Electrochemical Sensors Modified with Combinations of Sulfur Containing Phthalocyanines and Capped Gold Nanoparticles: A Study of the Influence of the Nature of the Interaction between Sensing Materials
Abstract
1. Introduction
2. Materials and Methods
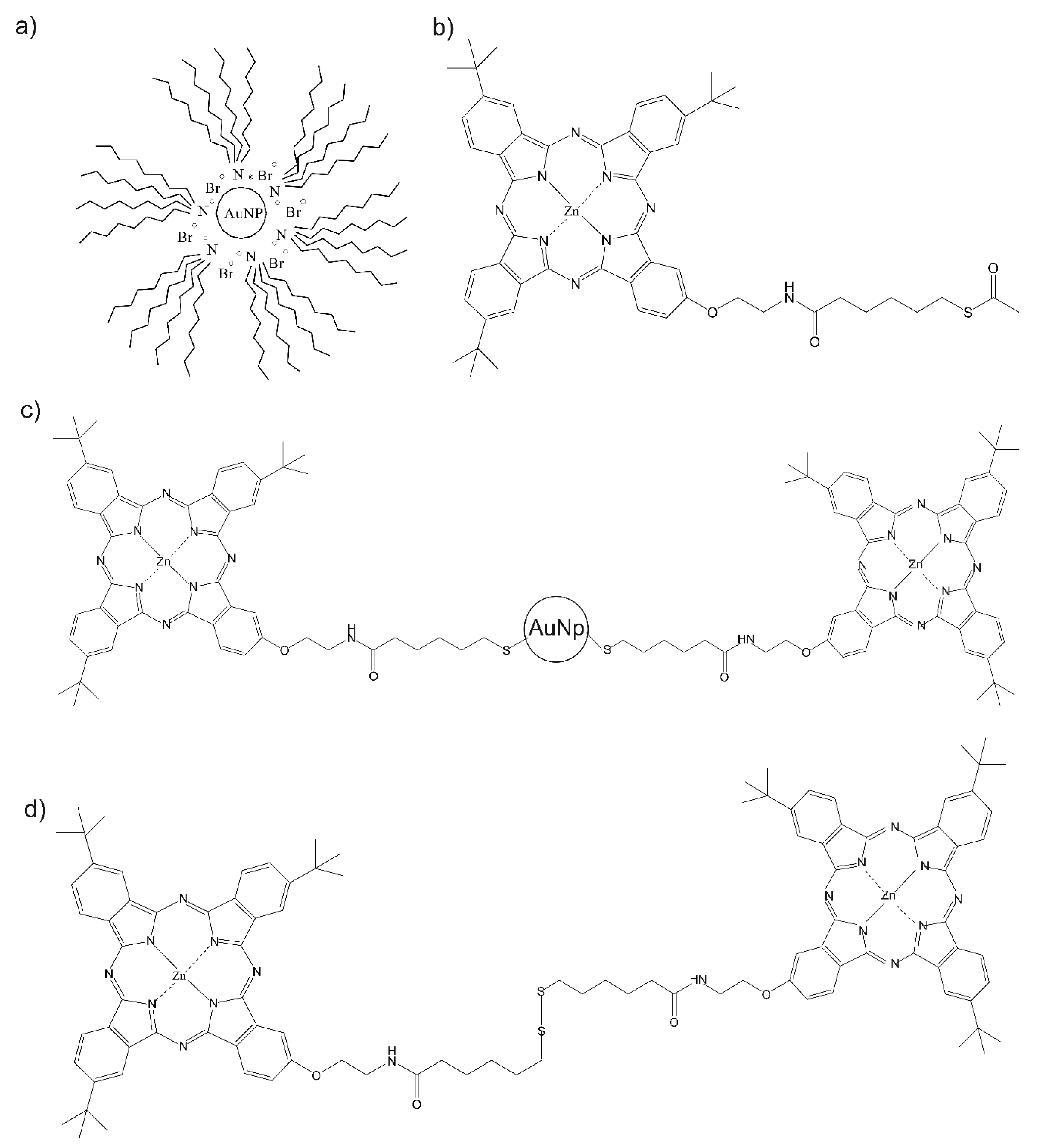
2.1. Synthesis of the Sensitive Materials
2.1.1. Tetraoctylammonium Bromide-Capped Gold Nanoparticles (AuNPtOcBr)
2.1.2. 6.6′-dithiodihexanoic Acid
2.1.3. Sulfur-Substituted Zinc Phthalocyanine: 2-{2′-[(5′′-Acetylthiopentyloxo)amino]ethoxy}-9(10),16(17),23(24)-tri-tert-butylphalocyaninate Zn(II) (ZnPcRS)
2.1.4. Dimeric Sulfur Substituted Zinc Bisphthalocyanine: (ZnPcR-S-ZnPcR)
2.1.5. AuNPtOcBr/ZnPcRS and AuNPtOcBr/ZnPcR-S-ZnPcR Mixtures
2.1.6. AuNPtOcBr-S-ZnPcR Covalent Adduct
2.2. Preparation of the Sensors
2.3. Sensing Properties
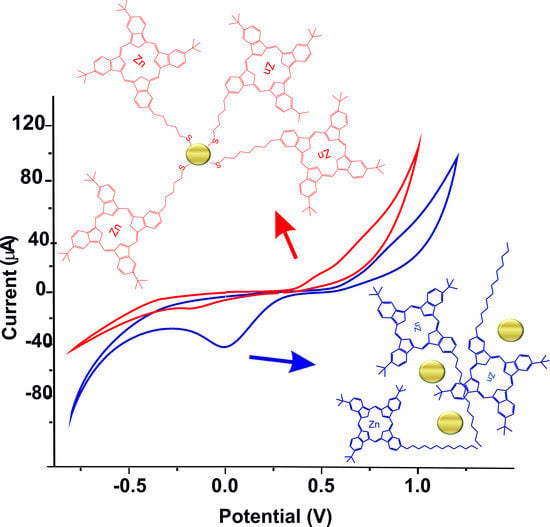
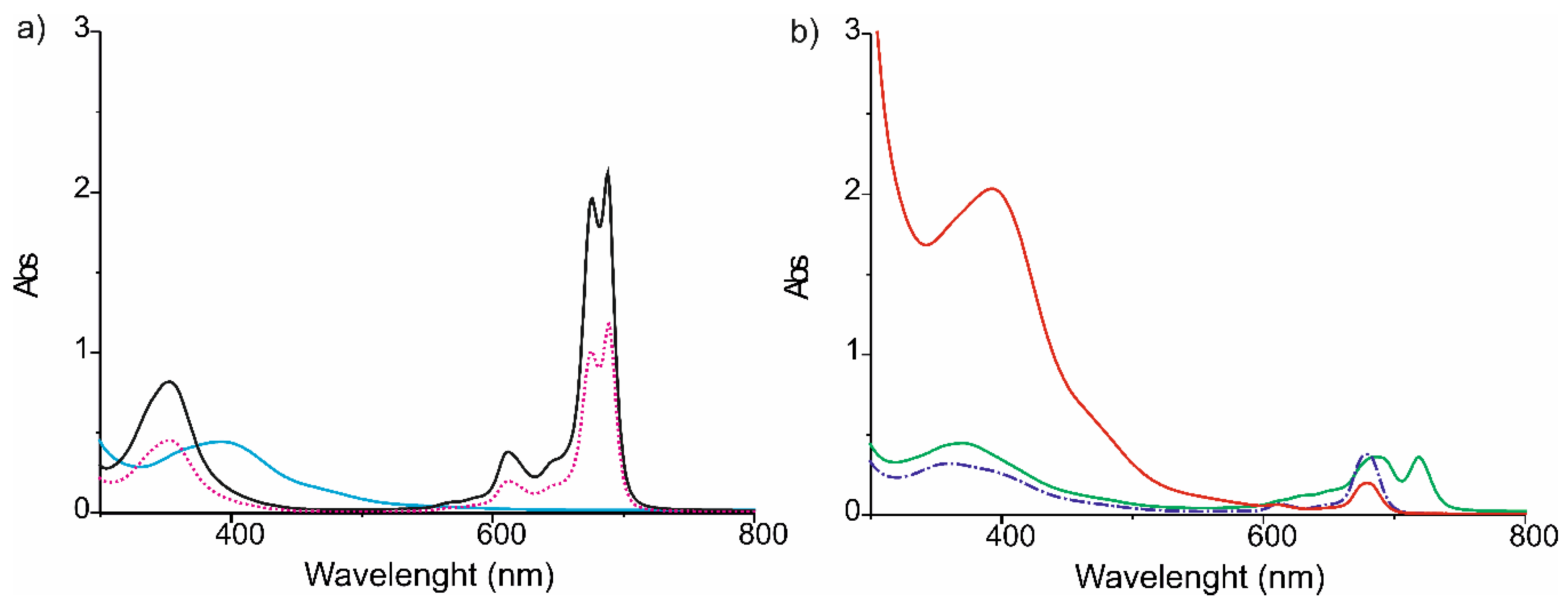
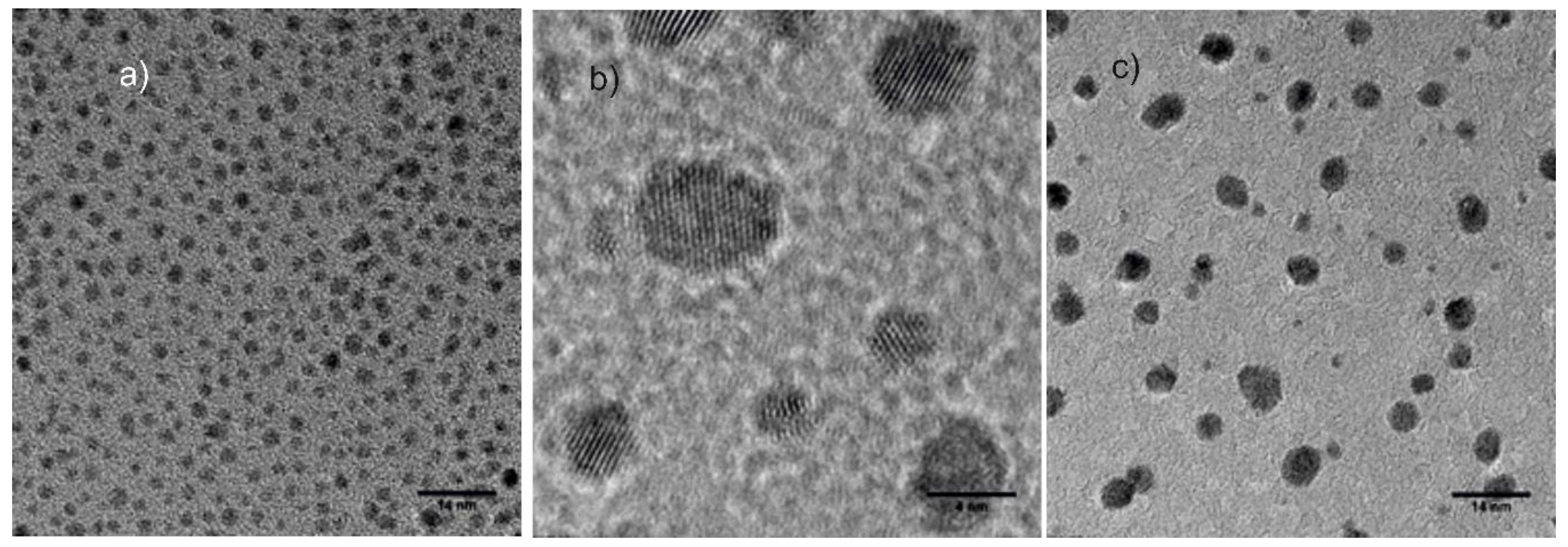
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blasco, A.J.; González Crevillén, A.; González, M.C.; Escarpa, A. Direct Electrochemical Sensing and Detection of Natural Antioxidants and Antioxidant Capacity in Vitro Systems. Electroanalysis 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; García-Cabezón, C.; García-Hernández, C.; Bramorski, C.; Blanco-Val, Y.; Martín-Pedrosa, F.; Kawai, T.; de Saja, J.A.; Rodríguez-Méndez, M.L. Analysis of organic acids and phenols of interest in the wine industry using Langmuir–Blodgett films based on functionalized nanoparticles. Anal. Chim. Acta 2015, 853, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Makhotkina, O.; Kilmartin, P.A. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Gay Martín, M.; de Saja, J.A.; Muñoz, R.; Rodríguez-Méndez, M.L. Multisensor system based on bisphthalocyanine nanowires for the detection of antioxidants. Electrochim. Acta 2012, 68, 88–94. [Google Scholar] [CrossRef]
