A Novel Strategy for Fabricating a Strong Nanoparticle Monolayer and Its Enhanced Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Methods
2.2.1. Preparation and Phase Transfer of Au NPs
2.2.2. Fabrication of Ultrathin Monolayer Au NP Membranes on Quartz Plate and Heat Treatment
2.2.3. Fabrication of Ultrathin Monolayer Au NP Membranes on Teflon Tape and Heat Treatment
2.2.4. Cross-Linking of Ultrathin Monolayer Au NP Membranes
3. Results and Discussion
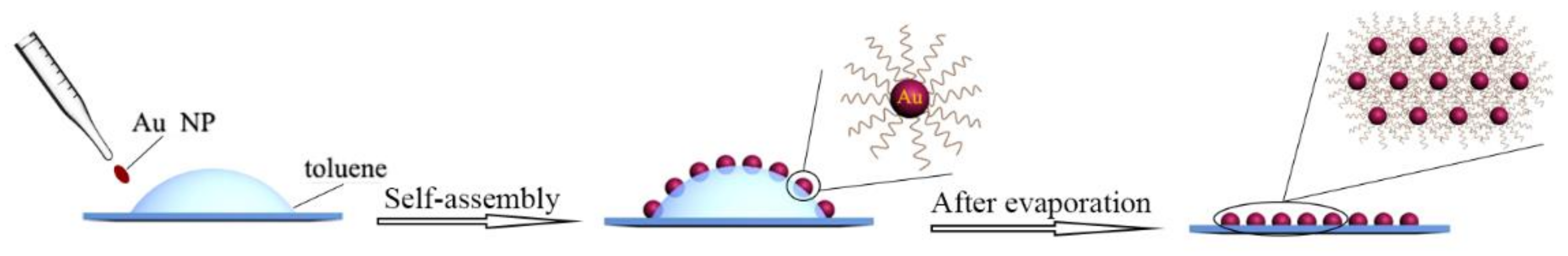
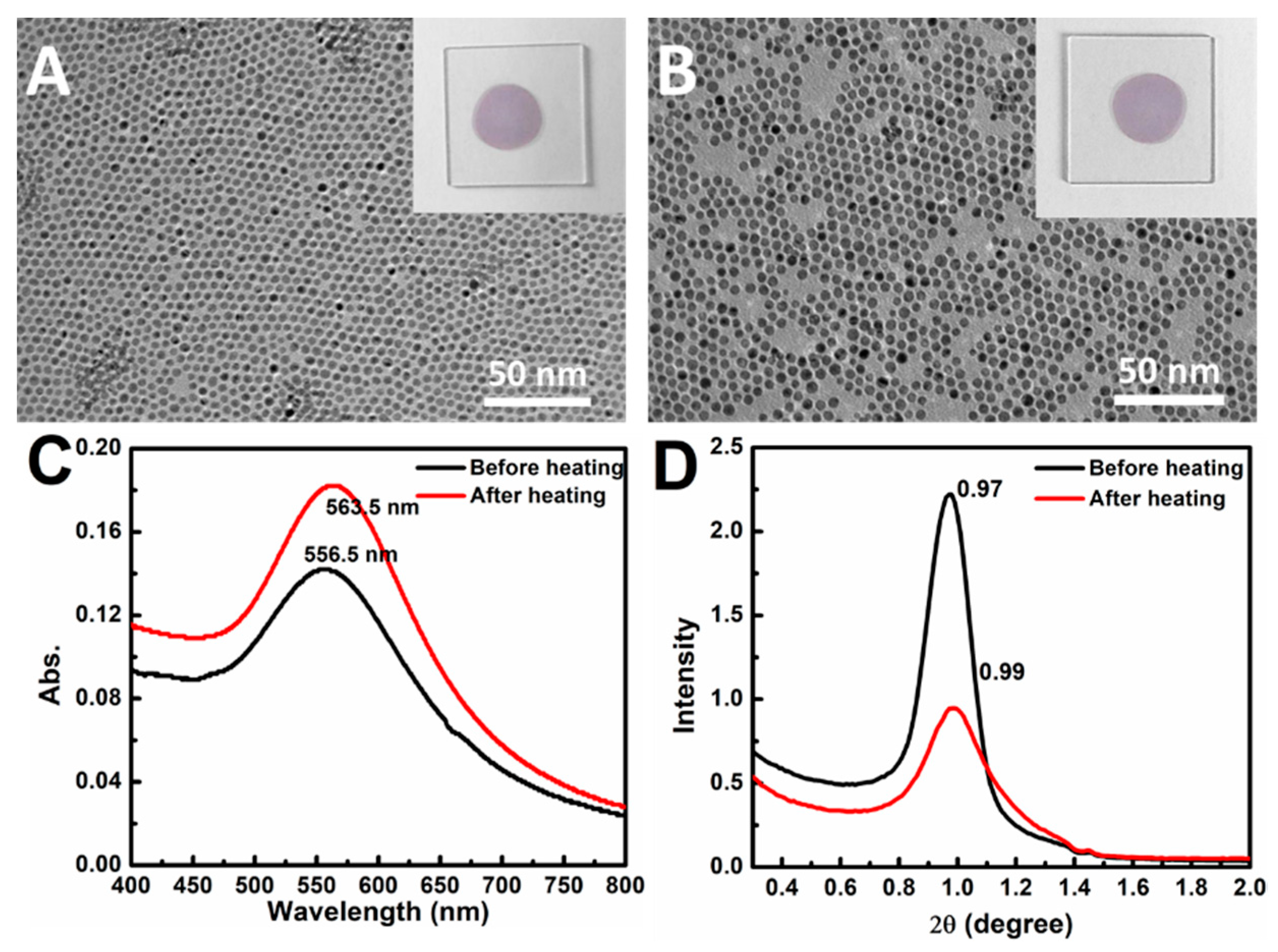
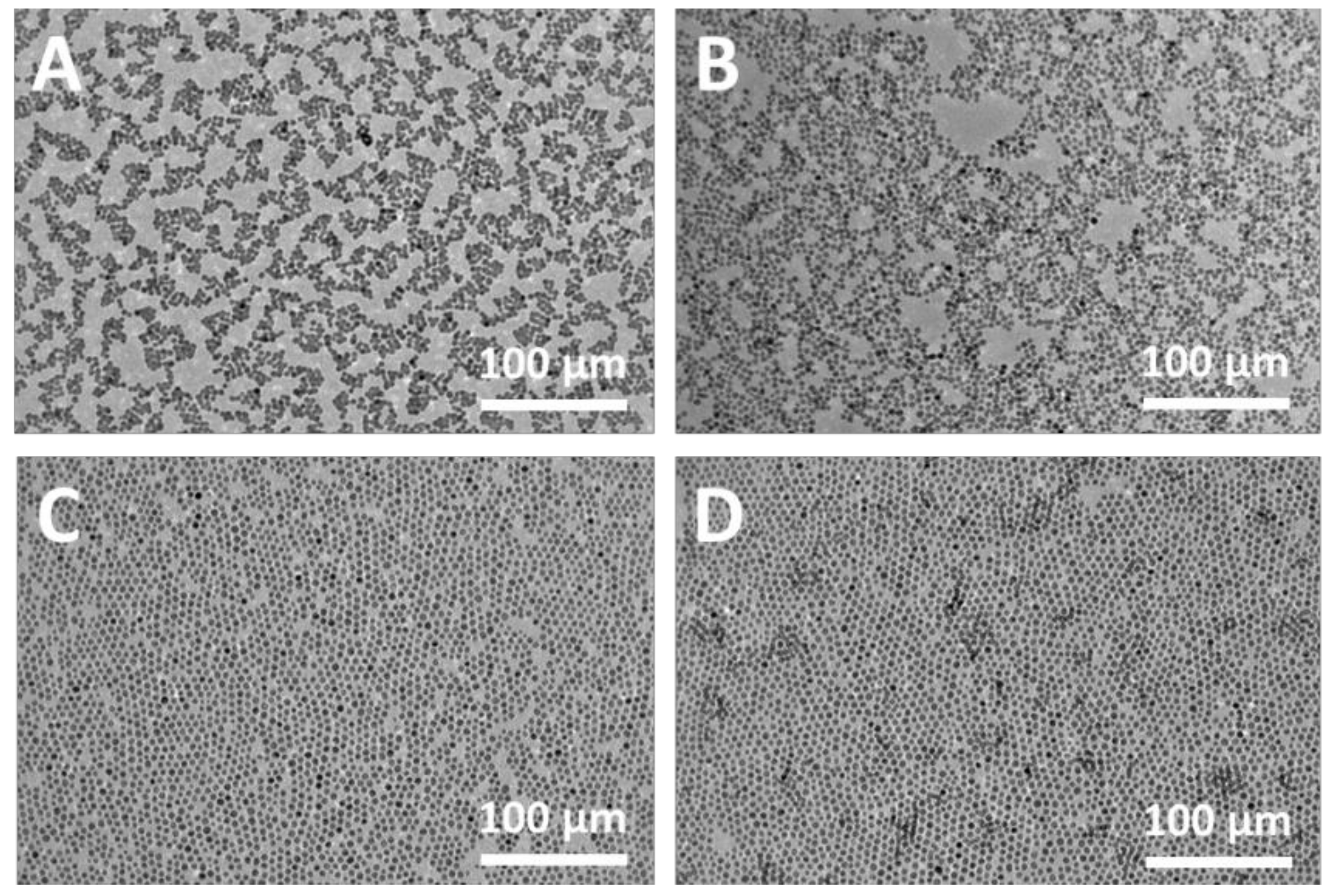
3.1. Preparation of Au NP Membranes and Heat Treatment
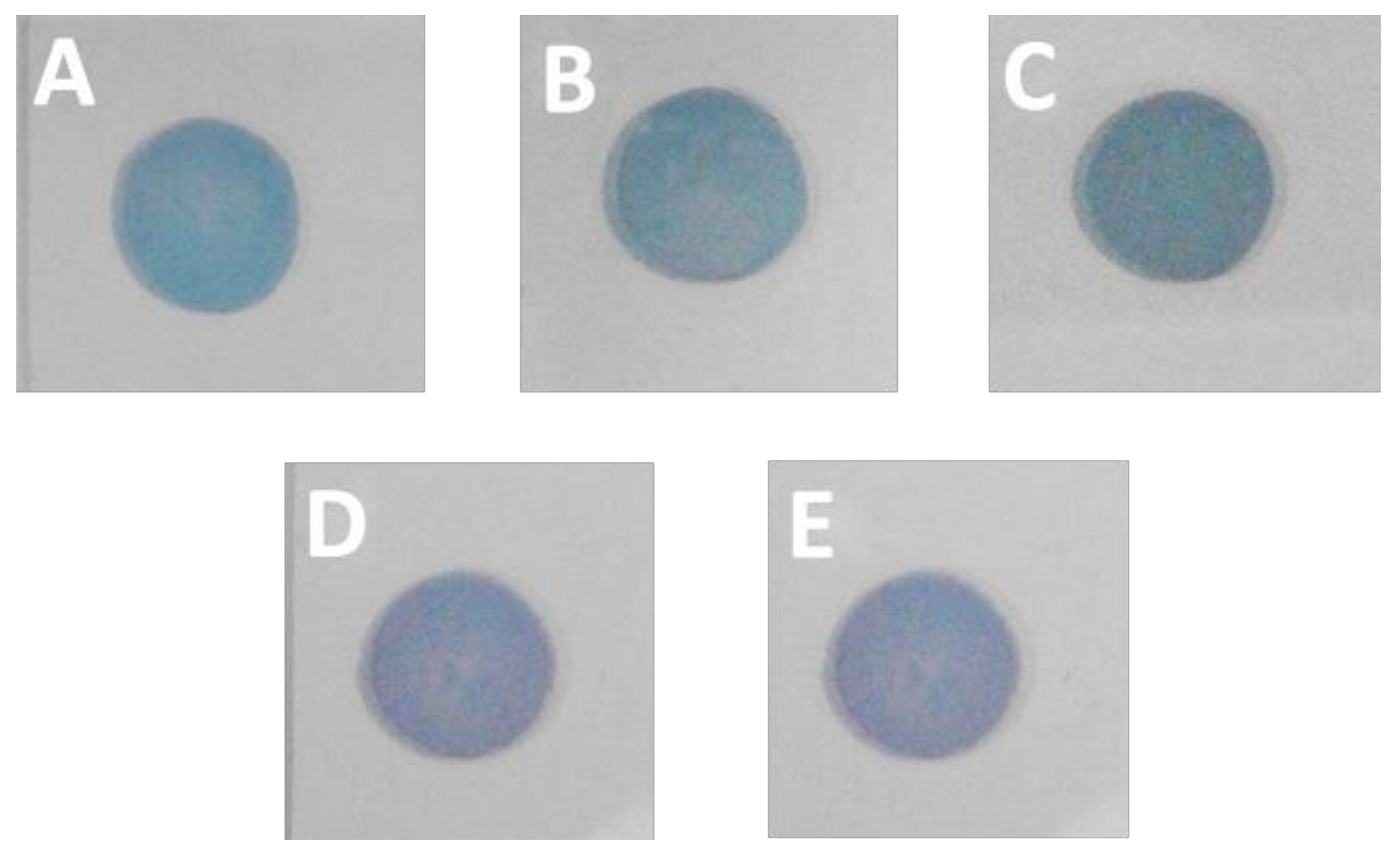
3.2. Cross-Linking of Au NP Membranes
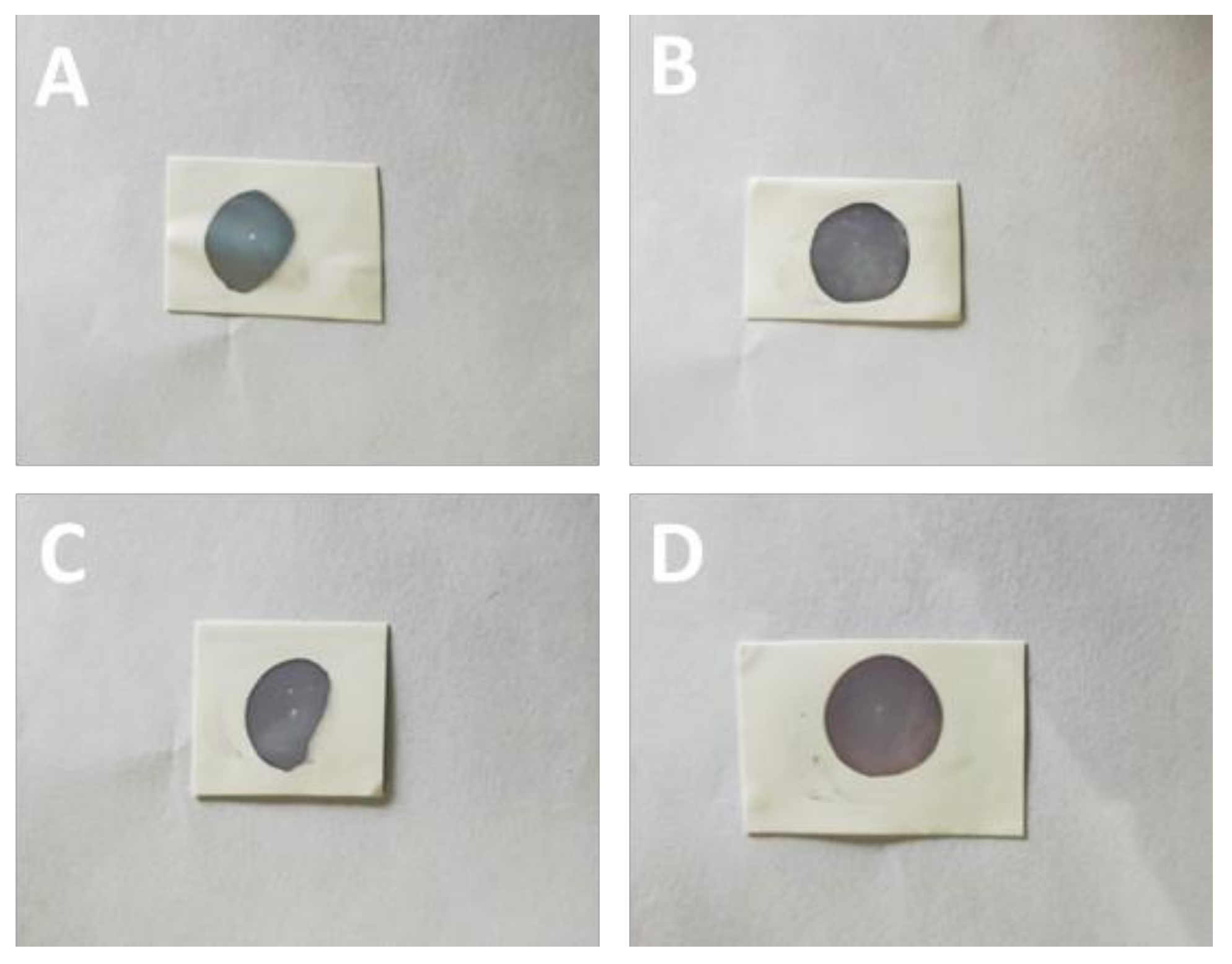
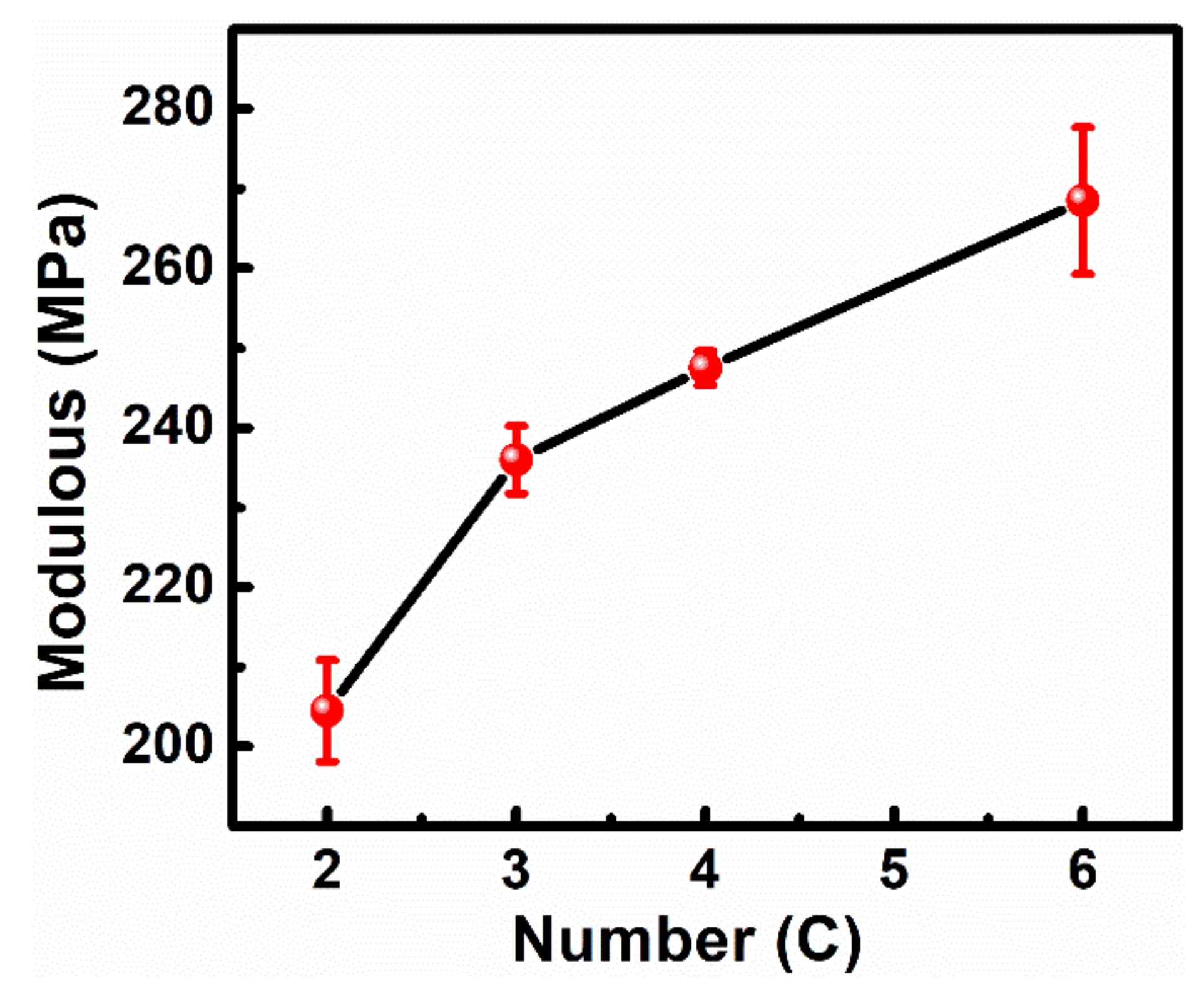
3.3. Mechanical Properties of the Cross-Linking Monolayer Film
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schlicke, H.; Battista, D.; Kunze, S.; Schröter, C.J.; Eich, M.; Vossmeyer, T. Freestanding Membranes of Cross-Linked Gold Nanoparticles: Novel Functional Materials for Electrostatic Actuators. ACS Appl. Mater. Interfaces 2015, 7, 15123–15128. [Google Scholar] [CrossRef]
