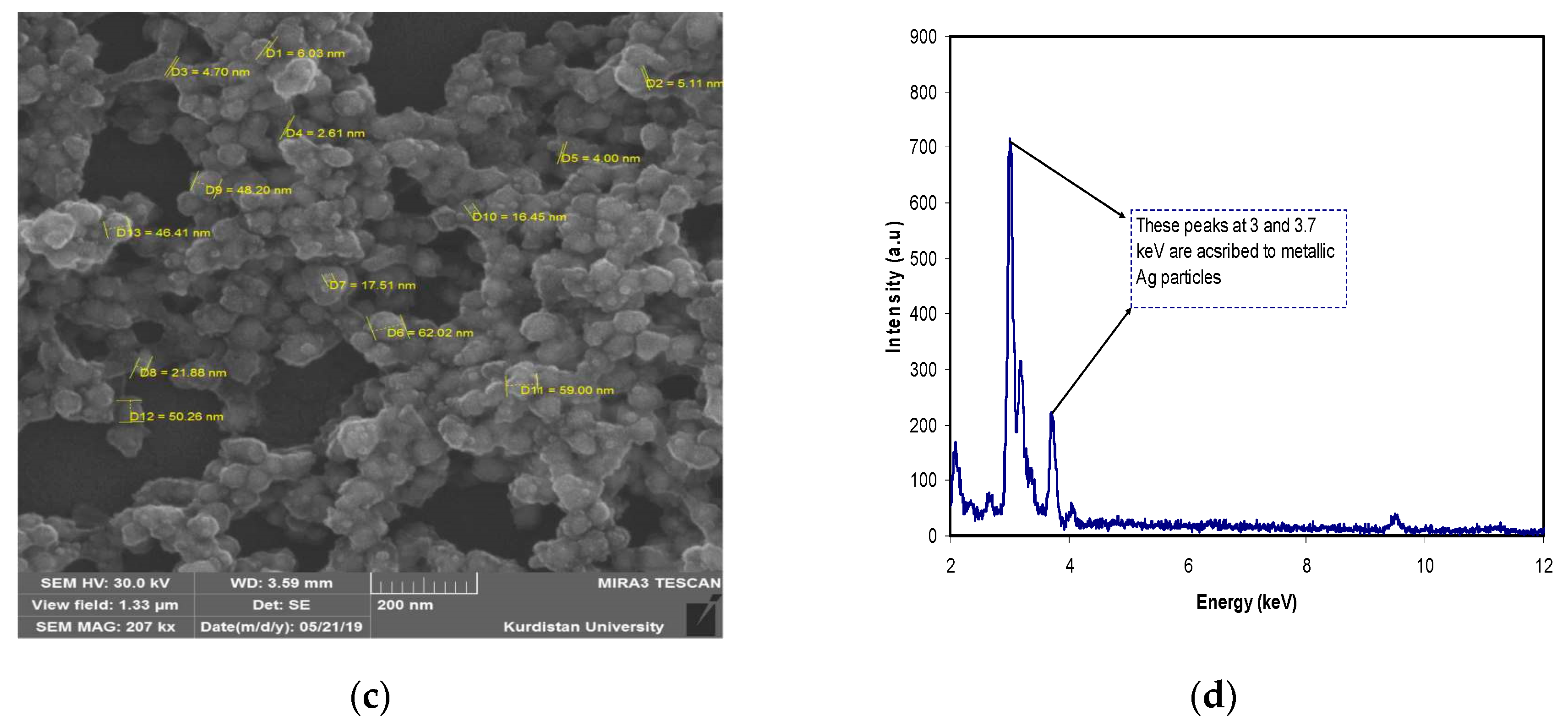
3.4. Morphological Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moodley:, J.S.; Krishna, S.B.; Pillay, K.; Govender, P. Green synthesis of silver nanoparticles from Moringaoleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Dmitruk, I.M.; Alexeenko, A.A.; Losytskyy, M.Y.; Kotko, A.V.; Pinchuk, A.O. Size-dependent surface-plasmon-enhanced photoluminescence from silver nanoparticles embedded in silica. Phys. Rev. B 2009, 79, 235438. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Yoon, W.J.; Jung, K.Y.; Liu, J.; Duraisamy, T.; Revur, R.; Teixeira, F.L.; Sengupta, S.; Berger, P.R. Plasmon-enhanced optical absorption and photocurrent in organic bulk heterojunction photovoltaic devices using self-assembled layer of silver nanoparticles. Sol. Energy Mater. Sol. Cells 2010, 94, 128–132. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, P.; Zhang, X.; Ravichandran, N.; Ying, H.; Yu, C.; Ying, H.; Xu, Y.; Yin, J.; Wang, K. New epigallocatechingallate (EGCG) nanocomplexes co-assembled with 3-mercapto-1-hexanol and β-lactoglobulin for improvement of antitumor activity. J. Biomed. Nanotechnol. 2017, 13, 805–814. [Google Scholar] [CrossRef]
- Khan, M.Z.; Tareq, F.K.; Hossen, M.A.; Roki, M.N. Green Synthesis and characterization of silver nanoparticles using coriandrumsativum leaf extract. J. Eng. Sci. Technol. 2018, 13, 158–166. [Google Scholar]
- Aziz, S.B.; Brza, M.A.; Mohamed, P.A.; Kadir, M.F.; Hamsan, M.H.; Abdulwahid, R.T.; Woo, H.J. Increase of metallic silver nanoparticles in Chitosan: AgNt based polymer electrolytes incorporated with alumina filler. Results Phys. 2019, 13, 102326. [Google Scholar] [CrossRef]
- Aziz, S.; Abdullah, R.; Rasheed, M.; Ahmed, H. Role of ion dissociation on DC conductivity and silver nanoparticle formation in PVA: AgNt based polymer electrolytes: Deep insights to ion transport mechanism. Polymers 2017, 9, 338. [Google Scholar] [CrossRef]
- Schaadt, D.M.; Feng, B.; Yu, E.T. Enhanced semiconductor optical absorption via surface plasmon excitation in metal nanoparticles. Appl. Phys. Lett. 2005, 86, 063106. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Chieng, B.W.; Nishibuchi, M.; Radu, S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia Sinensis. Int. J. Nanomed. 2012, 7, 4263–4267. [Google Scholar]
- Kouvaris, P.; Delimitis, A.; Zaspalis, V.; Papadopoulos, D.; Tsipas, S.A.; Michailidis, N. Green synthesis and characterization of silver nanoparticlesproduced using Arbutus Unedo leaf extract. Mater. Lett. 2012, 76, 18–20. [Google Scholar] [CrossRef]
- Khanna, P.K.; Subbarao, V.V. Synthesis of fine CdS powder from direct in-situ reduction of sulphur and cadmium salts in aqueous N, N\ -dimethylformamide. Mater. Lett. 2004, 58, 2801–2804. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Reduction of silver nanoparticles in DMF. Formation of monolayers and stable colloids. Pure Appl. Chem. 2000, 72, 83–90. [Google Scholar] [CrossRef]
- Piquemal, J.Y.; Viau, G.; Beaunier, P.; Bozon-Verduraz, F.; Fiévet, F. One-step construction of silver nanowires in hexagonal mesoporous silica using the polyol process. Mater. Res. Bull. 2003, 38, 389–394. [Google Scholar] [CrossRef]
- Ducamp-Sanguesa, C.; Herrera-Urbina, R.; Figlarz, M. Synthesis and characterization of fine and monodisperse silver particles of uniform shape. J. Solid State Chem. 1992, 100, 272–280. [Google Scholar] [CrossRef]
