Synthesis of g-C3N4-Decorated ZnO Porous Hollow Microspheres for Room-Temperature Detection of CH4 under UV-Light Illumination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
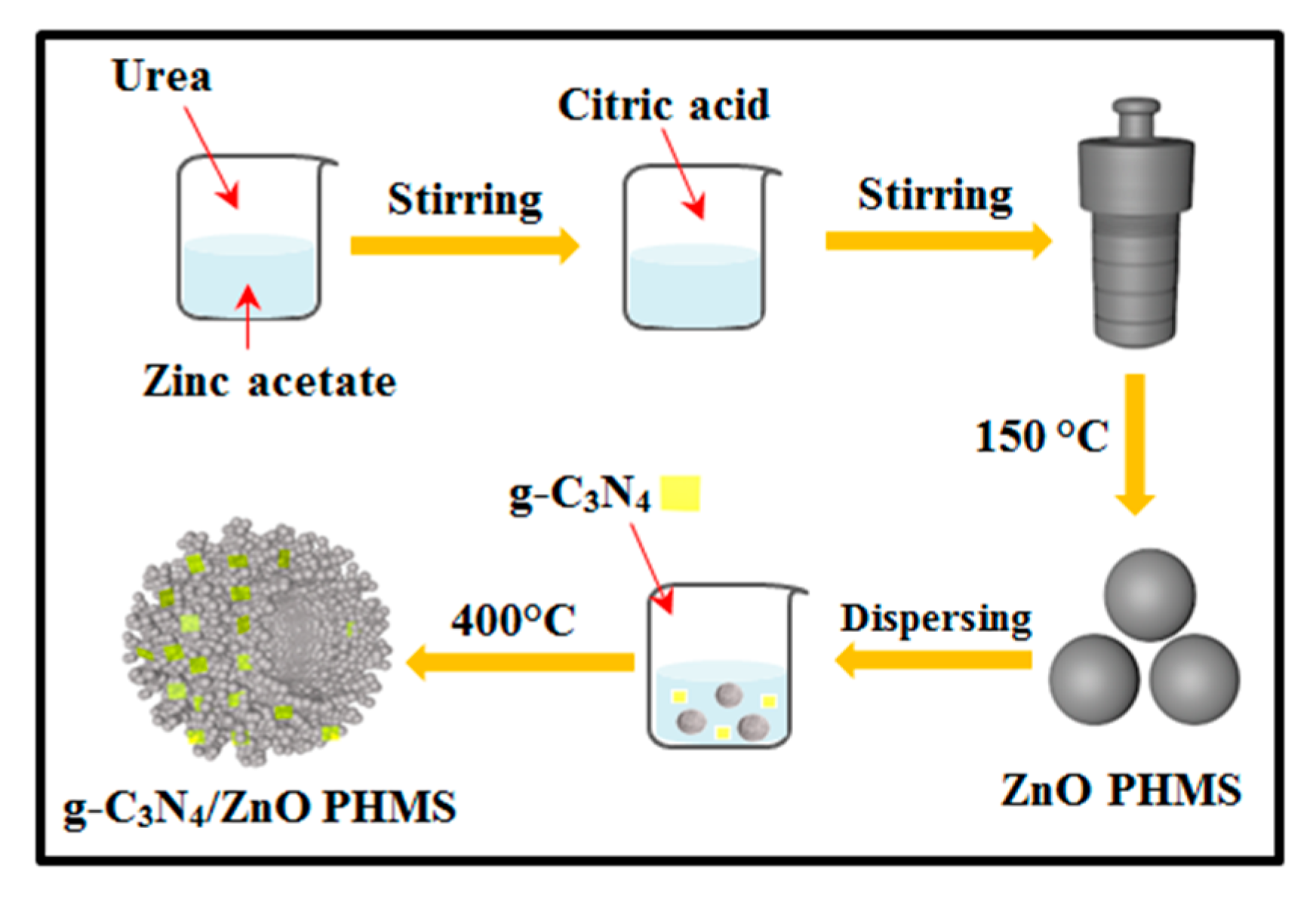
2.2. Synthesis of g-C3N4/ZnO PHMSs
2.3. Characterizations
2.4. Gas Sensor Fabrication and Analysis
3. Results and Discussion
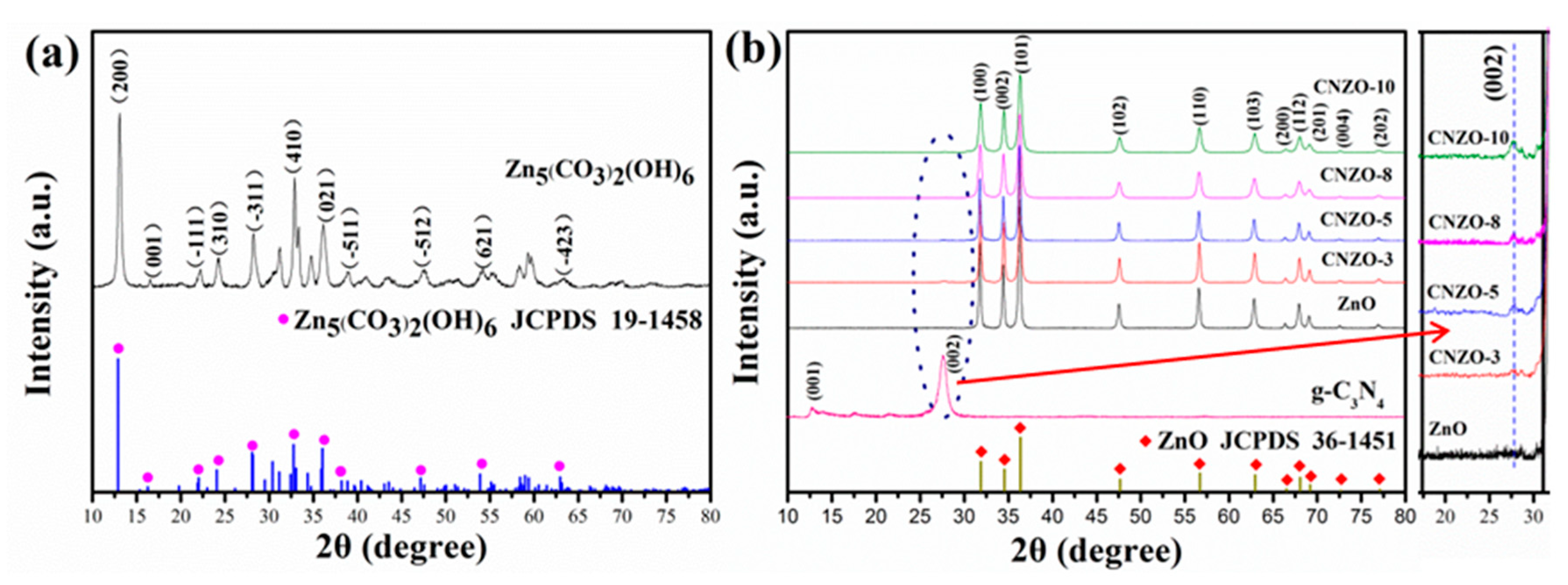
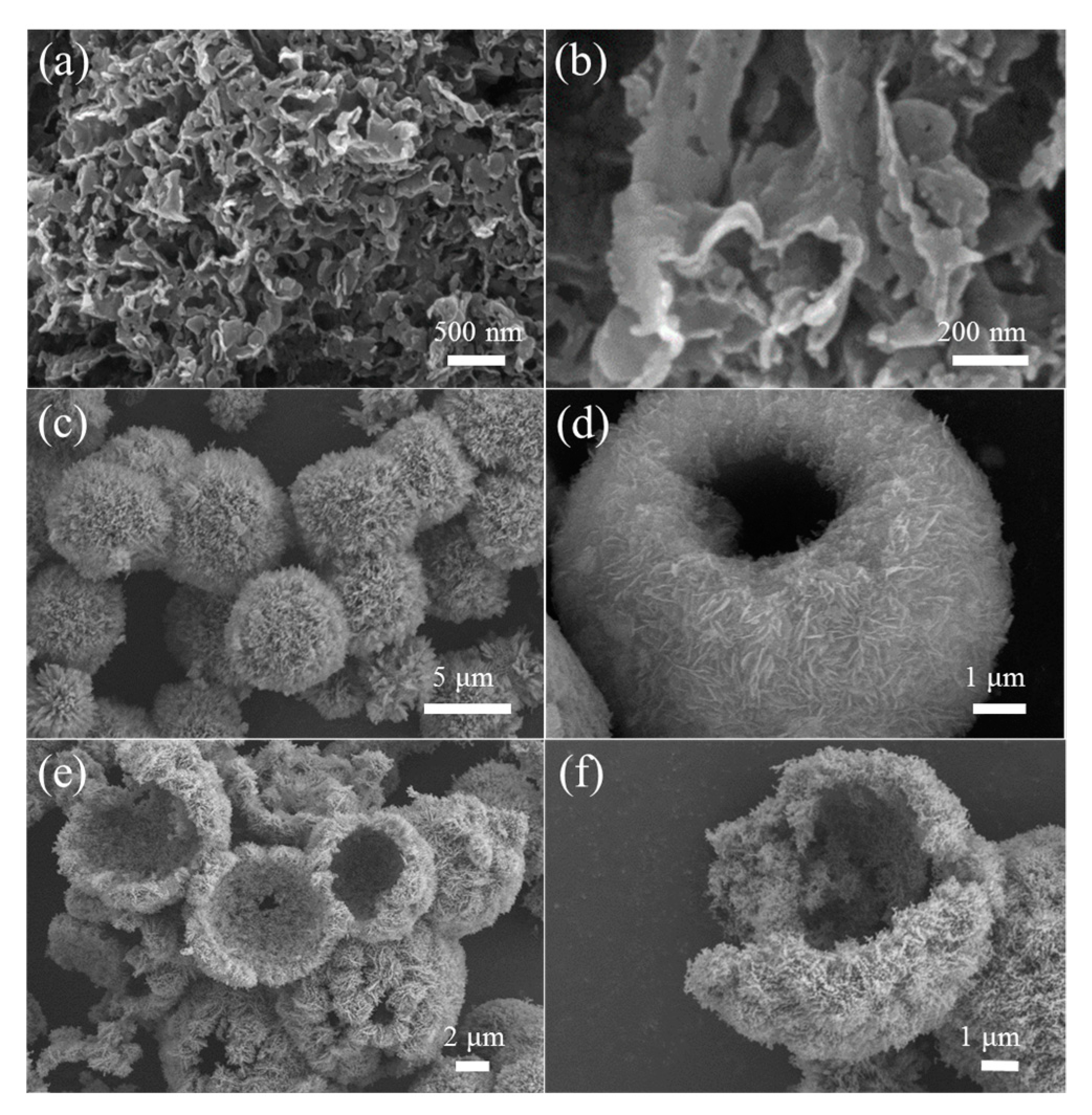
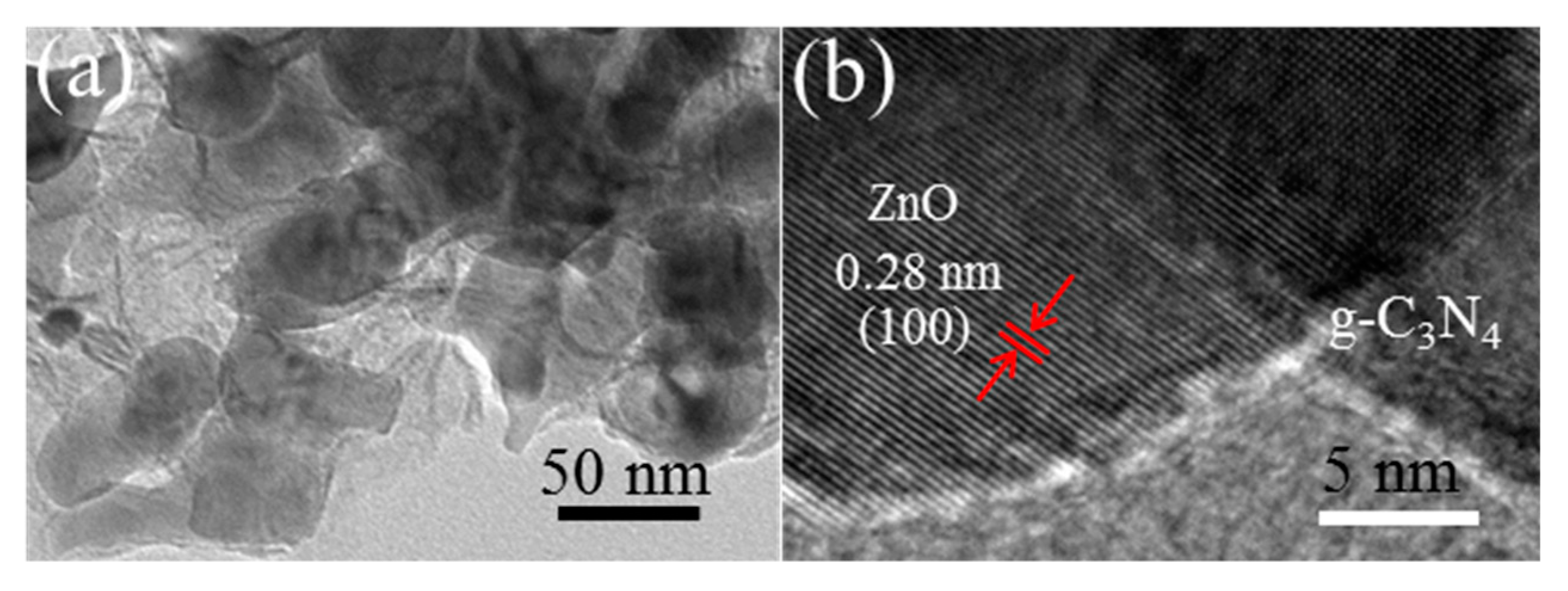
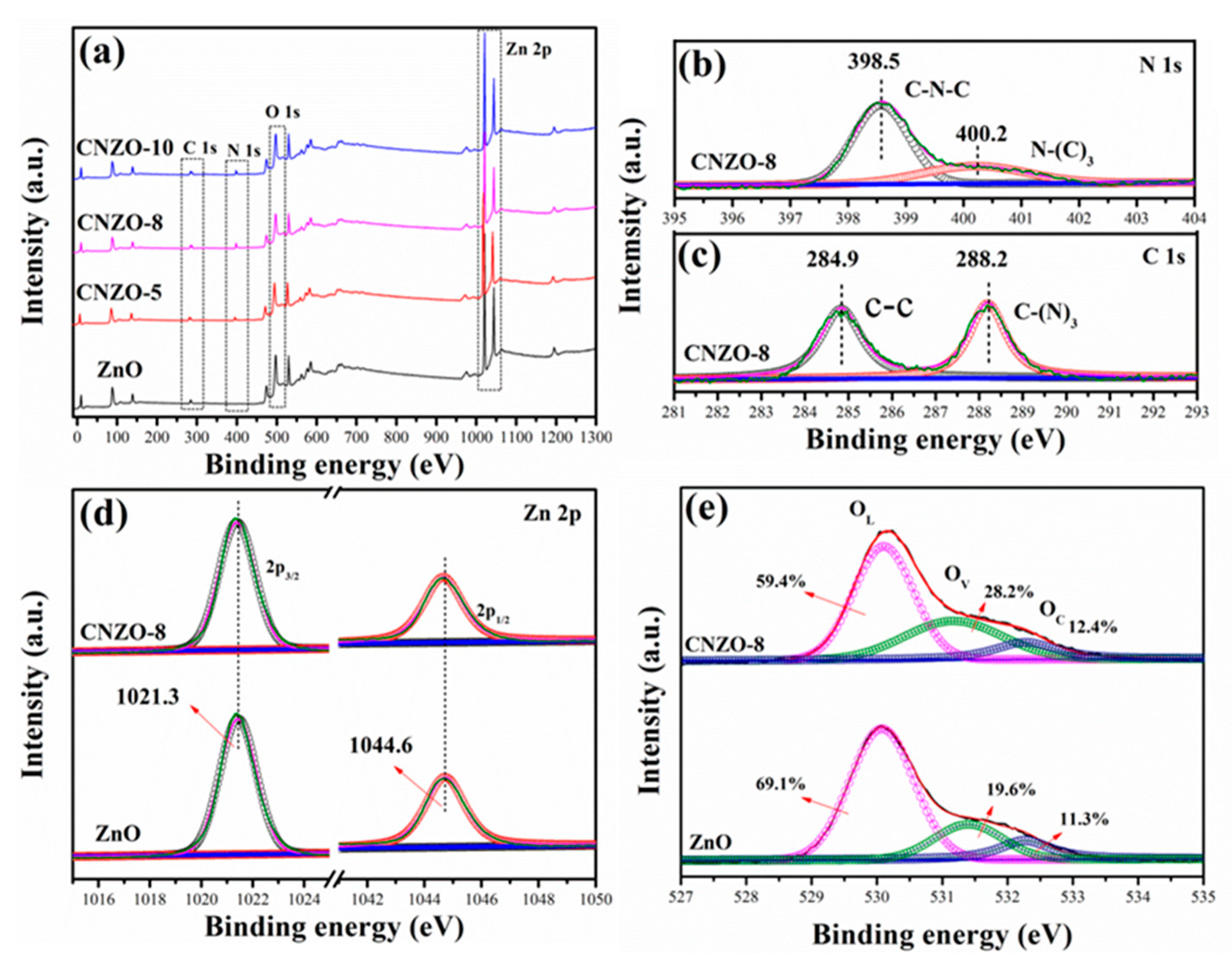
3.1. Morphology and Structure Characterization of the Prepared Samples
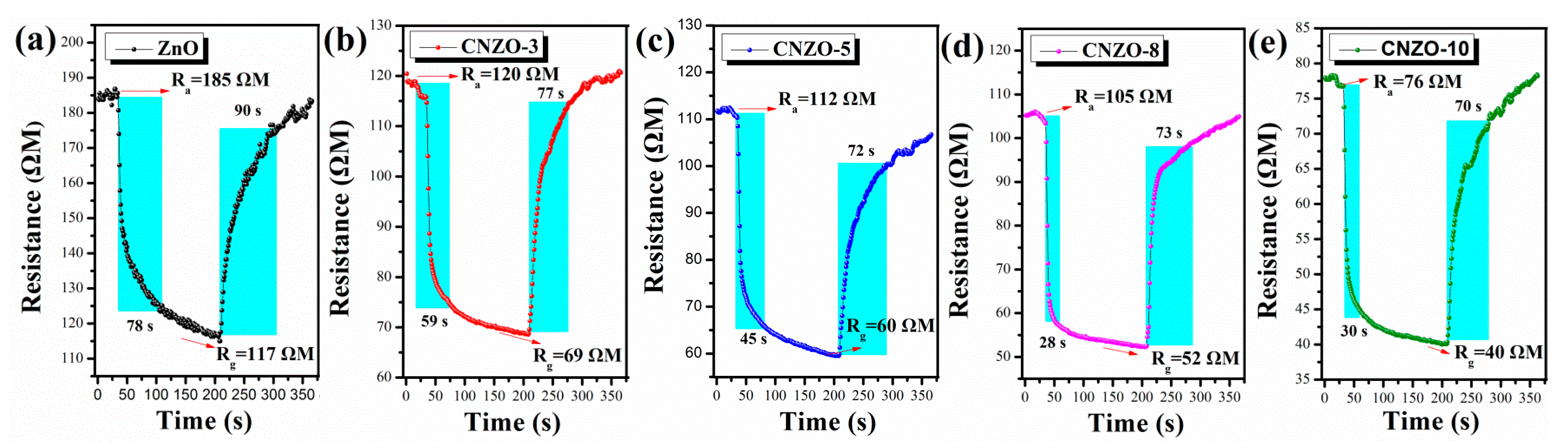
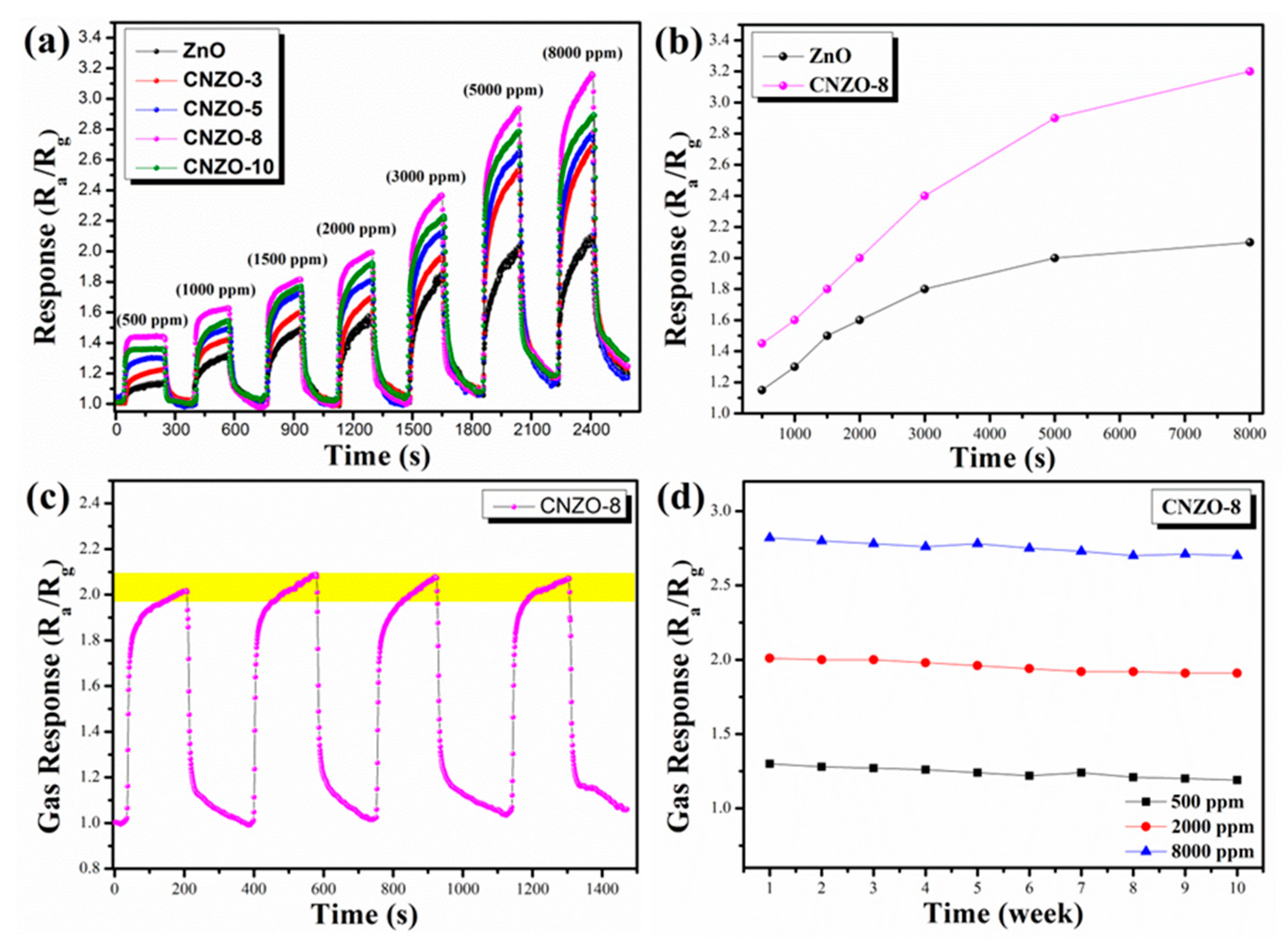
3.2. Gas Sensing Properties
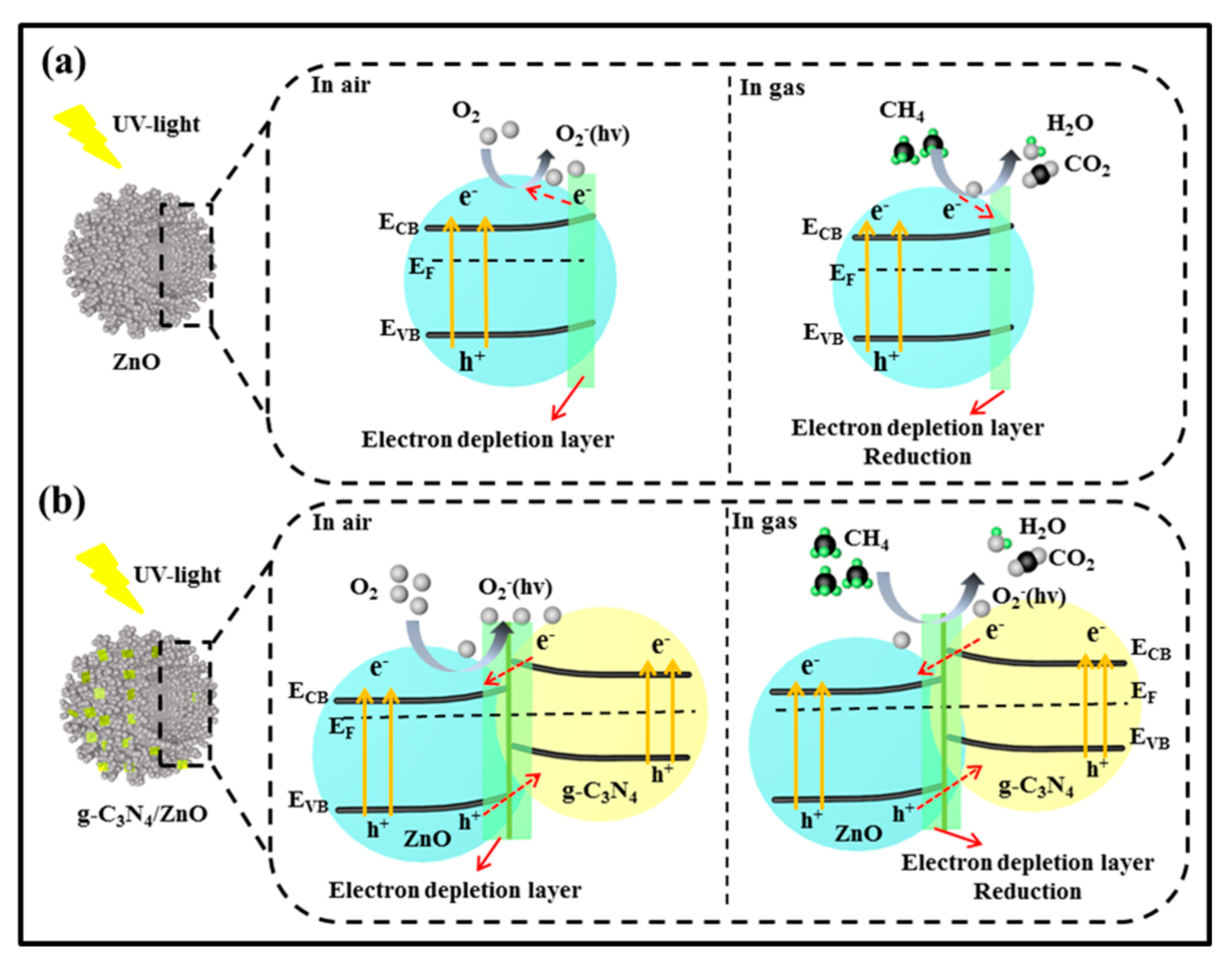
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Abakumov, A.M.; Gaskov, A.M. Visible light activated room temperature gas sensors based on nanocrystalline ZnO sensitized with CdSe quantum dots. Sens. Actuators B 2014, 205, 305–312. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ueda, T.; Kamada, K.