- Schlicke, H.; Leib, E.W.; Petrov, A.; Schröder, J.H.; Vossmeyer, T. Elastic and viscoelastic properties of cross-linked gold nanoparticles probed by AFM bulge tests. J. Phys. Chem. C 2014, 118, 4386–4395. [Google Scholar] [CrossRef]
- Shi, Q.; Gómez, D.E.; Dong, D.; Sikdar, D.; Fu, R.; Liu, Y.; Zhao, Y.; Smilgies, D.; Cheng, W. 2D Freestanding Janus Gold Nanocrystal Superlattices. Adv. Mater. 2019, 31, 1900989. [Google Scholar] [CrossRef]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar] [CrossRef]
- Wang, C.; Hwang, D.; Yu, Z.; Takei, K.; Park, J.; Chen, T.; Ma, B.; Javey, A. User-interactive electronic skin for instantaneous pressure visualization. Nat. Mater. 2013, 12, 899–904. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Xu, C.; Sun, Y.; Li, S.; Jiang, M.; Qin, G. Control of Catalytic Activity of Nano-Au through Tailoring the Fermi Level of Support. Small 2019, 15, 1901789. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, F.; Lin, W.; Zhao, F.; Zhou, J.; Li, S.; Qin, G. In situ synthesis of Ni/NiO composites with defect-rich ultrathin nanosheets for highly efficient biomass-derivative selective hydrogenation. J. Mater. Chem. A 2019, 7, 17834–17841. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, J.; Wang, H.; Li, S.; Wang, W.; Qin, G. Abnormal thermal stability of sub-10 nm Au nanoparticles and their high catalytic activity. J. Mater. Chem. A 2019, 7, 10980–10987. [Google Scholar] [CrossRef]
- Li, S.; Cai, J.; Liu, Y.; Gao, M.; Cao, F.; Qin, G. Tuning orientation of doped hematite photoanodes for enhanced photoelectrochemical water oxidation. Sol. Energy Mater. Sol. Cells 2018, 179, 328–333. [Google Scholar] [CrossRef]
- Pradhan, S.; Ghosh, D.; Xu, L.P.; Chen, S. Interparticle charge transfer mediated by π-π stacking of aromatic moieties. J. Am. Chem. Soc. 2007, 129, 10622–10623. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.; Wang, T.; Li, Y.; Zhang, J.; Lu, Z.; Fu, Y.; Li, F. Adjusting the inter-particle spacing of a nanoparticle array at the sub-nanometre scale by thermal annealing. Chem. Commun. 2014, 50, 14547–14549. [Google Scholar] [CrossRef]