- Torigoe, K.; Nakajima, Y.; Esumi, K. Preparation and characterization of colloidal silver-platinum alloys. J. Phys. Chem. 1993, 97, 8304–8309. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, J.H.; Son, H.T.; Won, C.W.; Maeng, D.Y. New and effective chemical reduction method for preparation of nanosized silver powder and colloid dispersion. Mater. Res. Bull. 2003, 38, 949–956. [Google Scholar] [CrossRef]
- Khanna, P.K.; Subbarao, V.V. Nanosized silver powder via reduction of silver nitrate by sodium formaldehydesulfoxylate in acidic pH medium. Mater. Lett. 2003, 57, 2242. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Charan, S.; Subbarao, V.V.; Gokhale, R.; Mulik, U.P. Synthesis and characterization of Ag/PVA nanocompositeby chemical reduction method. Mater. Chem. Phys. 2005, 93, 117–121. [Google Scholar] [CrossRef]
- Viorica, G.P.; Musat, V.; Pimentel, A.; Calmeiro, T.R.; Carlos, E.; Baroiu, L.; Martins, R.; Fortunato, E. Hybrid (Ag)ZnO/Cs/PMMA nanocomposite thin films. J. Alloy. Compd. 2019, 803, 922–933. [Google Scholar] [CrossRef]
- Pimentel, A.; Araújo, A.; Coelho, B.; Nunes, D.; Oliveira, M.; Mendes, M.; Águas, H.; Martins, R.; Fortunato, E. 3D ZnO/Ag Surface-Enhanced Raman Scattering on Disposable and Flexible Cardboard Platforms. Materials 2017, 10, 1351. [Google Scholar] [CrossRef]
- Senthilkumar, S.R.; Sivakumar, T. Green tea (Camellia Sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 6. [Google Scholar]
- Kamal, S.S.; Sahoo, P.K.; Vimala, J.; Premkumar, M.; Ram, S.; Durai, L. A Novel Green Chemical Route for Synthesis of Silver Nanoparticles Using Camellia Sinensis. Acta Chim. Slov. 2010, 57, 808–812. [Google Scholar] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Abdullah, R.M.; Aziz, S.B.; Mamand, S.M.; Hassan, A.Q.; Hussein, S.A.; Kadir, M.F. Reducing the Crystallite Size of Spherulites in PEO-Based Polymer Nanocomposites Mediated by Carbon Nanodots and Ag Nanoparticles. Nanomaterials 2019, 9, 874. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N.; Khan, B.A. The morphology, characteristics, and medicinalproperties of Camellia sinensis’ tea. J. Med. Plants Res. 2010, 4, 2028–2033. [Google Scholar]
- Mallikarjuna, K.; Narasimha, G.; Dillip, G.R.; Praveen, B.; Shreedhar, B.; Lakshmi, C.S.; Reddy, B.V.; Raju, B.D. Green synthesis of silver nanoparticles using ocimum leaf extract and their characterization. Dig. J. Nanomater. Biostruct. 2011, 6, 181–186. [Google Scholar]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Saber, D.R.; Rasheed, M.A.; Ahmed, H.M. Investigation of Metallic Silver Nanoparticles through UV-Vis and Optical Micrograph Techniques. Int. J. Electrochem. Sci. 2017, 12, 363–373. [Google Scholar] [CrossRef]
- Aziz, S.B.; Marif, R.B.; Brza, M.A.; Hassan, A.N.; Ahmad, H.A.; Faidhalla, Y.A.; Kadir, M.F. Structural, thermal, morphological and optical properties of PEO filled with biosynthesized Ag nanoparticles: New insights to band gap study. Results Phys. 2019, 13, 102220. [Google Scholar] [CrossRef]