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuators B 2016, 223, 429–439. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Chandra, R. Highly sensitive and selective CO gas sensor based on hydrophobic SnO2/CuO bilayer. RSC Adv. 2016, 6, 47178–47184. [Google Scholar] [CrossRef]
- Hassan, J.J.; Mahdi, M.A.; Chin, C.W.; Abu-Hassan, H.; Hassan, Z. A high-sensitivity room-temperature hydrogen gas sensor based on oblique and vertical ZnO nanorod arrays. Sens. Actuators B 2013, 176, 360–367. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Development of highly sensitive ZnO/In2O3 composite gas sensor activated by UV-LED. Sens. Actuators B 2017, 241, 828–839. [Google Scholar] [CrossRef]
- Gong, B.; Shi, T.; Zhu, W.; Liao, G.; Li, X.; Huang, J.; Zhou, T. UV irradiation-assisted ethanol detection operated by the gas sensor based on ZnO nanowires/optical fiber hybrid structure. Sens. Actuators B 2017, 245, 821–827. [Google Scholar] [CrossRef]
- Da Silva, L.F.; M’Peko, J.C.; Catto, A.C.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Ribeiro, C.; Longo, E. UV-enhanced Ozone Gas Sensing Response of ZnO-SnO2 Heterojunctions at Room Temperature. Sens. Actuators B 2016, 240, 573–579. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Yang, S.B.; Gong, Y.J.; Zhang, J.S.; Zhan, L.; Ma, L.L.; Fang, Z.Y.; Vajtai, R.; Wang, X.C.; Ajayan, P.M. Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Guo, W.; Chen, S. The α-Fe2O3/g-C3N4 heterostructural nanaocomposites with enhanced ethanol gas sensing performance. J. Alloys Compd. 2016, 685, 84–90. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, T.; Wang, C.; Liu, D. UV-light-assisted ethanol sensing characteristics of g-C3N4/ZnO composites at room temperature. Appl. Surf. Sci. 2018, 441, 317–323. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Geng, X.; Chen, S.; Lv, X.; Jiang, W.; Wang, T. Synthesis of g-C3N4/Bi5O7I microspheres with enhanced photocatalytic activity under visible light. Appl. Surf. Sci. 2018, 462, 18–28. [Google Scholar] [CrossRef]