- Edel, J.B.; Kornyshev, A.A.; Kucernak, A.R.; Urbakh, M. Fundamentals and applications of self-assembled plasmonic nanoparticles at interfaces. Chem. Soc. Rev. 2016, 45, 1581–1596. [Google Scholar] [CrossRef]
- Markutsya, S.; Jiang, C.; Pikus, Y.; Tsukruk, V.V. Freely suspended layer-by-layer nanomembranes: Testing micromechanical properties. Adv. Funct. Mater. 2005, 15, 771–780. [Google Scholar] [CrossRef]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The role of interparticle and external forces. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef]
- Cheng, W.; Campolongo, M.J.; Tan, S.J.; Luo, D. Freestanding ultrathin nano-membranes via self-assembly. Nano Today 2009, 4, 482–493. [Google Scholar] [CrossRef]
- Xia, H.; Wang, D. Fabrication of macroscopic freestanding films of metallic nanoparticle monolayers by interfacial self-assembly. Adv. Mater. 2008, 20, 4253–4256. [Google Scholar] [CrossRef]
- Lin, Y.; Skaff, H.; Böker, A.; Dinsmore, A.D.; Emrick, T.; Russell, T.P. Ultrathin Cross-Linked Nanoparticle Membranes. J. Am. Chem. Soc. 2003, 125, 12690–12691. [Google Scholar] [CrossRef]