- Liu, D.; Li, F.; Yu, D.; Yu, J.; Ding, Y.; Liu, D.; Li, F.; Yu, D.; Yu, J.; Ding, Y. Mesoporous Carbon and Ceria Nanoparticles Composite Modified Electrode for the Simultaneous Determination of Hydroquinone and Catechol. Nanomaterials 2019, 9, 54. [Google Scholar] [CrossRef]
- Krampa, F.; Aniweh, Y.; Awandare, G.; Kanyong, P.; Krampa, F.D.; Aniweh, Y.; Awandare, G.A.; Kanyong, P. A Disposable Amperometric Sensor Based on High-Performance PEDOT:PSS/Ionic Liquid Nanocomposite Thin Film-Modified Screen-Printed Electrode for the Analysis of Catechol in Natural Water Samples. Sensors 2017, 17, 1716. [Google Scholar] [CrossRef]
- Apetrei, C.; Casilli, S.; De Luca, M.; Valli, L.; Jiang, J.; Rodriguez-Méndez, M.L.; De Saja, J.A. Spectroelectrochemical characterisation of Langmuir-Schaefer films of heteroleptic phthalocyanine complexes. Potential applications. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 284–285, 574–582. [Google Scholar] [CrossRef]
- Rodriguez-Méndez, M.L.; Gay, M.; de Saja, J.A. New insights into sensors based on radical bisphthalocyanines. J. Porphyr. Phthalocyanines 2009, 13, 1159–1167. [Google Scholar] [CrossRef]
- Alessio, P.; Martin, C.S.; de Saja, J.A.; Rodriguez-Mendez, M.L. Mimetic biosensors composed by layer-by-layer films of phospholipid, phthalocyanine and silver nanoparticles to polyphenol detection. Sens. Actuators B Chem. 2016, 233, 654–666. [Google Scholar] [CrossRef]
- Matemadombo, F.; Apetrei, C.; Nyokong, T.; Rodríguez-Méndez, M.L.; de Saja, J.A. Comparison of carbon screen-printed and disk electrodes in the detection of antioxidants using CoPc derivatives. Sens. Actuators B Chem. 2012, 166, 457–466. [Google Scholar] [CrossRef]
