Mesoporous Tungsten Trioxide Photoanodes Modified with Nitrogen-Doped Carbon Quantum Dots for Enhanced Oxygen Evolution Photo-Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of CQDs, N-CQDs, and Mesoporous WO3 Hybrid Photoanodes
2.3. Material Characterization
2.4. Photoelectrochemical Measurements of the Hybrid Materials
3. Results and Discussion
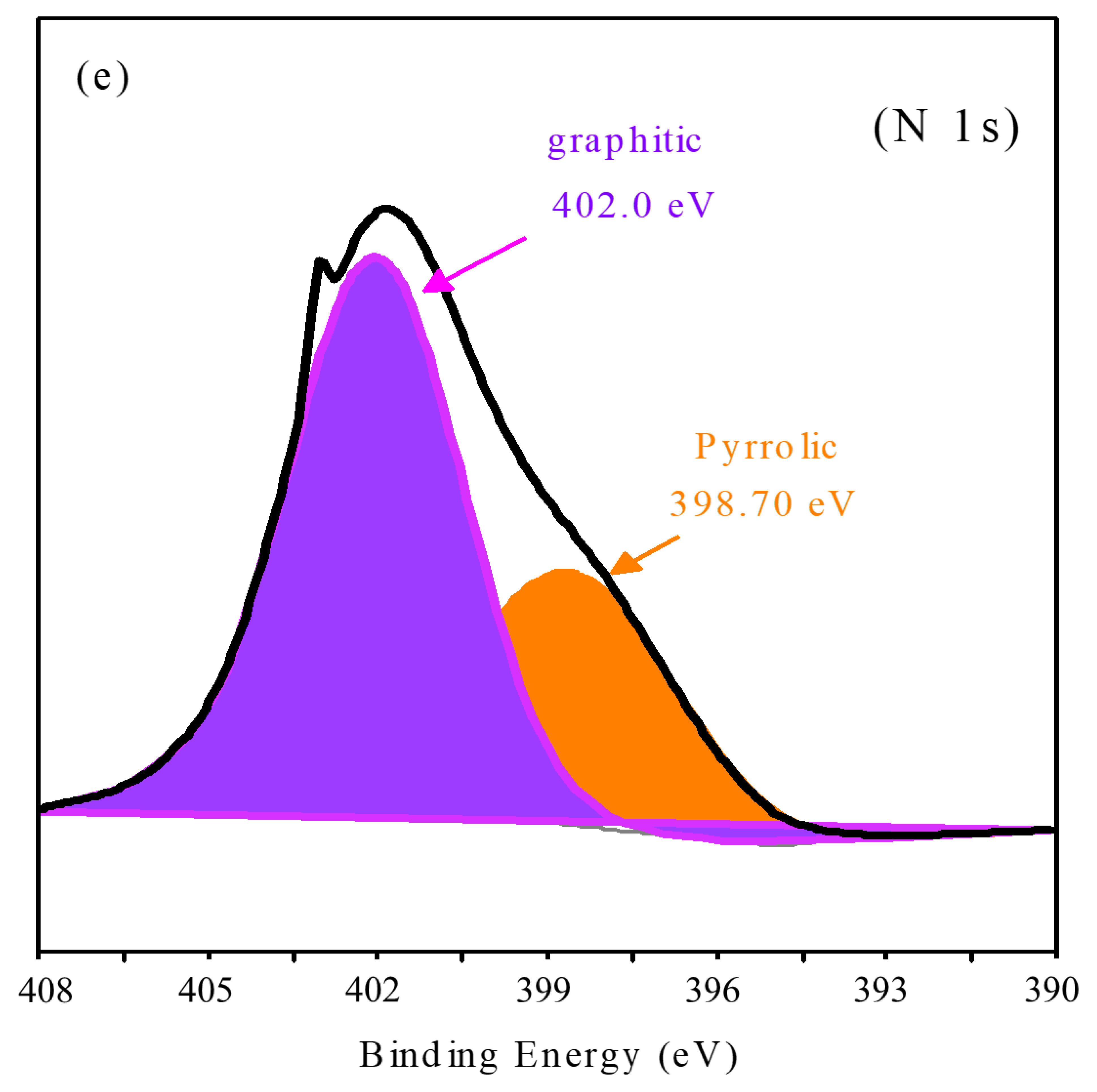
3.1. Characterization of the CQDs and N-CQDs
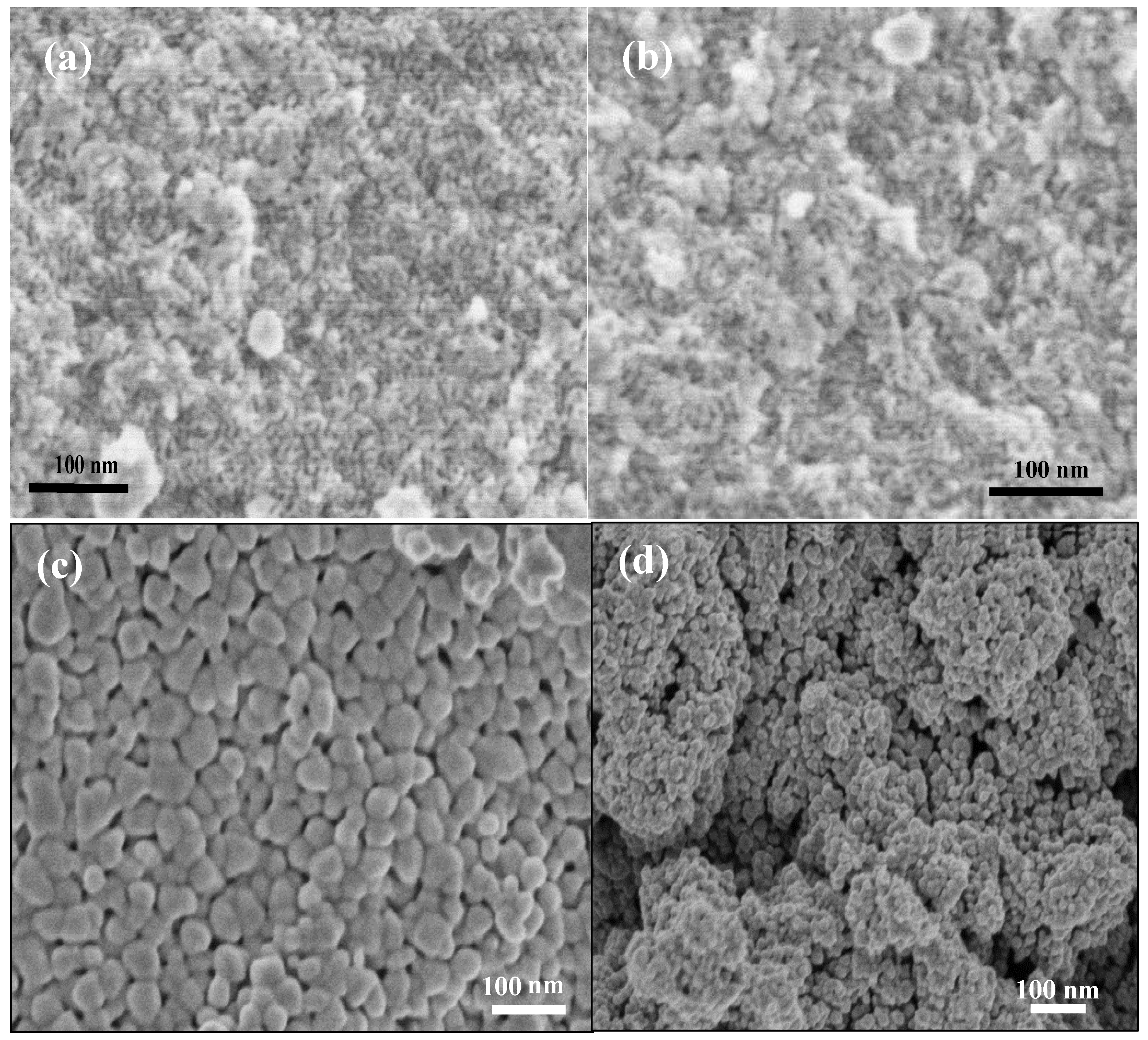
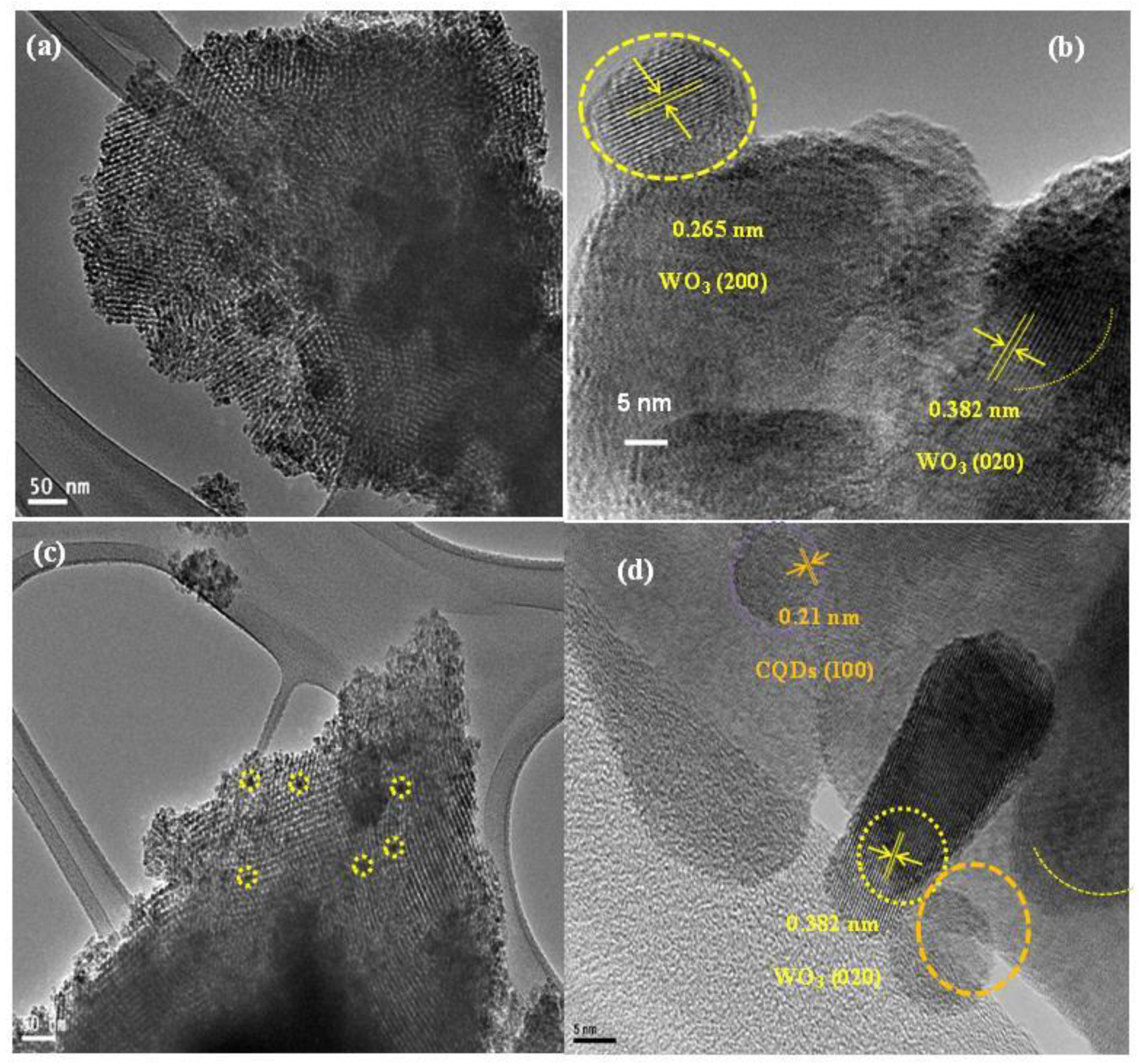
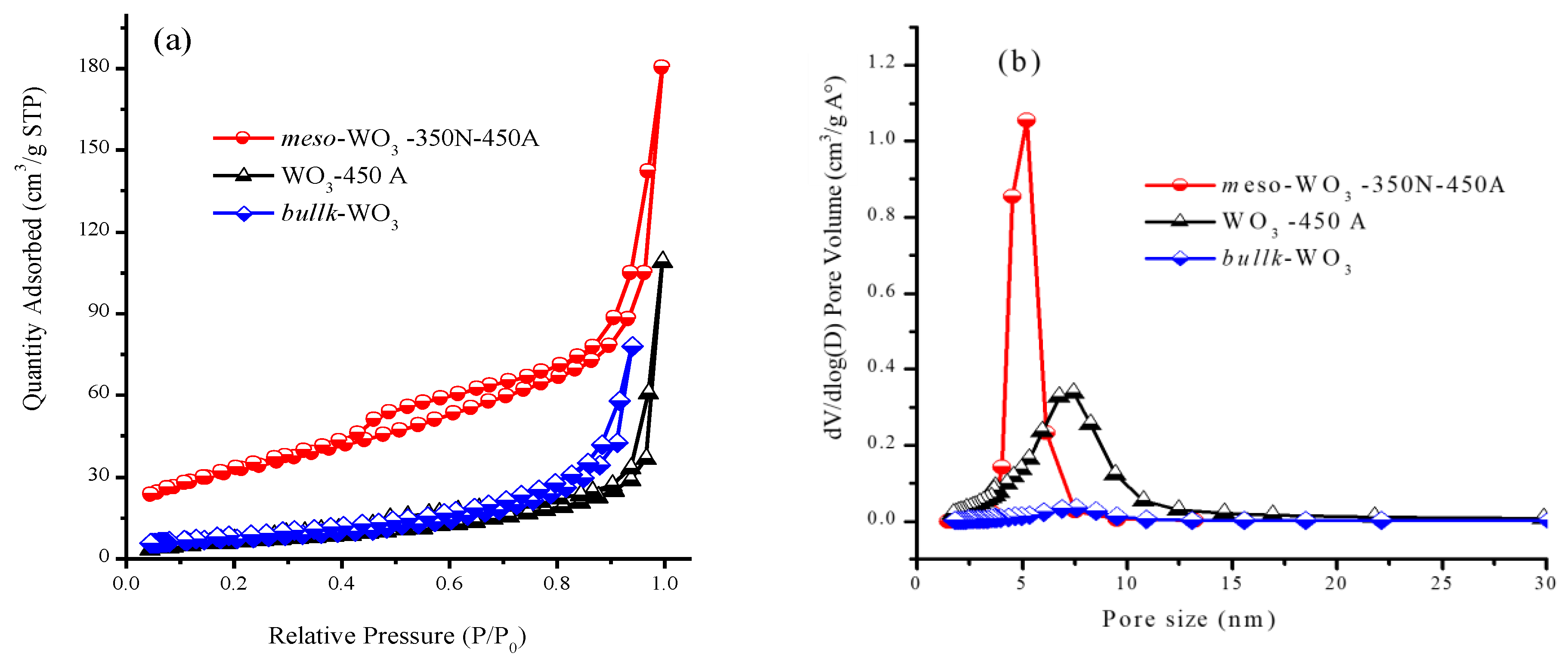
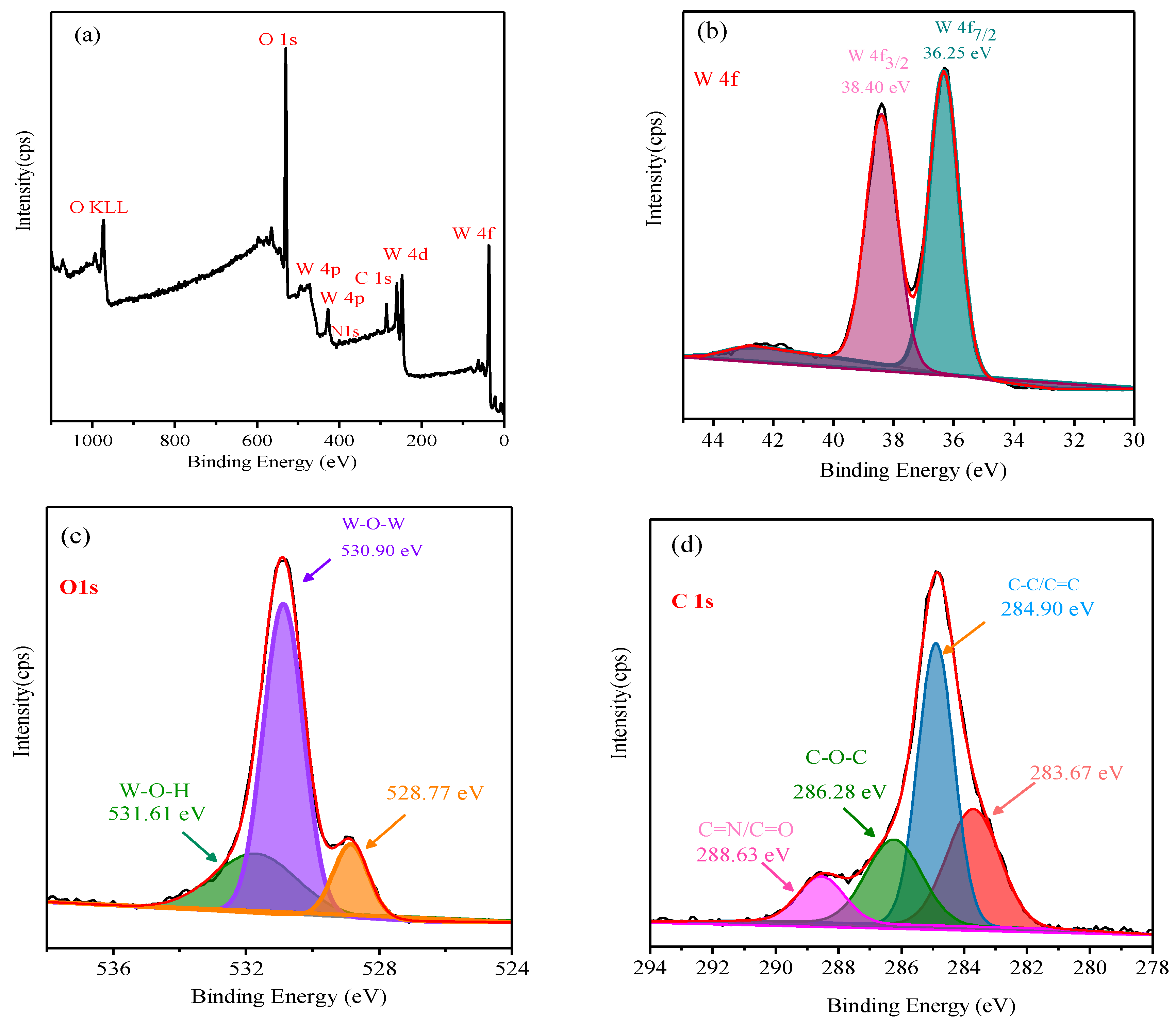
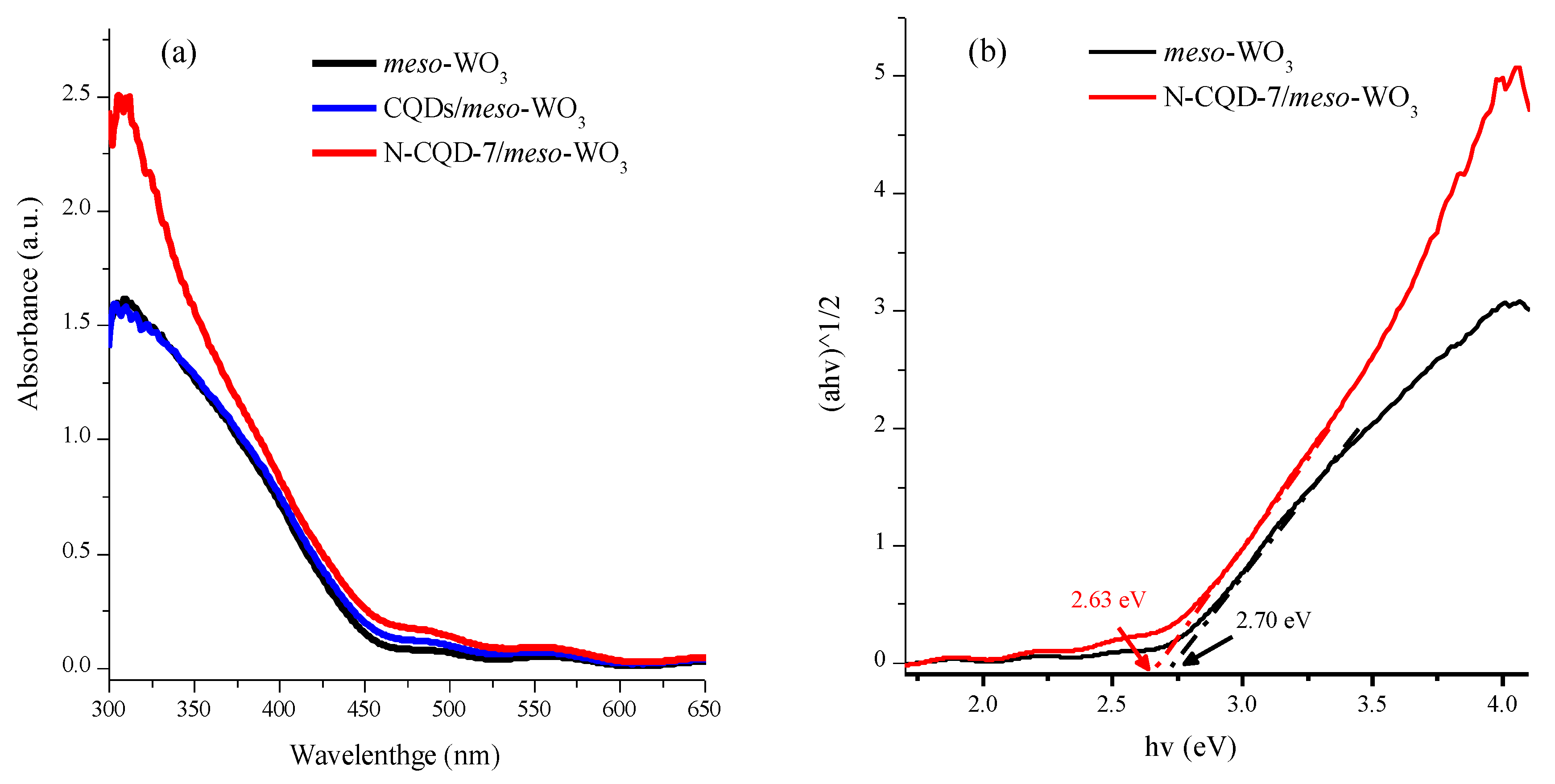
3.2. Characterization of Meso-WO3 and the CQD/Meso-WO3 and N-CQD/Meso-WO3 Hybrids
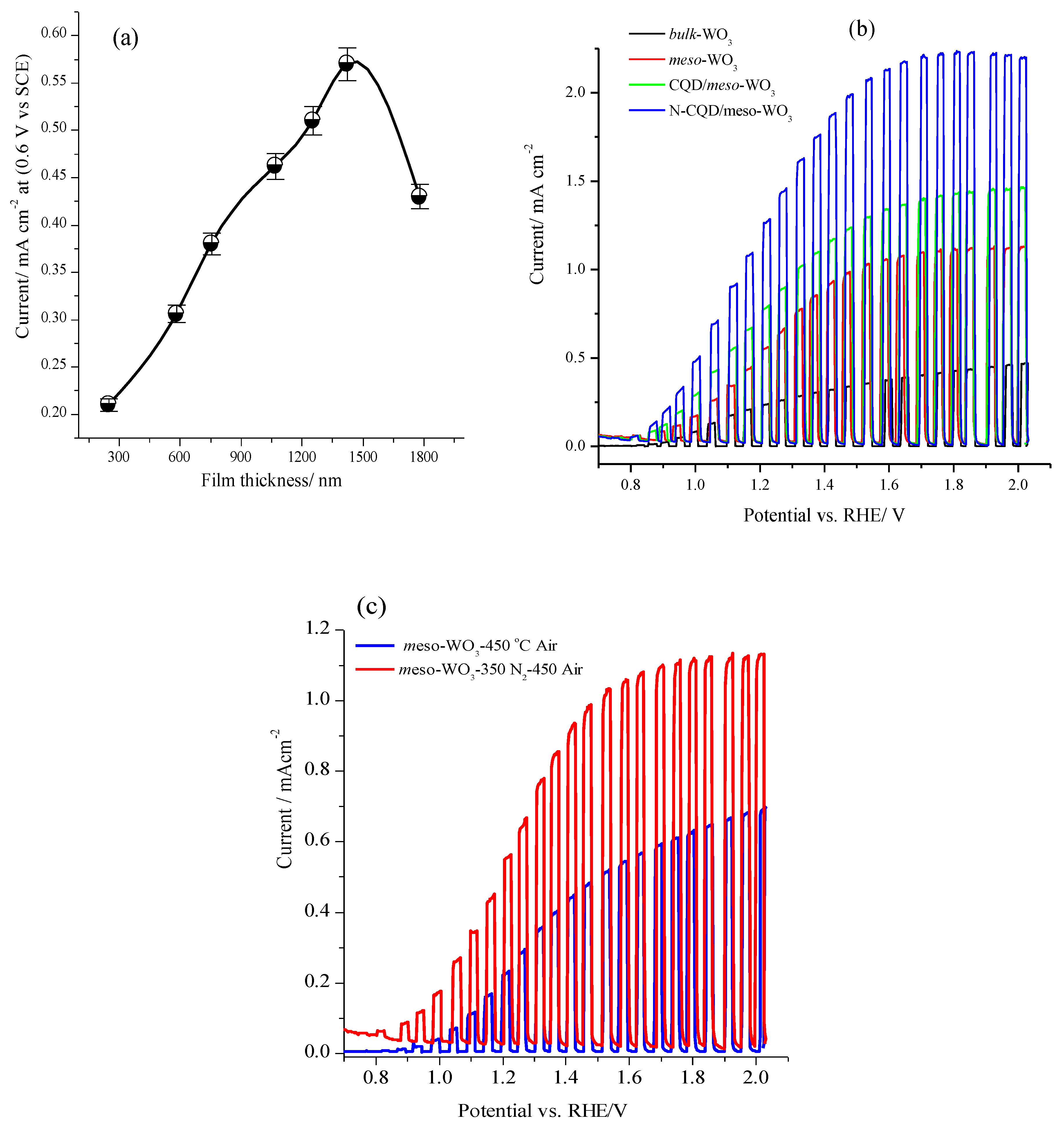
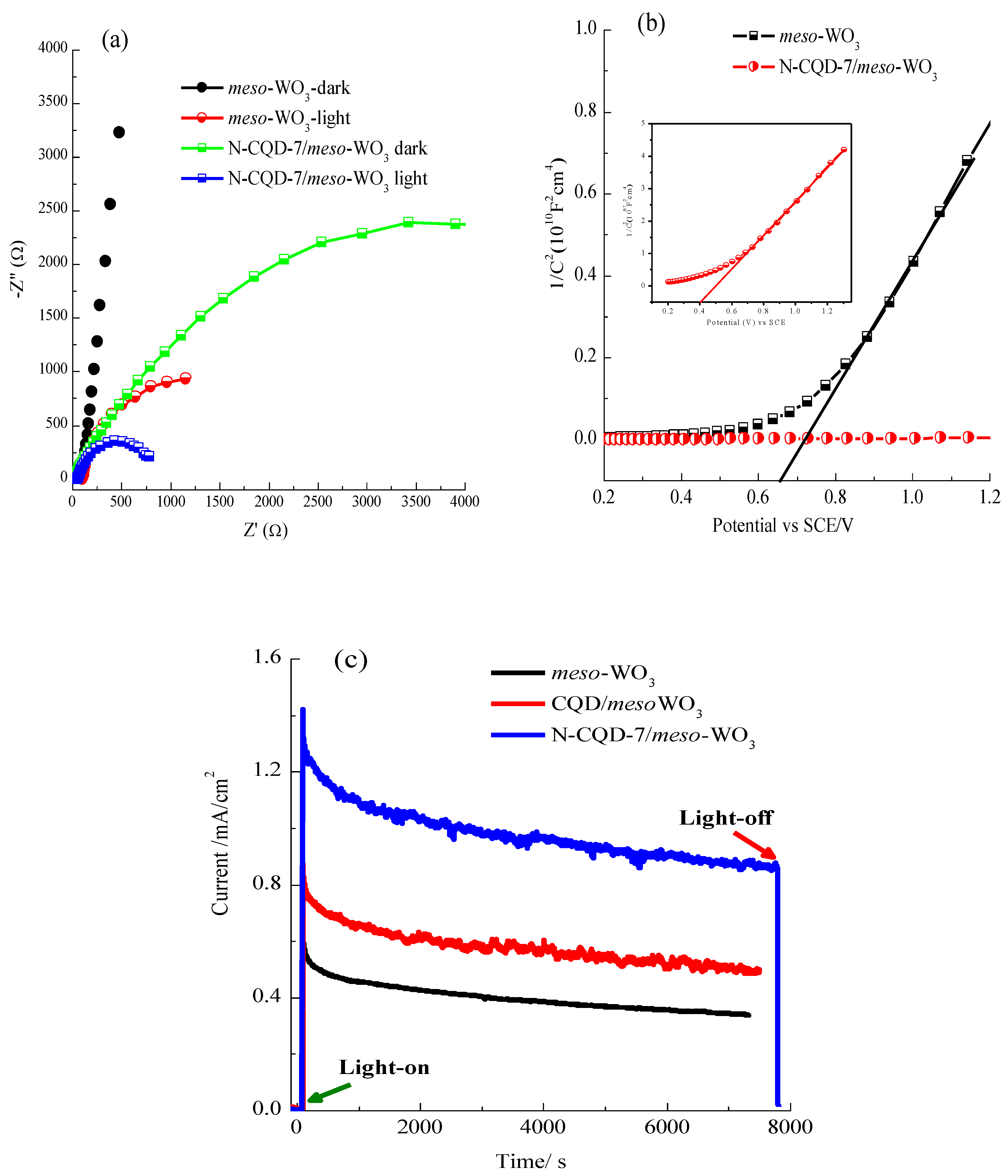
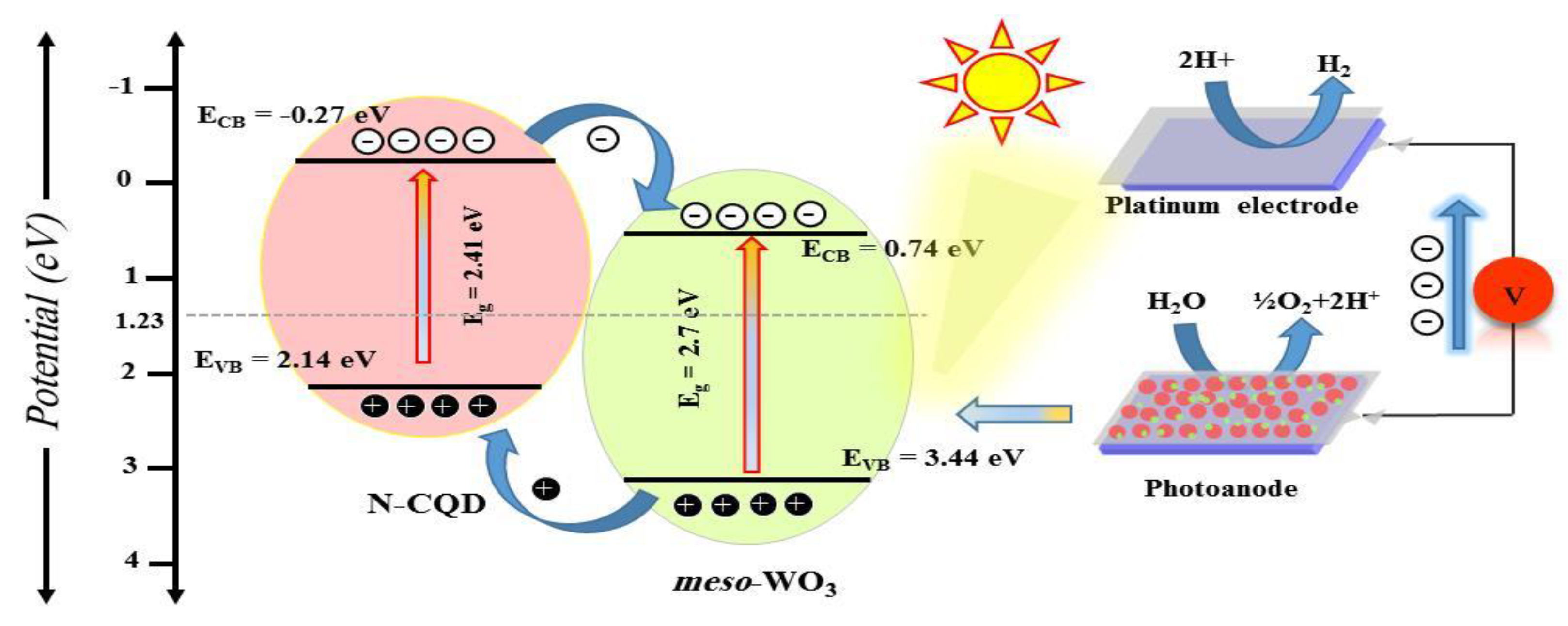
3.3. Photo-Electrochemical Properties of the Meso-WO3 Photoanodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Seitz, L.C.; Chen, Z.; Forman, A.J.; Pinaud, B.A.; Benck, J.D.; Jaramillo, T.F. Modeling practical performance limits of photoelectrochemical water splitting based on the current state of materials research. ChemSusChem 2014, 7, 1372–1385. [Google Scholar] [CrossRef]
- Cummings, C.Y.; Marken, F.; Peter, L.M.; Wijayantha, K.G.U.; Tahir, A.A. Study by Modulated Transmittance and Impedance Spectroscopies. J. Am. Chem. Soc. 2012, 134, 1228–1234. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, W.; Xu, S.; Wang, Z.L. Enhancing light emission of ZnO microwire-based diodes by piezo-phototronic effect. Nano Lett. 2011, 11, 4012–4017. [Google Scholar] [CrossRef]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015, 6, 8769. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, K.; Cho, S.M.; Lee, T.W.; Park, J.H. Synthesis of transparent mesoporous tungsten trioxide films with enhanced photoelectrochemical response: Application to unassisted solar water splitting. Energy Environ. Sci. 2011, 4, 1465–1470. [Google Scholar] [CrossRef]
- Chandra, D.; Saito, K.; Yui, T.; Yagi, M. Crystallization of tungsten trioxide having small mesopores: Highly efficient photoanode for visible-light-driven water oxidation. Angew. Chem. Int. Ed. 2013, 52, 12606–12609. [Google Scholar] [CrossRef]
- Hartmann, P.; Lee, D.K.; Smarsly, B.M.; Janek, J. Mesoporous TiO2: Comparison of classical sol-gel and nanoparticle based photoelectrodes for the water splitting reaction. ACS Nano 2010, 4, 3147–3154. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically oriented mesoporous WO3 films: Synthesis, characterization, and applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef]
- Liu, Y.; Koep, E.; Liu, M. A highly sensitive and fast-responding SnO2 sensor fabricated by combustion chemical vapor deposition. Chem. Mater. 2005, 17, 3997–4000. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Wu, X.; Jin, G.; Zhai, Z.; Liu, H. Inhomogeneous oxygen vacancy distribution in semiconductor gas sensors: Formation, migration and determination on gas sensing characteristics. Sensors 2017, 17, 1852. [Google Scholar] [CrossRef]