- Cao, J.L.; Gong, Y.X.; Wang, Y.; Zhang, B.; Zhang, H.L.; Sun, G.; Hari, B.; Zhang, Z.Y. Cocoon-like ZnO decorated graphitic carbon nitride nanocomposite: Hydrothermal synthesis and ethanol gas sensing application. Mater. Lett. 2017, 198, 76–80. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, Y.; Li, Z.; Yan, S.; Wang, N.; Zou, Z. High-yield synthesis of millimetre-long, semiconducting carbon nitride nanotubes with intense photoluminescence emission and reproducible photoconductivity. Nanoscale 2012, 4, 3687–3692. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Muller, J.O.; Schlogl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Zhang, G.; Wang, X. Synthesis of Carbon Nitride Semiconductors in Sulfur Flux for Water Photoredox Catalysis. ACS Catal. 2012, 2, 940–948. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Jia, L.; Xiao, Z. Origin of the catalytic activity of graphite nitride for the electrochemical reduction of oxygen: Geometric factors vs. electronic factors. Phys. Chem. Chem. Phys. 2009, 11, 2730–2740. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Hong, Z.; Shen, B.; Lin, B.; Gao, B. Origin of the enhanced visible-light photocatalytic activity of CNT modified g-C3N4 for H2 production. Phys. Chem. Chem. Phys. 2014, 16, 8106–8113. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Raizada, P.; Singh, P.; Kumar, A.; Sharma, G.; Pare, B.; Jonnalagadda, S.B.; Thakur, P. Solar photocatalytic activity of nano-ZnO supported on activated carbon or brick grain particles: Role of adsorption in dye degradation. Appl. Catal. A 2014, 486, 159–169. [Google Scholar] [CrossRef]
- Li, G.J.; Zhang, X.H.; Kawi, S. Relationships between sensitivity, catalytic activity, and surface areas of SnO2 gas sensors. Sens. Actuators B 1999, 60, 64–70. [Google Scholar] [CrossRef]
- Fang, J.W.; Fan, H.Q.; Li, M.M.; Long, C.B. Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 13819–13826. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, R.F.; Liu, S.H.; Song, J.; Chen, J.S.; Dong, B.; Song, H.W. NiO@ZnO Heterostructured Nanotubes: Coelectrospinning Fabrication, Characterization, and Highly Enhanced Gas Sensing Properties. Inorg. Chem. 2012, 51, 7733–7740. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Alshammari, A.S.; Jayawardena, K.D.G.I.; Beliatis, M.J.; Henley, S.J.; Silva, S.R.P. Role of the Exposed Polar Facets in the Performance of Thermally and UV Activated ZnO Nanostructured Gas Sensors. J. Phys. Chem. C 2013, 117, 17850–17858. [Google Scholar] [CrossRef]
- Wang, T.; Kou, X.; Zhao, L.; Sun, P.; Liu, C.; Wang, Y.; Shimanoe, K.; Yamazoe, N.; Lu, G. Flower-like ZnO hollow microspheres loaded with CdO nanoparticles as high performance sensing material for gas sensors. Sens. Actuators B 2017, 250, 692–702. [Google Scholar] [CrossRef]
- Park, S.; Sun, G.J.; Kheel, H.; Hyun, S.K.; Jin, C.; Lee, C. Hydrogen gas sensing of CO3O4-Decorated WO3 nanowires. Met. Mater. Int. 2016, 22, 156–162. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Fu, X.; Li, Z.; Han, W.; Wang, X. Relationship between oxygen defects and the photocatalytic property of ZnO nanocrystals in nafion membranes. Langmuir 2008, 25, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, A.M.; Hou, Y.; Jayatissa, A.H. The effect of UV irradiation on nanocrysatlline zinc oxide thin films related to gas sensing characteristics. Appl. Surf. Sci. 2011, 257, 5398–5402. [Google Scholar] [CrossRef]