- Fernandes, E.G.R.; Brazaca, L.C.; Rodríguez-Mendez, M.L.; Saja, J.A.; de Zucolotto, V. Immobilization of lutetium bisphthalocyanine in nanostructured biomimetic sensors using the LbL technique for phenol detection. Biosens. Bioelectron. 2011, 26, 4715–4719. [Google Scholar] [CrossRef] [PubMed]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Tkachenko, N.V.; Efimov, A.; Lemmetyinen, H. Covalent phthalocyanine-fullerene dyads: Synthesis, electron transfer in solutions and molecular films. J. Porphyr. Phthalocyanines 2011, 15, 780–790. [Google Scholar] [CrossRef]
- Martín-Gomis, L.; Peralta-Ruiz, F.; Thomas, M.B.; Fernández-Lázaro, F.; D’Souza, F.; Sastre-Santos, Á. Multichromophoric Perylenediimide-Silicon Phthalocyanine-C 60 System as an Artificial Photosynthetic Analogue. Chem. A Eur. J. 2017, 23, 3863–3874. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K.; Ortiz, J.; Gutiérrez, A.M.; Fernández-Lázaro, F.; Sastre-Santos, Á. Control of Photoinduced Electron Transfer in Zinc Phthalocyanine-Perylenediimide Dyad and Triad by the Magnesium Ion. J. Phys. Chem. A 2008, 112, 10744–10752. [Google Scholar] [CrossRef]
- Blas-Ferrando, V.M.; Ortiz, J.; Ohkubo, K.; Fukuzumi, S.; Fernández-Lázaro, F.; Sastre-Santos, Á. Submillisecond-lived photoinduced charge separation in a fully conjugated phthalocyanine–perylenebenzimidazole dyad. Chem. Sci. 2014, 5, 4785–4793. [Google Scholar] [CrossRef]
- Aragão, J.S.; Ribeiro, F.W.P.; Portela, R.R.; Santos, V.N.; Sousa, C.P.; Becker, H.; Correia, A.N.; de Lima-Neto, P. Electrochemical determination diethylstilbestrol by a multi-walled carbon nanotube/cobalt phthalocyanine film electrode. Sens. Actuators B Chem. 2017, 239, 933–942. [Google Scholar] [CrossRef]
- Jubete, E.; Żelechowska, K.; Loaiza, O.A.; Lamas, P.J.; Ochoteco, E.; Farmer, K.D.; Roberts, K.P.; Biernat, J.F. Derivatization of SWCNTs with cobalt phthalocyanine residues and applications in screen printed electrodes for electrochemical detection of thiocholine. Electrochim. Acta 2011, 56, 3988–3995. [Google Scholar] [CrossRef]