- Tian, B.; Liu, X.; Solovyov, L.A.; Liu, Z.; Yang, H.; Zhang, Z.; Xie, S.; Zhang, F.; Tu, B.; Yu, C.; et al. Facile Synthesis and Characterization of Novel Mesoporous and Mesorelief Oxides with Gyroidal Structures. J. Am. Chem. Soc. 2004, 126, 865–875. [Google Scholar] [CrossRef]
- Deng, X.; Chen, K.; Tüysüz, H. Protocol for the Nanocasting Method: Preparation of Ordered Mesoporous Metal Oxides. Chem. Mater. 2017, 29, 40–52. [Google Scholar] [CrossRef]
- Jo, C.; Hwang, J.; Song, H.; Dao, A.H.; Kim, Y.T.; Lee, S.H.; Hong, S.W.; Yoon, S.; Lee, J. Block-copolymer-assisted one-pot synthesis of ordered mesoporous WO3-x/carbon nanocomposites as high-rate-performance electrodes for pseudocapacitors. Adv. Funct. Mater. 2013, 23, 3747–3754. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 1998, 396, 152–155. [Google Scholar] [CrossRef]
- Zhou, W.; Li, W.; Wang, J.Q.; Qu, Y.; Yang, Y.; Xie, Y.; Zhang, K.; Wang, L.; Fu, H.; Zhao, D. Ordered mesoporous black TiO2 as highly efficient hydrogen evolution photocatalyst. J. Am. Chem. Soc. 2014, 136, 9280–9283. [Google Scholar] [CrossRef]
- Cheng, W.; Baudrin, E.; Dunn, B.; Zink, J.I. Synthesis and electrochromic properties of mesoporous tungsten oxide. J. Mater. Chem. 2001, 11, 92–97. [Google Scholar] [CrossRef]
- Walcarius, A. Mesoporous materials and electrochemistry. Chem. Soc. Rev. 2013, 42, 4098–4140. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; García-Martínez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef]