- Ramya, M.; Subapriya, M.S. Green synthesis of silver nanoparticles. Int. J. Pharm. Med. Biol. Sci. 2012, 1, 1. [Google Scholar]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M.; Silva, B.M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydoniaoblonga) leaf: A comparative study with green tea (Camellia sinensis). Food Chem.Toxicol. 2009, 47, 860. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Synthesis of silver nanoparticles using Acalyphaindica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Aziz, S.B. Modifying poly (vinyl alcohol)(PVA) from insulator to small-bandgap polymer: A novel approach for organic solar cells and optoelectronic devices. J. Electron. Mater. 2016, 45, 736. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, A.M.; Ahmed, H.M. From insulating PMMA polymer to conjugated double bond behavior: Green chemistry as a novel approach to fabricate small band gap polymers. Polymers 2017, 9, 626. [Google Scholar] [CrossRef]
- Shukla, R.; Shukla, S.; Bivolarski, V.; Iliev, I.; Ivanova, I.; Goyal, A. Structural Characterization of Insoluble Dextran Produced by Leuconostocmesenteroides NRRL B-1149 in the Presence of Maltose. Food Technol. Biotechnol. 2011, 49, 291–296. [Google Scholar]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant Mediated Green Synthesis of CuO Nanoparticles: Comparison of Toxicity of Engineered and Plant Mediated CuO Nanoparticles towards Daphnia magna. Nanomaterials 2016, 6, 205. [Google Scholar] [CrossRef]
- Dubey, S.; Sillanpaa, M.; Varma, R. Reduction of Hexavalent Chromium Using Sorbariasorbifolia Aqueous Leaf Extract. Appl. Sci. 2017, 7, 715. [Google Scholar] [CrossRef]
- Cai, J.X.; Wang, Y.F.; Xi, X.G.; Li, H.; Wei, X.L. Using FTIR spectra and pattern recognition for discrimination of tea varieties. Int. J. Biol. Macromol. 2015, 78, 439–446. [Google Scholar] [CrossRef]
- Huang, L.; Weng, X.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Huang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles by green tea extract and theirdegradation of malachite. Ind. Crops Prod. 2013, 51, 342–347. [Google Scholar] [CrossRef]
- Wu, D.; Bird, M.R. The interaction of protein and polyphenol species in ready to drink black tea liquor production. J. Food Process Eng. 2010, 33, 481–505. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Carvalho, M.; Valentão, P.; Andrade, P.B.; Silva, B.M. Targeted metabolites and biological activities of Cydoniaoblonga Miller leaves. Food Res. Int. 2012, 46, 496–504. [Google Scholar] [CrossRef]
- Xu, L.Q.; Neoh, K.G.; Kang, E.T. Natural Polyphenols as Versatile Platforms for Material Engineering and Surface Functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Muzzarelli, R. Potential of Chitin/Chitosan-Bearing Materials for Uranium Recovery: An Interdisciplinary Review. Carbohydr. Polym. 2011, 84, 54–63. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant Activity of Tea Polyphenols In Vivo: Evidence from Animal Studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Ucun, F.; Saglam, A.; Guclu, V. Molecular strutures and vibrational frequencies of Xanthine and its methyl derivatives (caffeine and theobromine) by ab initio hatreefock and density functional theory calculations. J. Spectrochim. Part A 2007, 67, 342–349. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Hicham, E.H. Synthesis and Characterization of caffeine Complexes [M (caf) 4X2] M =Ni(II), Cu(II), Zn(II), Cd(II) X = SCN-, CN-; caf: Caffeine. Res. J. Chem. Sci. 2014, 4, 42–48. [Google Scholar]
- Mendoza-Reséndez, R.; Núñez, N.O.; Barriga-Castro, E.D.; Luna, C. Synthesis of metallic silver nanoparticles and silver organometallic nanodisks mediated by extracts of Capsicum annuum var. aviculare (piquin) fruits. RSC Adv. 2013, 3, 20765. [Google Scholar]