- Nombona, N.; Antunes, E.; Litwinski, C.; Nyokong, T. Synthesis and photophysical studies of phthalocyanine-gold nanoparticle conjugates. Dalt Trans. 2011, 40, 11876–11884. [Google Scholar] [CrossRef]
- Blas-Ferrando, V.M.; Ortiz, J.; Fernández-Lázaro, F.; Sastre-Santos, Á. Synthesis and characterization of a sulfur-containing phthalocyanine-gold nanoparticle hybrid. J. Porphyr. Phthalocyanines 2015, 19, 335–343. [Google Scholar] [CrossRef]
- Baldovi, H.G.; Blas-Ferrando, V.M.; Ortiz, J.; Garcia, H.; Fernández-Lázaro, F.; Sastre-Santos, Á. Phthalocyanine-Gold Nanoparticle Hybrids: Modulating Quenching with a Silica Matrix Shell. ChemPhysChem 2016, 17, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liang, X.; Yao, L.; Wang, X.; Jing, Y.; Ma, J.; Fei, Y.; Chen, L.; Mi, L.; Pan, X.; et al. Study of the Photodynamic Activity of N-Doped TiO2 Nanoparticles Conjugated with Aluminum Phthalocyanine. Nanomaterials 2017, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Yu, C.; Wang, Q.; Qian, D.; Li, W.; Huang, Y.; Chen, F.; Bao, N.; Gu, H. An ITO electrode modified with electrodeposited graphene oxide and gold nanoclusters for detecting the release of H2O2 from bupivacaine-injured neuroblastoma cells. Microchim. Acta 2016, 183, 3167–3175. [Google Scholar] [CrossRef]
- Singh, S.; Jain, D.S.M. Sol–gel based composite of gold nanoparticles as matix for tyrosinase for amperometric catechol biosensor. Sens. Actuators B Chem. 2013, 182, 161–169. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Rodriguez-Mendez, M.L.; Sutter, P.; Tong, X.; Sutter, E. Nanoscale Au-In Alloy-Oxide Core-Shell Particles as Electrocatalysts for Efficient Hydroquinone Detection. J. Phys. Chem. C 2015, 119, 25100–25107. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. Glassy carbon electrodes modified with gold nanoparticles for the simultaneous determination of three food antioxidants. Anal. Chim. Acta 2013, 765, 54–62. [Google Scholar] [CrossRef]