- Ye, Y.; Jo, C.; Jeong, I.; Lee, J. Functional mesoporous materials for energy applications: Solar cells, fuel cells, and batteries. Nanoscale 2013, 5, 4584–4605. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, Z.; Luo, W.; Li, Y.; Elzatahry, A.A.; Al-Enizi, A.M.; Deng, Y.; Zhao, D. New insight into the synthesis of large-pore ordered mesoporous materials. J. Am. Chem. Soc. 2017, 139, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, W.; Qin, N.; Dong, J.; Wei, J.; Li, W.; Feng, S.; Chen, J.; Xu, J.; Elzatahry, A.A.; et al. Highly ordered mesoporous tungsten oxides with a large pore size and crystalline framework for H2S sensing. Angew. Chem. Int. Ed. 2014, 53, 9035–9040. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Y.; Ma, J.; Cheng, X.; Xie, J.; Xu, P.; Liu, H.; Liu, H.; Zhang, H.; Wu, M.; et al. Mesoporous Tungsten Oxides with Crystalline Framework for Highly Sensitive and Selective Detection of Foodborne Pathogens. J. Am. Chem. Soc. 2017, 139, 10365–10373. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.; Amer, M.S.; Ghanem, M.A.; Al-Mayouf, A.M.; Zhao, D. Activation effect of silver nanoparticles on the photoelectrochemical performance of mesoporous TiO2 nanospheres photoanodes for water oxidation reaction. Int. J. Hydrog. Energy 2017, 42, 11346–11355. [Google Scholar] [CrossRef]
- Hu, D.; Diao, P.; Xu, D.; Wu, Q. Gold/WO3 nanocomposite photoanodes for plasmonic solar water splitting. Nano Res. 2016, 9, 1735–1751. [Google Scholar] [CrossRef]
- Ghanem, M.A.; Arunachalam, P.; Amer, M.S.; Al-Mayouf, A.M. Mesoporous titanium dioxide photoanodes decorated with gold nanoparticles for boosting the photoelectrochemical alkali water oxidation. Mater. Chem. Phys. 2018, 213, 56–66. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, S.; Liu, B.K.; Wang, D.J.; Xie, T.F. Highly efficient CdS/WO3 photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic H2 evolution under visible light. ACS Catal. 2014, 4, 3724–3729. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Chen, L.; Cao, F.; Guo, J.; Li, L. Three-Dimensional WO3 Nanoplate/Bi2S3 Nanorod Heterojunction as a Highly Efficient Photoanode for Improved Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 40235–40243. [Google Scholar] [CrossRef]
- Lee, Y.L.; Huang, B.M.; Chien, H.T. Highly efficient CdSe-sensitized TiO2 photoelectrode for quantum-dot-sensitized solar cell applications. Chem. Mater. 2008, 20, 6903–6905. [Google Scholar] [CrossRef]
- Nozik, A.J. Quantum Dot Solar Cells. Phys. E Low Dimens. Syst. Nanostructures 2002, 14, 115–120. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lv, X.; Shen, Y. BiOI/WO3 photoanode with enhanced photoelectrochemical water splitting activity. Front. Optoelectron. 2018, 11, 367–374. [Google Scholar] [CrossRef]
- Zhao, Z.; Butburee, T.; Peerakiatkhajohn, P.; Lyu, M.; Wang, S.; Wang, L.; Zheng, H. Carbon Quantum Dots sensitized Vertical WO3 Nanoplates with Enhanced Photoelectrochemical Properties. ChemistrySelect 2016, 1, 2772–2777. [Google Scholar] [CrossRef]
- Miao, R.; Luo, Z.; Zhong, W.; Chen, S.Y.; Jiang, T.; Dutta, B.; Nasr, Y.; Zhang, Y.; Suib, S.L. Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst. Appl. Catal. B Environ. 2016, 189, 26–38. [Google Scholar] [CrossRef]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Kong, B.; Tang, J.; Zhang, Y.; Jiang, T.; Gong, X.; Peng, C.; Wei, J.; Yang, J.; Wang, Y.; Wang, X.; et al. Incorporation of well-dispersed sub-5-nm graphitic pencil nanodots into ordered mesoporous frameworks. Nat. Chem. 2016, 8, 171–177. [Google Scholar] [CrossRef]
