Facile Synthesis of FeS@C Particles Toward High-Performance Anodes for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Material Characterization
2.3. Electrode Preparation and Electrochemical Measurement
3. Results and Discussion
3.1. Morphology and Structure
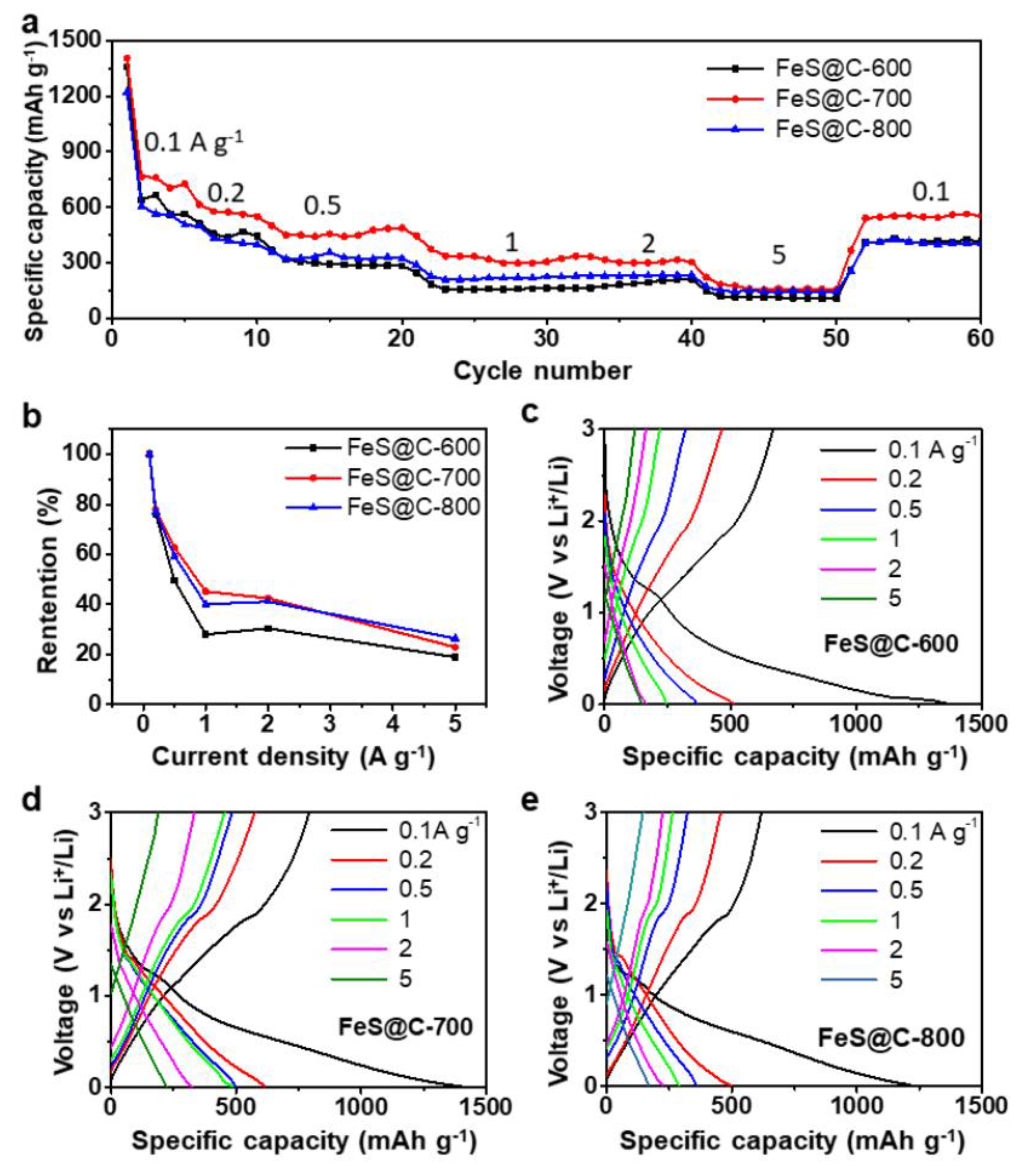
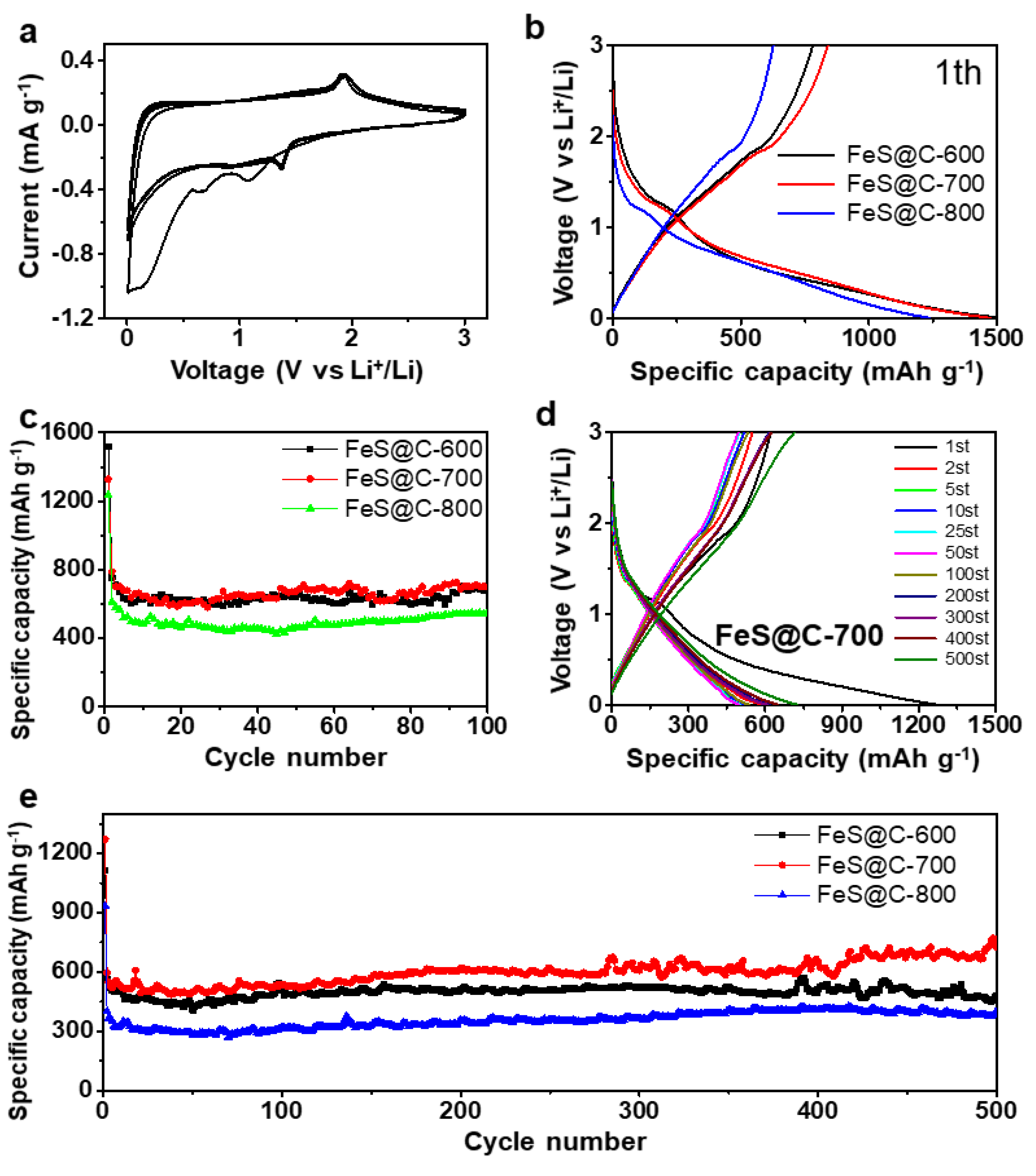
3.2. Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, K.; Lee, H.-W.; Lu, Z.; Liu, N.; Cui, Y. Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nat. Energy 2016, 1, 15029. [Google Scholar] [CrossRef]
- Rahman, M.A.; Song, G.; Bhatt, A.I.; Wong, Y.C.; Wen, C. Nanostructured Silicon Anodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Ma, J.; Kim, N.; Lee, H.-W.; Cui, Y.; Cho, J. Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries. Nat. Energy 2016, 1, 16113. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Noonan, O.; Zhou, L.; Yu, C. Lithium-Ion Batteries: Tailored Yolk-Shell Sn@C Nanoboxes for High-Performance Lithium Storage. Adv. Funct. Mater. 2017, 27, 1606023. [Google Scholar] [CrossRef]
- Cook, J.B.; Lin, T.C.; Detsi, E.; Weker, J.N.; Tolbert, S.H. Using X-ray Microscopy to Understand How Nanoporous Materials Can Be Used to Reduce the Large Volume Change in Alloy Anodes. Nano Lett. 2017, 17, 870–877. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A Review on Design Strategies for Carbon Based Metal Oxides and Sulfides Nanocomposites for High Performance Li and Na Ion Battery Anodes. Adv. Energy Mater. 2016, 7, 1601424. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wang, Z.; Zou, M.; Zhang, H.; Zhao, W.; Yousaf, M.; Yang, L.; Cao, A.; Han, R.P.S. Densification by Compaction as an Effective Low-Cost Method to Attain a High Areal Lithium Storage Capacity in a CNT@Co3O4 Sponge. Adv. Energy Mater. 2018, 8, 1702981. [Google Scholar] [CrossRef]
- Kwon, T.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, H.; Chen, Z.; McDowell, M.T.; Cui, Y.; Bao, Z. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 2013, 5, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, W.; Kim, Y.; Cho, J. Nanostructured transition metal sulfides for lithium ion batteries: Progress and challenges. Nano Today 2014, 9, 604–630. [Google Scholar] [CrossRef]