- Zielinski, A.A.; Haminiuk, C.W.; Alberti, A.; Nogueira, A.; Demiate, I.M.; Granato, D. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res. Int. 2014, 60, 246–254. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, R.C. Facile and Eco-Friendly Fabrication of Colored and Bioactive Silk Materials Using Silver Nanoparticles Synthesized by Two Flavonoids. Polymers 2018, 10, 404. [Google Scholar] [CrossRef]
- Latif, U.; Al-Rubeaan, K.; Saeb, A.T. A Review on Antimicrobial Chitosan-Silver Nanocomposites: A Roadmap Toward Pathogen Targeted Synthesis. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 448–458. [Google Scholar] [CrossRef]
- Goodman, B.A.; Severino, J.F.; Pirker, K.F. Reactions of green and black teas with Cu(II). Food Funct. 2012, 3, 399. [Google Scholar] [CrossRef]
- Kaur, H.; Jaryal, N. Utilization of biogenic tea waste silver nanoparticles for the reduction of organic dyes. Mater. Res. Express 2018, 5, 055402. [Google Scholar] [CrossRef]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Beevi, A.H.; Anand, M.; Ramakritinan, C.M.; Kumaraguru, A.K. Synthesis of chitosan mediated silver nanoparticles (AgNPs) for potential antimicrobial applications. Front. Labor. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.; Arof, A.K. Effect of silver nanoparticles on the DC conductivity in chitosan–silver triflate polymer electrolyte. Phys. B Condens. Matter 2010, 405, 4429. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.; Kadir, M.F. Innovative method to avoid the reduction of silver ions to silver nanoparticles in silver ion conducting based polymer electrolytes. Phys. Scr. 2015, 90, 035808. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green Synthesis and Characterization of Silver/Chitosan/Polyethylene Glycol Nanocompositeswithout any Reducing Agent. Int. J. Mol. Sci. 2011, 12, 4872–4884. [Google Scholar] [CrossRef] [PubMed]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Babu, N.V.; Veerabhadram, G. A novel green one-step synthesis of silver nanoparticles using chitosan: Catalytic activity and antimicrobial studies. Appl. Nanosci. 2014, 4, 113–119. [Google Scholar] [CrossRef]
- Yasir, M.; Singh, J.; Tripathi, M.K.; Singh, P.; Shrivastava, R. Green Synthesis of Silver Nanoparticles Using Leaf Extract of Common Arrowhead Houseplant and Its Anticandidal Activity. Pharmacogn Mag. 2017, 13, S840–S844. [Google Scholar]
- Behravan, M.; Panahi, A.H.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Junsu, P.; Younghun, K. Effect of Shape of Silver Nanoplates on the Enhancement of Surface Plasmon Resonance (SPR) Signals. J. Nanosci. Nanotechnol. 2008, 8, 5026–5029. [Google Scholar]
- Murray, W.A.; Astilean, S.; Barnes, W.L. Transition from localized surface plasmon resonance to extended surface plasmon-polariton as metallic nanoparticles merge to form a periodic hole array. Phys. Rev. B 2004, 69, 165407. [Google Scholar] [CrossRef]
- Rhodes, C.; Franzen, S.; Maria, J.P.; Losego, M.; Leonard, D.N.; Laughlin, B.; Duscher, G.; Weibel, S. Surface plasmon resonance in conducting metal oxides. J. Appl. Phys. 2006, 100, 054905. [Google Scholar] [CrossRef]
