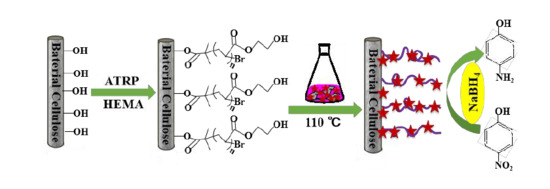
Reusable Surface-Modified Bacterial Cellulose Based on Atom Transfer Radical Polymerization Technology with Excellent Catalytic Properties
Abstract
:1. Introduction
2. Experiment
2.1. Materials
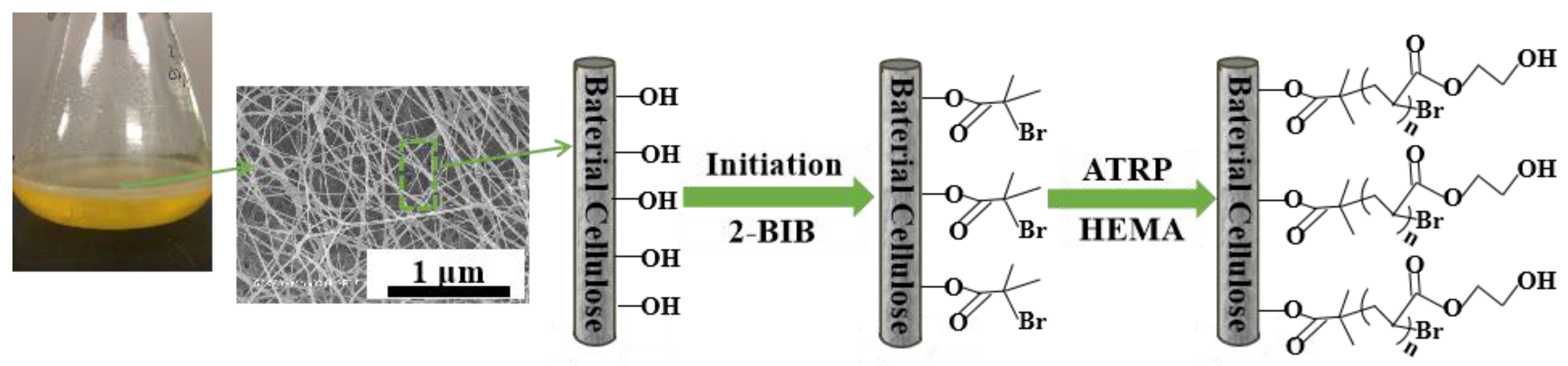
2.2. Preparation of Bacterial Cellulose 2-Hydroxyethyl Methacrylate (BC-Poly(HEMA))
2.2.1. Initiation
2.2.2. Surface Grafting