- Yuan, H.; Kong, L.; Li, T.; Zhang, Q. A review of transition metal chalcogenide/graphene nanocomposites for energy storage and conversion. Chin. Chem. Lett. 2017, 28, 2180–2194. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Li, K.; Tahir, M.; Wang, Y.-T.; Pan, L.; Wang, L.; Zhang, X.; Zou, J.-J. Hollow Cobalt-Based Bimetallic Sulfide Polyhedra for Efficient All-pH-Value Electrochemical and Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2016, 138, 1359–1365. [Google Scholar] [CrossRef]
- Chang, K.; Hai, X.; Ye, J. Transition Metal Disulfides as Noble-Metal-Alternative Co-Catalysts for Solar Hydrogen Production. Adv. Energy Mater. 2016, 6, 1502555. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2015, 28, 1917–1933. [Google Scholar] [CrossRef]
- Yin, L.W.; Lee, S.T. Wurtzite-Twinning-Induced Growth of Three-Dimensional II-VI Ternary Alloyed Nanoarchitectures and their Tunable Band Gap Energy Properties. Nano Lett. 2009, 9, 957–963. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Pan, A.; Yang, H.; Liu, R.; Yu, R.; Zou, B.; Wang, Z. Color-Tunable Photoluminescence of Alloyed CdSxSe1-xNanobelts. J. Am. Chem. Soc. 2005, 127, 15692–15693. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lu, M.-Y.; Chen, L.-J. Metal sulfide nanostructures: Synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 2012, 22, 19–30. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Yu, L.; Lou, X.W. Metal Sulfide Hollow Nanostructures for Electrochemical Energy Storage. Adv. Energy Mater. 2015, 6, 1501333. [Google Scholar] [CrossRef]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef]
- Zhu, C.; Kopold, P.; Li, W.; Aken, P.A.V.; Maier, J.; Yu, Y. Metal Sulphides: A General Strategy to Fabricate Carbon-Coated 3D Porous Interconnected Metal Sulfides: Case Study of SnS/C Nanocomposite for High-Performance Lithium and Sodium Ion Batteries. Adv. Sci. 2015, 2, 1500200. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.; Zhou, J.; Chen, X. Iron sulfide-embedded carbon microsphere anode material with high-rate performance for lithium-ion batteries. Chem. Commun. 2011, 47, 8653–8655. [Google Scholar] [CrossRef]
- Wu, C.; Maier, J.; Yu, Y. Generalizable Synthesis of Metal-Sulfides/Carbon Hybrids with Multiscale, Hierarchically Ordered Structures as Advanced Electrodes for Lithium Storage. Adv. Mater. 2016, 28, 174–180. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, Y.; Rui, X.; Xiao, N.; Zhu, J.; Zhang, W.; Chen, J.; Liu, W.; Tan, H.; Hng, H.H.; et al. Controlled Soft-Template Synthesis of Ultrathin C@FeS Nanosheets with High-Li-Storage Performance. ACS Nano 2012, 6, 4713–4721. [Google Scholar] [CrossRef]