- Shujahadeen, B.A. Morphological and optical characteristics of chitosan (1− x): Cuox (4≤ x≤ 12) based polymer nano-composites: Optical dielectric loss as an alternative method for tauc’s model. Nanomaterials 2017, 7, 444. [Google Scholar] [CrossRef]
- Thomas, S.; Nair, S.K.; Jamal, E.M.; Al-Harthi, S.H.; Varma, M.R.; Anantharaman, M.R. Size-dependent surface plasmon resonance in silver silica nanocomposites. Nanotechnology 2008, 19, 075710. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hassan, A.Q.; Mohammed, S.J.; Karim, W.O.; Kadir, M.F.; Tajuddin, H.A.; Chan, N.N. Structural and optical characteristics of PVA: C-Dot composites: Tuning the absorption of ultra violet (UV) region. Nanomaterials 2019, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Abidin, Z.H. Electrical and morphological analysis of chitosan: AgTf solid electrolyte. Mater. Chem. Phys. 2014, 144, 280. [Google Scholar] [CrossRef]
- Aziz, S.B.; Mamand, S.M.; Saed, S.R.; Abdullah, R.M.; Hussein, S.A. New method for the development of plasmonic metal-semiconductor interface layer: Polymer composites with reduced energy band gap. J. Nanomater. 2017, 2017, 8140693. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Kadir, M.F.; Ahmed, H.M. Non suitability of silver ion conducting polymer electrolytes based on chitosan mediated by barium titanate (BaTiO3) for electrochemical device applications. Electrochim. Acta 2019, 296, 494. [Google Scholar] [CrossRef]
- Susilowati, E. Sunlight-assisted synthesis of colloidal silver nanoparticles using chitosan as reducing agent. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 349, p. 012019. [Google Scholar]
- Alwan, A.M.; Yousif, A.A.; Wali, L.A. A Study on the Morphology of the Silver Nanoparticles Deposited on the n-Type Porous Silicon Prepared Under Different Illumination Types. Plasmonics 2018, 13, 1191–1199. [Google Scholar] [CrossRef]
- Schneidewind, H.; Schüler, T.; Strelau, K.K.; Weber, K.; Cialla, D.; Diegel, M.; Mattheis, R.; Berger, A.; Möller, R.; Popp, J. The morphology of silver nanoparticles prepared by enzyme-induced reduction. Beilstein J. Nanotechnol. 2012, 3, 404–414. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A. A novel polymer composite with a small optical band gap: New approaches for photonics and optoelectronics. J. Appl. Polym. Sci. 2017, 134, 44847. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
B. Aziz, S.; Hussein, G.; Brza, M.A.; J. Mohammed, S.; T. Abdulwahid, R.; Raza Saeed, S.; Hassanzadeh, A. Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution. Nanomaterials 2019, 9, 1557. https://doi.org/10.3390/nano9111557
B. Aziz S, Hussein G, Brza MA, J. Mohammed S, T. Abdulwahid R, Raza Saeed S, Hassanzadeh A. Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution. Nanomaterials. 2019; 9(11):1557. https://doi.org/10.3390/nano9111557
Chicago/Turabian StyleB. Aziz, Shujahadeen, Govar Hussein, M. A. Brza, Sewara J. Mohammed, R. T. Abdulwahid, Salah Raza Saeed, and Abdollah Hassanzadeh. 2019. "Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution" Nanomaterials 9, no. 11: 1557. https://doi.org/10.3390/nano9111557
APA StyleB. Aziz, S., Hussein, G., Brza, M. A., J. Mohammed, S., T. Abdulwahid, R., Raza Saeed, S., & Hassanzadeh, A. (2019). Fabrication of Interconnected Plasmonic Spherical Silver Nanoparticles with Enhanced Localized Surface Plasmon Resonance (LSPR) Peaks Using Quince Leaf Extract Solution. Nanomaterials, 9(11), 1557. https://doi.org/10.3390/nano9111557