- Li, X.; Ye, X.; Li, C.; Wu, K. Substitution group effects of 2-mercaptobenzothiazole on gold nanoparticles toward electrochemical oxidation and sensing of tetrabromobisphenol A. Electrochim. Acta 2018, 270, 517–525. [Google Scholar] [CrossRef]
- Steinebrunner, D.; Schnurpfeil, G.; Wichmann, A.; Wöhrle, D.; Wittstock, A. Synergistic Effect in Zinc Phthalocyanine—Nanoporous Gold Hybrid Materials for Enhanced Photocatalytic Oxidations. Catalysts 2019, 9, 555. [Google Scholar] [CrossRef]
- Nyokong, T.; Antunes, E. Influence of nanoparticle materials on the photophysical behavior of phthalocyanines. Coord. Chem. Rev. 2013, 257, 2401–2418. [Google Scholar] [CrossRef]
- Gonzalez-Anton, R.; Osipova, M.M.; Garcia-Hernandez, C.; Dubinina, T.V.; Tomilova, L.G.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Subphthalocyanines as electron mediators in biosensors based on phenol oxidases: Application to the analysis of red wines. Electrochim. Acta 2017, 255, 239–247. [Google Scholar] [CrossRef]
- Muthukumar, P.; Abraham John, S. Synergistic effect of gold nanoparticles and amine functionalized cobalt porphyrin on electrochemical oxidation of hydrazine. New J. Chem. 2014, 38, 3473–3479. [Google Scholar] [CrossRef]
- Muthukumar, P.; John, S.A. Effect of amine substituted at ortho and para positions on the electrochemical and electrocatalytic properties of cobalt porphyrins self-assembled on glassy carbon surface. Electrochim. Acta 2014, 115, 197–205. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Furini, L.N.; Constantino, C.J.L.; De Saja, J.A.; Rodriguez-Mendez, M.L. Synergistic electrocatalytic effect of nanostructured mixed films formed by functionalised gold nanoparticles and bisphthalocyanines. Anal. Chim. Acta 2014, 851, 95–102. [Google Scholar] [CrossRef]