- Qiu, W.; Xia, J.; Zhong, H.; He, S.; Lai, S.; Chen, L. L-Cysteine-Assisted Synthesis of Cubic Pyrite/Nitrogen-Doped Graphene Composite as Anode Material for Lithium-ion Batteries. Electrochim. Acta 2014, 137, 197–205. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, X.; Suo, L.; Luo, C.; Gao, T.; Wang, C. Electrospun FeS2@Carbon Fiber Electrode as a High Energy Density Cathode for Rechargeable Lithium Batteries. ACS Nano 2016, 10, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, W.; Zhang, F.; Zhang, X.; Zhang, W.; Lee, C.-S.; Tang, Y. In situ incorporation of FeS nanoparticles/carbon nanosheets composite with an interconnected porous structure as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2016, 4, 3697–3703. [Google Scholar] [CrossRef]
- Lou, P.; Tan, Y.; Lu, P.; Cui, Z.; Guo, X. Novel one-step gas-phase reaction synthesis of transition metal sulfide nanoparticles embedded in carbon matrices for reversible lithium storage. J. Mater. Chem. A 2016, 4, 16849–16855. [Google Scholar] [CrossRef]
- Pasini, D.; Takeuchi, D. Cyclopolymerizations: Synthetic Tools for the Precision Synthesis of Macromolecular Architectures. Chem. Rev. 2018, 118, 8983–9057. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, L.; Marangoni, T.; Miletić, T.; Rubio-Magnieto, J.; Mohanraj, J.; Amenitsch, H.; Pasini, D.; Liaros, N.; Couris, S.; Armaroli, N.; et al. Solvent Molding of Organic Morphologies Made of Supramolecular Chiral Polymers. J. Am. Chem. Soc. 2015, 137, 8150–8160. [Google Scholar] [CrossRef]
- Sharma, A.K.; Caricato, M.; Quartarone, E.; Edizer, S.; Schieroni, A.G.; Mendichi, R.; Pasini, D. Polystyrene-based self-aggregating polymers based on UPy units. Polym. Bull. 2019, 69, 911–923. [Google Scholar] [CrossRef]
- Bu, F.; Xiao, P.; Chen, J.; Aly Aboud, M.F.; Shakir, I.; Xu, Y. Rational design of three-dimensional graphene encapsulated core–shell FeS@carbon nanocomposite as a flexible high-performance anode for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 6414–6421. [Google Scholar] [CrossRef]