- Yeh, T.F.; Teng, C.Y.; Chen, S.J.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef]
- Arcudi, F.; Dordevic, L.; Prato, M. Synthesis, separation, and characterization of small and highly fluorescent nitrogen-doped carbon nanodots. Angew. Chem. Int. Ed. 2016, 55, 2107–2112. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Infrared Spectroscopy. In Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 71–125. [Google Scholar]
- Hsu, P.C.; Chang, H.T. Synthesis of high-quality carbon nanodots from hydrophilic compounds: Role of functional groups. Chem. Commun. 2012, 48, 3984–3986. [Google Scholar] [CrossRef]
- Shi, R.; Li, Z.; Yu, H.; Shang, L.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Zhang, T. Effect of Nitrogen Doping Level on the Performance of N-Doped Carbon Quantum Dot/TiO2 Composites for Photocatalytic Hydrogen Evolution. ChemSusChem 2017, 10, 4650–4656. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H.; Liu, X.; Li, B.; Li, Y.; Yu, W.; et al. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Long, B.; Huang, Y.; Li, H.; Zhao, F.; Rui, Z.; Liu, Z.; Tong, Y.; Ji, H. Carbon Dots Sensitized BiOI with Dominant {001} Facets for Superior Photocatalytic Performance. Ind. Eng. Chem. Res. 2015, 54, 12788–12794. [Google Scholar] [CrossRef]