- Alessio, P.; Aoki, P.H.B.; De Saja Saez, J.A.; Rodríguez-Méndez, M.L.; Constantino, C.J.L. Combining SERRS and electrochemistry to characterize sensors based on biomembrane mimetic models formed by phospholipids. RSC Adv. 2011, 1, 211–218. [Google Scholar] [CrossRef]
- García-Hernández, C.; García-Cabezón, C.; Medina-Plaza, C.; Martín-Pedrosa, F.; Blanco, Y.; de Saja, J.A.; Rodríguez-Méndez, M.L. Electrochemical behavior of polypyrrol/AuNP composites deposited by different electrochemical methods: Sensing properties towards catechol. Beilstein J. Nanotechnol. 2015, 6, 2052–2061. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R.; Kirkland, A.I.; Logan, D.E. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid?Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Roth, P.J.; Theato, P. Versatile Synthesis of Functional Gold Nanoparticles: Grafting Polymers From and Onto. Chem. Mater. 2008, 20, 1614–1621. [Google Scholar] [CrossRef]
- Alessio, P.; Pavinatto, F.J.; Oliveira, O.N.; de Saja, J.A.; Constantino, J.C.L.; Rodriguez-Mendez, M.L. Detection of catechol using mixed Langmuir–Blodgett films of a phospholipid and phthalocyanines as voltammetric sensors. Analyst 2010, 135, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Maximino, M.D.; Martin, C.S.; Paulovich, F.V.; Alessio, P. Layer-by-Layer Thin Film of Iron Phthalocyanine as a Simple and Fast Sensor for Polyphenol Determination in Tea Samples. J. Food. Sci. 2016, 81, C2344–C2351. [Google Scholar] [CrossRef] [PubMed]
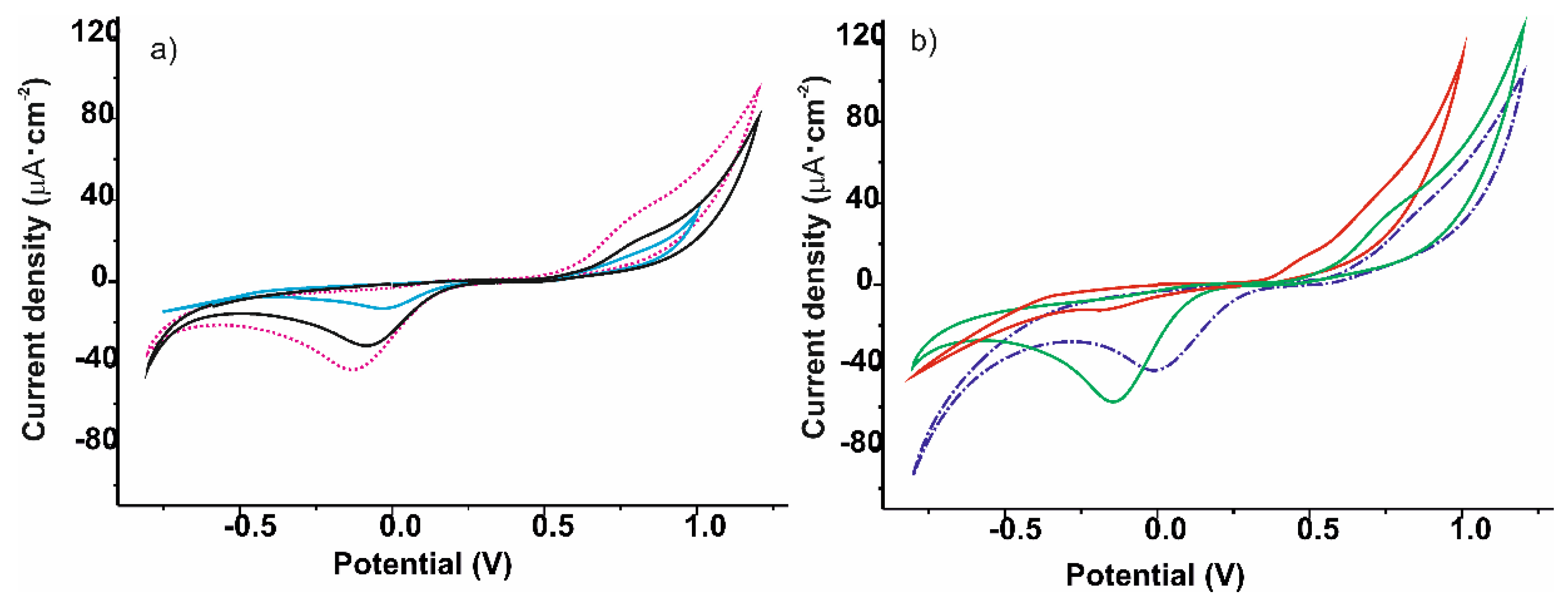
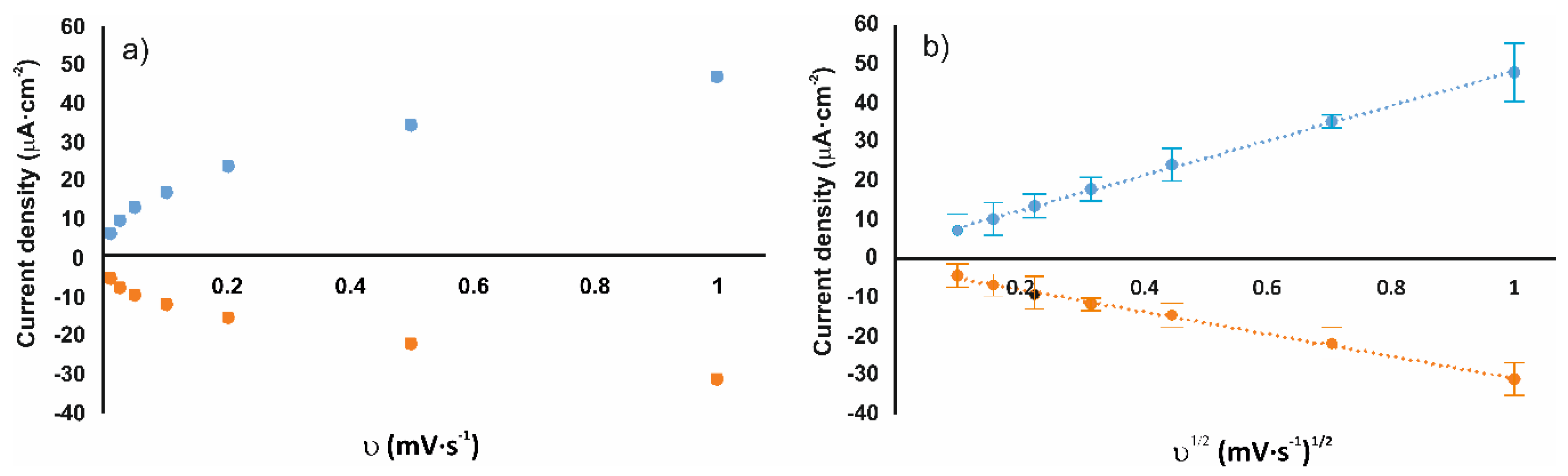
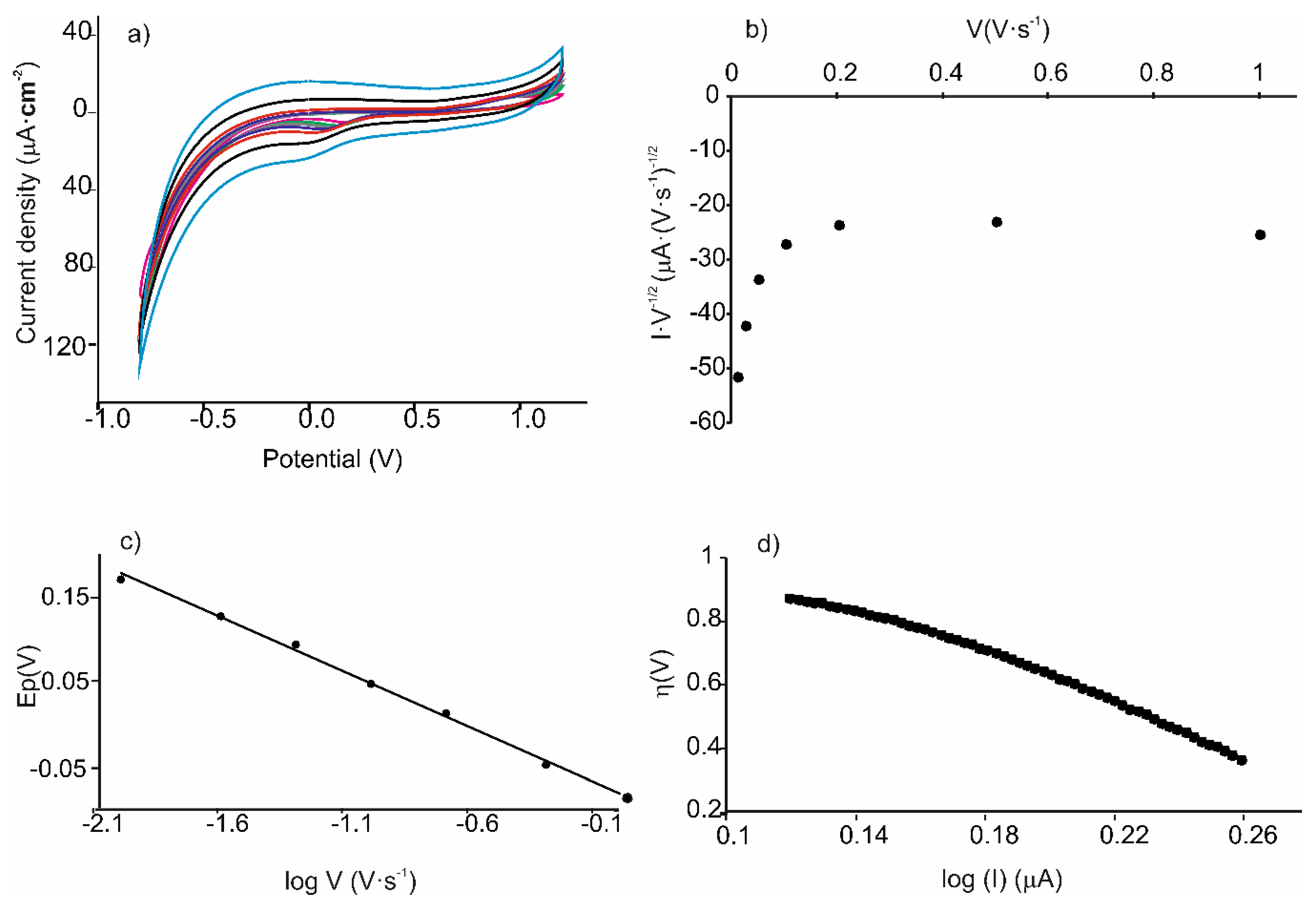
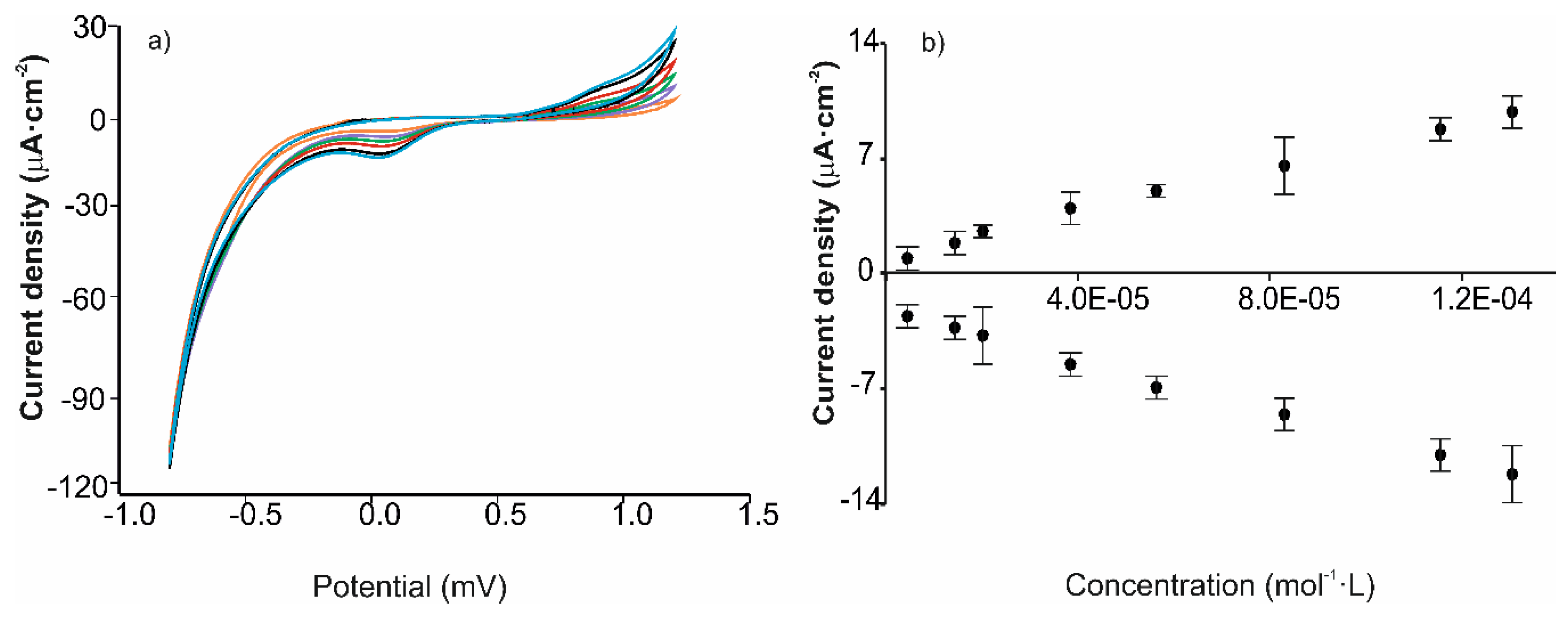
| Cathodic Wave at ca. –0.15 V | ||||||
|---|---|---|---|---|---|---|
| Laviron Model: I= f(ν), Ic (µA∙cm−2) vs. ν (V/s) | Randless–Sevcik Model I = f (sqrt(ν)) Ic (µA∙cm−2) vs. ν1/2 (V/s)1/2 | |||||
| Sensor | Slope | Intercept | R2 | Slope | Intercept | R2 |
| AuNPtOcBr/ZnPcRS | −19.32 | −6.11 | 0.993 | −21.31 | −2.32 | 0.973 |