- Cummins, D.R.; Russell, H.R.; Jasinski, J.B.; Menon, M.; Sunkara, M.K. Iron Sulfide (FeS) Nanotubes Using Sulfurization of Hematite Nanowires. Nano Lett. 2013, 13, 2423–2430. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. Adv. Mater. 2017, 29, 1604942. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Geng, H.; Zhu, L.; Li, W.; Liu, H.; Quan, L.; Xi, F.; Su, X. FeS/nickel foam as stable and efficient counter electrode material for quantum dot sensitized solar cells. J. Power Sources 2015, 281, 204–210. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, Y.; Zhao, Y.; Chen, N.; Zhang, Z.; Cao, M.; Qu, L. Highly nitrogen-doped carbon capsules: Scalable preparation and high-performance applications in fuel cells and lithium ion batteries. Nanoscale 2013, 5, 2726–2733. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.-Y.; Belharouak, I.; Sun, Y.-K. Na Storage Capability Investigation of a Carbon Nanotube-Encapsulated Fe1–xS Composite. ACS Energy Lett. 2017, 2, 364–372. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Dai, Q.; Wang, M.; Qu, L.; Dai, L. Earth-abundant carbon catalysts for renewable generation of clean energy from sunlight and water. Nano Energy 2017, 41, 367–376. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Park, K.; Zhou, L.; Huang, H.; Mai, Y.W.; Goodenough, J.B. Hollow Nanotubes of N-Doped Carbon on CoS. Angew. Chem. Int. Ed. 2016, 55, 15831–15834. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wen, Y.; Aken, P.A.V.; Maier, J.; Yu, Y. High Lithium Storage Performance of FeS Nanodots in Porous Graphitic Carbon Nanowires. Adv. Funct. Mater. 2015, 25, 2335–2342. [Google Scholar] [CrossRef]
- See, K.A.; Lumley, M.A.; Stucky, G.D.; Grey, C.P.; Seshadrib, R. Reversible Capacity of Conductive Carbon Additives at Low Potentials: Caveats for Testing Alternative Anode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A327–A333. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Yang, Z.; Guo, A.; Liu, D. Facile Synthesis of FeS@C Particles Toward High-Performance Anodes for Lithium-Ion Batteries. Nanomaterials 2019, 9, 1467. https://doi.org/10.3390/nano9101467
Lin X, Yang Z, Guo A, Liu D. Facile Synthesis of FeS@C Particles Toward High-Performance Anodes for Lithium-Ion Batteries. Nanomaterials. 2019; 9(10):1467. https://doi.org/10.3390/nano9101467
Chicago/Turabian StyleLin, Xuanni, Zhuoyi Yang, Anru Guo, and Dong Liu. 2019. "Facile Synthesis of FeS@C Particles Toward High-Performance Anodes for Lithium-Ion Batteries" Nanomaterials 9, no. 10: 1467. https://doi.org/10.3390/nano9101467
APA StyleLin, X., Yang, Z., Guo, A., & Liu, D. (2019). Facile Synthesis of FeS@C Particles Toward High-Performance Anodes for Lithium-Ion Batteries. Nanomaterials, 9(10), 1467. https://doi.org/10.3390/nano9101467