- Le Ouay, B.; Guldin, S.; Luo, Z.; Allegri, S.; Stellacci, F. Freestanding Ultrathin Nanoparticle Membranes Assembled at Transient Liquid-Liquid Interfaces. Adv. Mater. Interfaces 2016, 3, 1600191. [Google Scholar] [CrossRef]
- Schlicke, H.; Rebber, M.; Kunze, S.; Vossmeyer, T. Resistive pressure sensors based on freestanding membranes of gold nanoparticles. Nanoscale 2016, 8, 183–186. [Google Scholar] [CrossRef]
- Schlicke, H.; Schröter, C.J.; Vossmeyer, T. Electrostatically driven drumhead resonators based on freestanding membranes of cross-linked gold nanoparticles. Nanoscale 2016, 8, 15880–15887. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Apodaca, M.M.; Nakanishi, H.; Smoukov, S.K.; Grzybowski, B.A. Lift-Off and Micropatterning of Mono- and Multilayer Nanoparticle Films. Small 2009, 5, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Law, M.; Song, Q.; Perkins, C.L.; Beard, M.C.; Nozik, A.J. Structural, Optical, and Electrical Properties of Self-Assembled Films of PbSe Nanocrystals Treated with 1,2-Ethanedithiol. ACS Nano 2008, 2, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Tangirala, R.; Baker, J.L.; Alivisatos, A.P.; Milliron, D.J. Modular Inorganic Nanocomposites by Conversion of Nanocrystal Superlattices. Angew. Chemie Int. Ed. 2010, 49, 2878–2882. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ni, J.; Song, Y.; Chen, B.; Li, Y.; Zhang, Y.; Li, F.; Jiao, Y.; Fu, Y. Transfer of ordered nanoparticle array and its application in high-modulus membrane fabrication. J. Mater. Chem. C 2014, 2, 6410–6414. [Google Scholar] [CrossRef]
- Zhou, J.; Song, G.; Li, Y.; Song, Y.; Chen, B.; Zhang, X.; Wang, T.; Fu, Y.; Li, F. Thermal Annealing: A Facile Way of Conferring Responsivity to Inert Alkyl-Chain-Passivated Nanoparticle Arrays. Langmuir 2014, 30, 13052–13057. [Google Scholar] [CrossRef]
- Martin, M.N.; Basham, J.I.; Chando, P.; Eah, S.-K. Charged Gold Nanoparticles in Non-Polar Solvents: 10-min Synthesis and 2D Self-Assembly. Langmuir 2010, 26, 7410–7417. [Google Scholar] [CrossRef]


| Types of Ligands | Concentrations (mmol·L−1) | Volume (μ L) |
|---|---|---|
| 1,2-ethanedithiol (C2H6S2) | 20 | 17 |