| AuNPtOcBr/ZnPcR-S-ZnPcR | −24.78 | −7.59 | 0.953 | −28.26 | −2.34 | 0.997 |
| AuNPtOcBr-S-ZnPcR | −54.46 | −5.33 | 0.915 | −31.65 | −1.64 | 0.982 |
| Anodic wave at ca. 0.8 V | ||||||
| Ic (µA∙cm−2) vs. ν (V/s) | Ic (µA∙cm−2) vs. ν1/2 (V/s)1/2 | |||||
| Sensor | Slope | Intercept | R2 | Slope | Intercept | R2 |
| AuNPtOcBr/ZnPcRS | 42.12 | 6.34 | 0.980 | 37.20 | −0.06 | 0.962 |
| AuNPtOcBr/ZnPcR-S-ZnPcR | 38.85 | 11.71 | 0.935 | 44.76 | 3.30 | 0.998 |
| AuNPtOcBr-S-ZnPcR | 210.91 | 10.45 | 0.934 | 92.17 | 1.82 | 0.987 |
| Cathodic Peak | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ic/ν1/2 vs. ν | Log I vs. ƞ | Ec vs. log ν | |||||||
| Sensor | Slope | R2 | Slope | R2 | α | Slope | R2 | αn | n |
| AuNPtOcBr/ZnPcAcS | 252.39 | 0.882 | 3.73 | 0.986 | 0.28 | 0.130 | 0.997 | 0.452 | 2.05 |
| AuNPtOcBr/ZnPcR-S-ZnPcR | 177.68 | 0.947 | 4.43 | 0.979 | 0.26 | −0.105 | 0.997 | 0.562 | 2.14 |
| AuNPtOcBr-S-ZnPcR | 102.29 | 0.958 | 2.912 | 0.997 | 0.17 | 0.136 | 0.990 | 0.434 | 2.43 |
| Anodic Peak | |||||||||
| Ic/ν1/2 vs. ν | Log I vs. ƞ | Ec vs. log ν | |||||||
| Sensor | Slope | R2 | Slope | R2 | α | Slope | R2 | αn | n |
| AuNPtOcBr/ZnPcAcS | −244.14 | 0.872 | 2.34 | 0.998 | 0.76 | 0.189 | 0.953 | 0.313 | 2.28 |
| AuNPtOcBr/ZnPcR-S-ZnPcR | −153.15 | 0.888 | 3.88 | 0.999 | 0.77 | 0.113 | 0.985 | 0.522 | 2.28 |
| AuNPtOcBr-S-ZnPcR | −137.50 | 0.964 | 1.579 | 0.999 | 0.90 | 0.331 | 0.992 | 0.165 | 1.76 |
| Sensor | Sensitivity (µA∙cm−2/mol∙L−1) | LOD (× 10−6 mol∙L−1) | R2 | |
|---|---|---|---|---|
| Cathodic peak | AuNPtOcBr | −23,747 | 4.0 | 0.992 |
| ZnPcRS | −87,223 | 2.0 | 0.987 | |
| AuNPtOcBr/ZnPcRS | −76,350 | 0.9 | 0.997 | |
| AuNPtOcBr/ZnPcR-S-ZnPcR | −99,039 | 1.2 | 0.989 | |
| AuNPtOcBr-S-ZnPcR | −32,419 | 8.3 | 0.989 | |
| Anodic peak | AuNPtOcBr | 10,539 | 4.4 | 0.992 |
| ZnPcRS | 28,343 | 2.9 | 0.985 | |
| AuNPtOcBr/ZnPcRS | 68,170 | 2.2 | 0.994 | |
| AuNPtOcBr/ZnPcR-S-ZnPcR | 44,337 | 2.07 | 0.981 | |