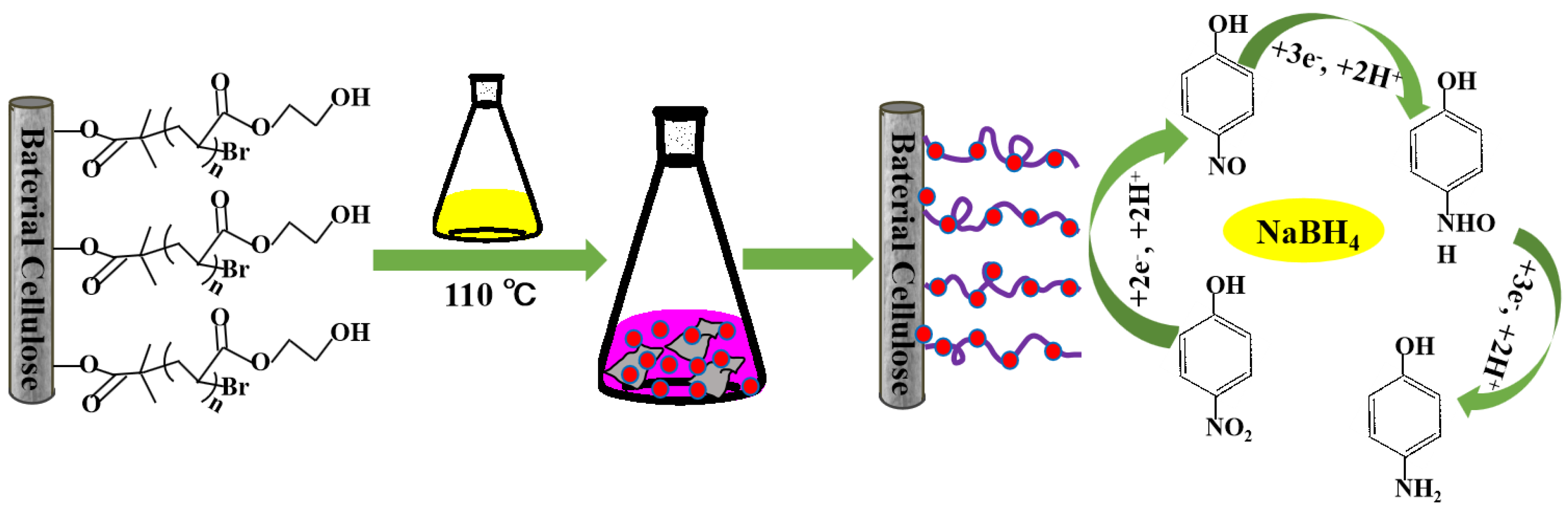
2.3. Synthesis of Gold Nanoparticles (AuNPs)/BC-Poly(HEMA)
2.4. Catalytic Reduction of 4-Nitrophenol (4-NP)
2.5. Materials Characterization
3. Results and Discussion
3.1. Morphology Analysis