| 1,3-dimercaptopropane (C3H8S2) | 20 | 20 |
| 1,4-Butanedithiol (C4H10S2) | 20 | 23 |
| 1,5-pentanedithiol (C5H12S2) | 20 | 27 |
| 1,6-hexanedithiol (C6H14S2) | 20 | 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Cao, X.; Li, L.; Cui, X.; Fu, Y. A Novel Strategy for Fabricating a Strong Nanoparticle Monolayer and Its Enhanced Mechanism. Nanomaterials 2019, 9, 1468. https://doi.org/10.3390/nano9101468
Zhou J, Cao X, Li L, Cui X, Fu Y. A Novel Strategy for Fabricating a Strong Nanoparticle Monolayer and Its Enhanced Mechanism. Nanomaterials. 2019; 9(10):1468. https://doi.org/10.3390/nano9101468
Chicago/Turabian StyleZhou, Jun, Xiaoqing Cao, Linlin Li, Xingcheng Cui, and Yu Fu. 2019. "A Novel Strategy for Fabricating a Strong Nanoparticle Monolayer and Its Enhanced Mechanism" Nanomaterials 9, no. 10: 1468. https://doi.org/10.3390/nano9101468
APA StyleZhou, J., Cao, X., Li, L., Cui, X., & Fu, Y. (2019). A Novel Strategy for Fabricating a Strong Nanoparticle Monolayer and Its Enhanced Mechanism. Nanomaterials, 9(10), 1468. https://doi.org/10.3390/nano9101468