- Tang, C.; Liu, E.; Wan, J.; Hu, X.; Fan, J. Co3O4 nanoparticles decorated Ag3PO4 tetrapods as an efficient visible-light-driven heterojunction photocatalyst. Appl. Catal. B Environ. 2016, 181, 707–715. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Liu, J.; Wang, Y.; Duan, D.; Fan, C. Facile regeneration and photocatalytic activity of CuO-modified silver bromide photocatalyst. Mater. Sci. Semicond. Process. 2015, 40, 613–620. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Gao, S.; Shang, J.K. Template-free solvothermal synthesis of WO3/WO3•H2O hollow spheres and their enhanced photocatalytic activity from the mixture phase effect. CrystEngComm 2014, 16, 7493–7501. [Google Scholar] [CrossRef]
- Hilliard, S.; Baldinozzi, G.; Friedrich, D.; Kressman, S.; Strub, H.; Artero, V.; Laberty-Robert, C. Mesoporous thin film WO3 photoanode for photoelectrochemical water splitting: A sol-gel dip coating approach. Sustain. Energy Fuels 2017, 1, 145–153. [Google Scholar]
- Ye, K.H.; Wang, Z.; Gu, J.; Xiao, S.; Yuan, Y.; Zhu, Y.; Zhang, Y.; Mai, W.; Yang, S. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes. Energy Environ. Sci. 2017, 10, 772–779. [Google Scholar] [CrossRef]
- Zhong, D.K.; Sun, J.; Inumaru, H.; Gamelin, D.R. Solar Water Oxidation by Composite Catalyst / r -Fe2O3 Photoanodes. J. Am. Chem. Soc. 2009, 131, 6086–6087. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Chang, X.; Zhang, L.; Gong, J. Synergistic Cocatalytic Effect of Carbon Nanodots and Co3O4 Nanoclusters for the Photoelectrochemical Water Oxidation on Hematite. Angew. Chem. Int. Ed. 2016, 55, 5851–5855. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Energy-level structure of nitrogen-doped graphene quantum dots. J. Mater. Chem. C 2013, 1, 4908–4915. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, X.; Liu, S.; Zhou, Y.; Chang, B.; Zhang, S.; Fan, H.; Yang, B. N Doped Carbon Dot Modified WO3 Nanoflakes for Efficient Photoelectrochemical Water Oxidation. Adv. Mater. Interfaces 2019, 6, 1801653. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Prediction of Flat band Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, M.S.; Arunachalam, P.; Al-Mayouf, A.M.; Prasad, S.; Alshalwi, M.N.; Ghanem, M.A. Mesoporous Tungsten Trioxide Photoanodes Modified with Nitrogen-Doped Carbon Quantum Dots for Enhanced Oxygen Evolution Photo-Reaction. Nanomaterials 2019, 9, 1502. https://doi.org/10.3390/nano9101502
Amer MS, Arunachalam P, Al-Mayouf AM, Prasad S, Alshalwi MN, Ghanem MA. Mesoporous Tungsten Trioxide Photoanodes Modified with Nitrogen-Doped Carbon Quantum Dots for Enhanced Oxygen Evolution Photo-Reaction. Nanomaterials. 2019; 9(10):1502. https://doi.org/10.3390/nano9101502
Chicago/Turabian StyleAmer, Mabrook S., Prabhakarn Arunachalam, Abdullah M. Al-Mayouf, Saradh Prasad, Matar N. Alshalwi, and Mohamed A. Ghanem. 2019. "Mesoporous Tungsten Trioxide Photoanodes Modified with Nitrogen-Doped Carbon Quantum Dots for Enhanced Oxygen Evolution Photo-Reaction" Nanomaterials 9, no. 10: 1502. https://doi.org/10.3390/nano9101502
APA StyleAmer, M. S., Arunachalam, P., Al-Mayouf, A. M., Prasad, S., Alshalwi, M. N., & Ghanem, M. A. (2019). Mesoporous Tungsten Trioxide Photoanodes Modified with Nitrogen-Doped Carbon Quantum Dots for Enhanced Oxygen Evolution Photo-Reaction. Nanomaterials, 9(10), 1502. https://doi.org/10.3390/nano9101502