| AuNPtOcBr-S-ZnPcR | 45,498 | 0.13 | 0.993 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Carmuega, A.I.; Garcia-Hernandez, C.; Ortiz, J.; Garcia-Cabezon, C.; Martin-Pedrosa, F.; Sastre-Santos, Á.; Rodríguez-Perez, M.A.; Rodriguez-Mendez, M.L. Electrochemical Sensors Modified with Combinations of Sulfur Containing Phthalocyanines and Capped Gold Nanoparticles: A Study of the Influence of the Nature of the Interaction between Sensing Materials. Nanomaterials 2019, 9, 1506. https://doi.org/10.3390/nano9111506
Ruiz-Carmuega AI, Garcia-Hernandez C, Ortiz J, Garcia-Cabezon C, Martin-Pedrosa F, Sastre-Santos Á, Rodríguez-Perez MA, Rodriguez-Mendez ML. Electrochemical Sensors Modified with Combinations of Sulfur Containing Phthalocyanines and Capped Gold Nanoparticles: A Study of the Influence of the Nature of the Interaction between Sensing Materials. Nanomaterials. 2019; 9(11):1506. https://doi.org/10.3390/nano9111506
Chicago/Turabian StyleRuiz-Carmuega, Ana Isabel, Celia Garcia-Hernandez, Javier Ortiz, Cristina Garcia-Cabezon, Fernando Martin-Pedrosa, Ángela Sastre-Santos, Miguel Angel Rodríguez-Perez, and Maria Luz Rodriguez-Mendez. 2019. "Electrochemical Sensors Modified with Combinations of Sulfur Containing Phthalocyanines and Capped Gold Nanoparticles: A Study of the Influence of the Nature of the Interaction between Sensing Materials" Nanomaterials 9, no. 11: 1506. https://doi.org/10.3390/nano9111506
APA StyleRuiz-Carmuega, A. I., Garcia-Hernandez, C., Ortiz, J., Garcia-Cabezon, C., Martin-Pedrosa, F., Sastre-Santos, Á., Rodríguez-Perez, M. A., & Rodriguez-Mendez, M. L. (2019). Electrochemical Sensors Modified with Combinations of Sulfur Containing Phthalocyanines and Capped Gold Nanoparticles: A Study of the Influence of the Nature of the Interaction between Sensing Materials. Nanomaterials, 9(11), 1506. https://doi.org/10.3390/nano9111506