- Hyodo, T.; Urata, K.; Kamada, K.; Ueda, T.; Shimizu, Y. Semiconductor-type SnO2-based NO2 sensors operated at room temperature under UV-light. Sens. Actuators, B 2017, 253, 630–640. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.P.; Liu, J.; Feng, P. Branched WO3 Nanosheet Array with Layered C3N4 Heterojunctions and CoOx Nanoparticles as a Flexible Photoanode for Efficient Photoelectrochemical Water Oxidation. Adv. Mater. 2014, 26, 5043–5049. [Google Scholar] [CrossRef]
- Fan, S.W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Xue, Z.; Cheng, Z.; Xu, J.; Xiang, Q.; Wang, X.; Xu, J. Controllable Evolution of Dual Defect Zni and VO Associate-Rich ZnO Nanodishes with (0001) Exposed Facet and Its Multiple Sensitization Effect for Ethanol Detection. ACS Appl. Mater. Interfaces 2017, 9, 41559–41567. [Google Scholar] [CrossRef]
- Lü, Y.; Zhan, W.; He, Y.; Wang, Y.; Kong, X.; Kuang, Q.; Xie, Z.; Zheng, L. MOF-Templated Synthesis of Porous CO3O4 Concave Nanocubes with High Specific Surface Area and Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Li, Y.; Zhang, B.; Sun, G.; Zhang, Z. Synthesis of g-C3N4-Decorated ZnO Porous Hollow Microspheres for Room-Temperature Detection of CH4 under UV-Light Illumination. Nanomaterials 2019, 9, 1507. https://doi.org/10.3390/nano9111507
Xiao M, Li Y, Zhang B, Sun G, Zhang Z. Synthesis of g-C3N4-Decorated ZnO Porous Hollow Microspheres for Room-Temperature Detection of CH4 under UV-Light Illumination. Nanomaterials. 2019; 9(11):1507. https://doi.org/10.3390/nano9111507
Chicago/Turabian StyleXiao, Min, Yanwei Li, Bo Zhang, Guang Sun, and Zhanying Zhang. 2019. "Synthesis of g-C3N4-Decorated ZnO Porous Hollow Microspheres for Room-Temperature Detection of CH4 under UV-Light Illumination" Nanomaterials 9, no. 11: 1507. https://doi.org/10.3390/nano9111507
APA StyleXiao, M., Li, Y., Zhang, B., Sun, G., & Zhang, Z. (2019). Synthesis of g-C3N4-Decorated ZnO Porous Hollow Microspheres for Room-Temperature Detection of CH4 under UV-Light Illumination. Nanomaterials, 9(11), 1507. https://doi.org/10.3390/nano9111507