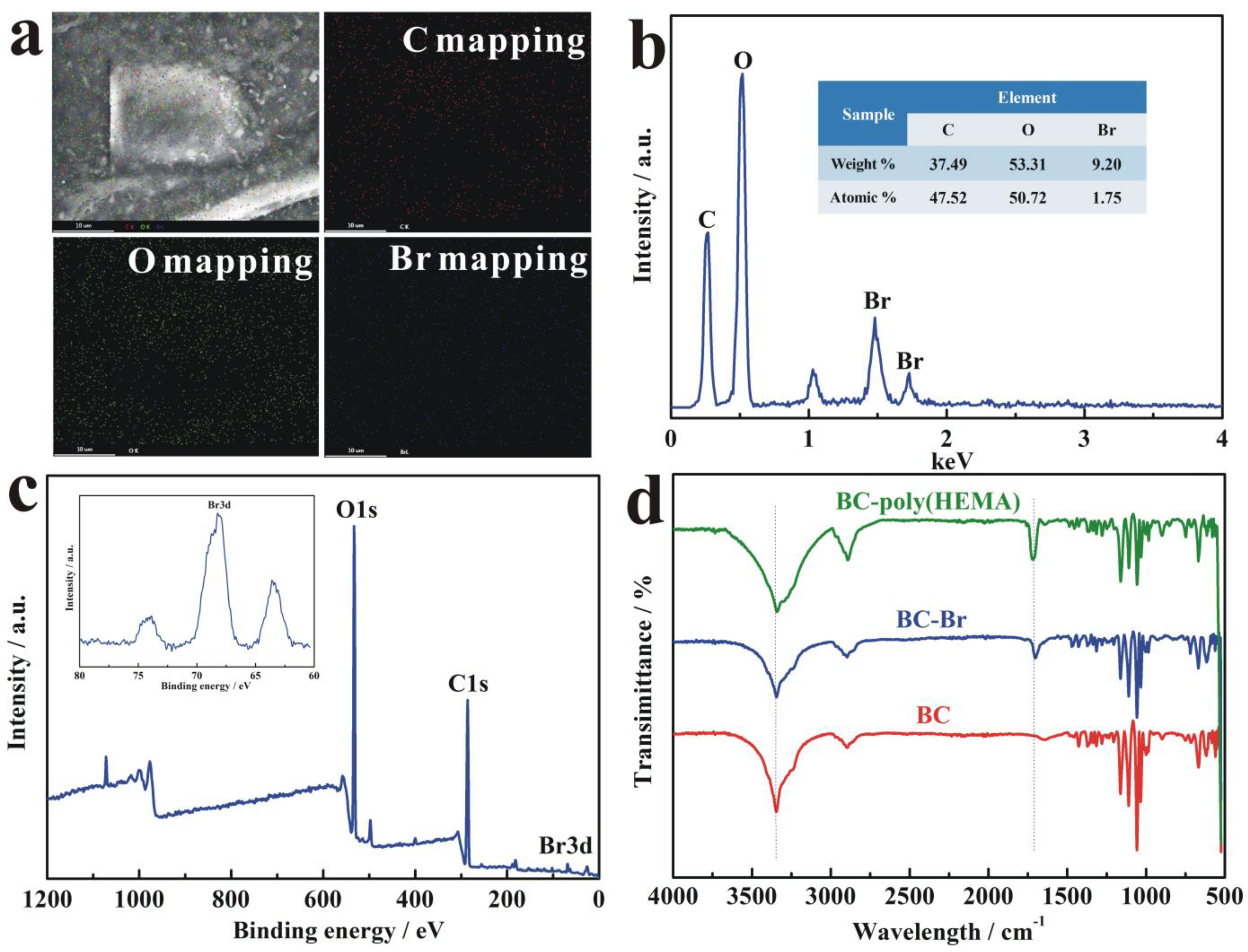
3.2. Characterization of BC-Poly(HEMA)
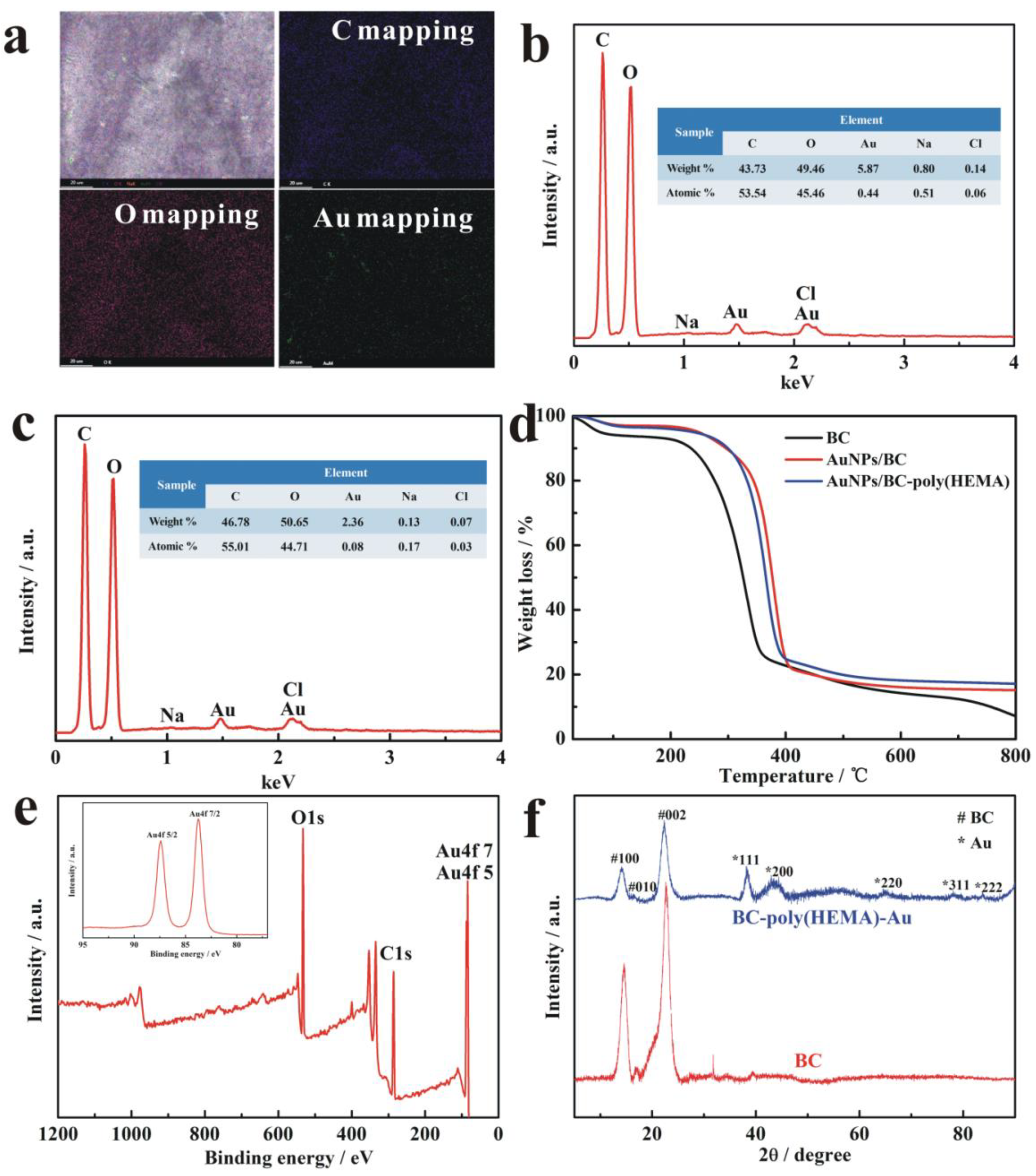
3.3. Characterization of AuNPs/BC-Poly(HEMA)
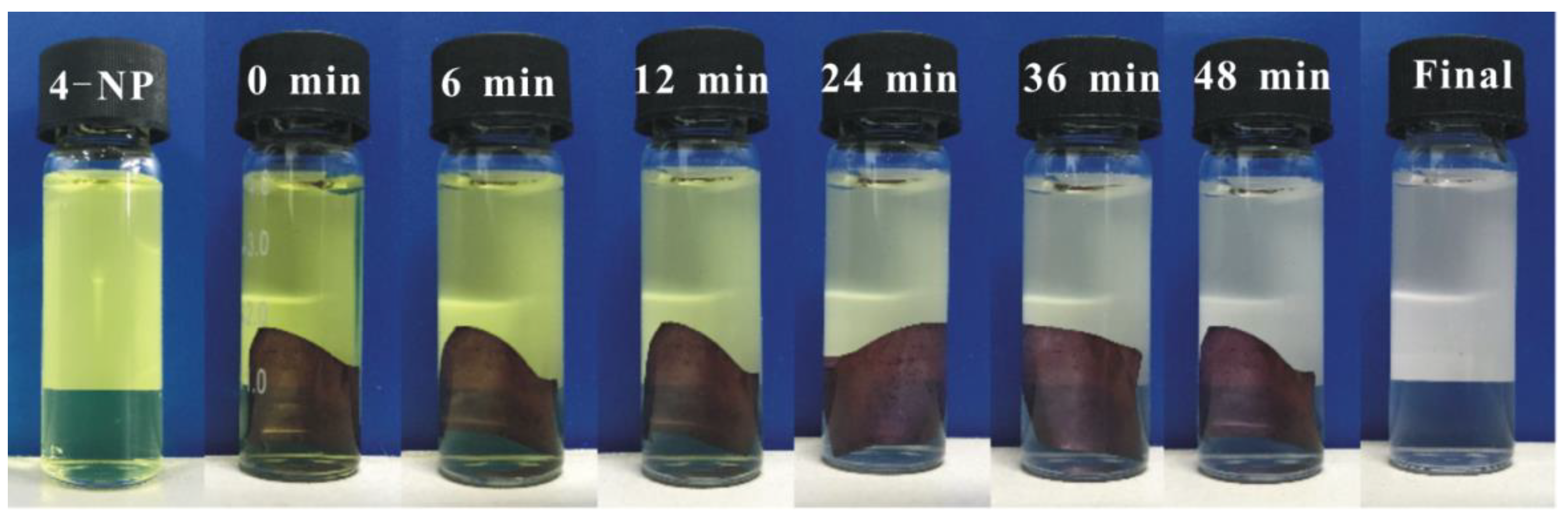
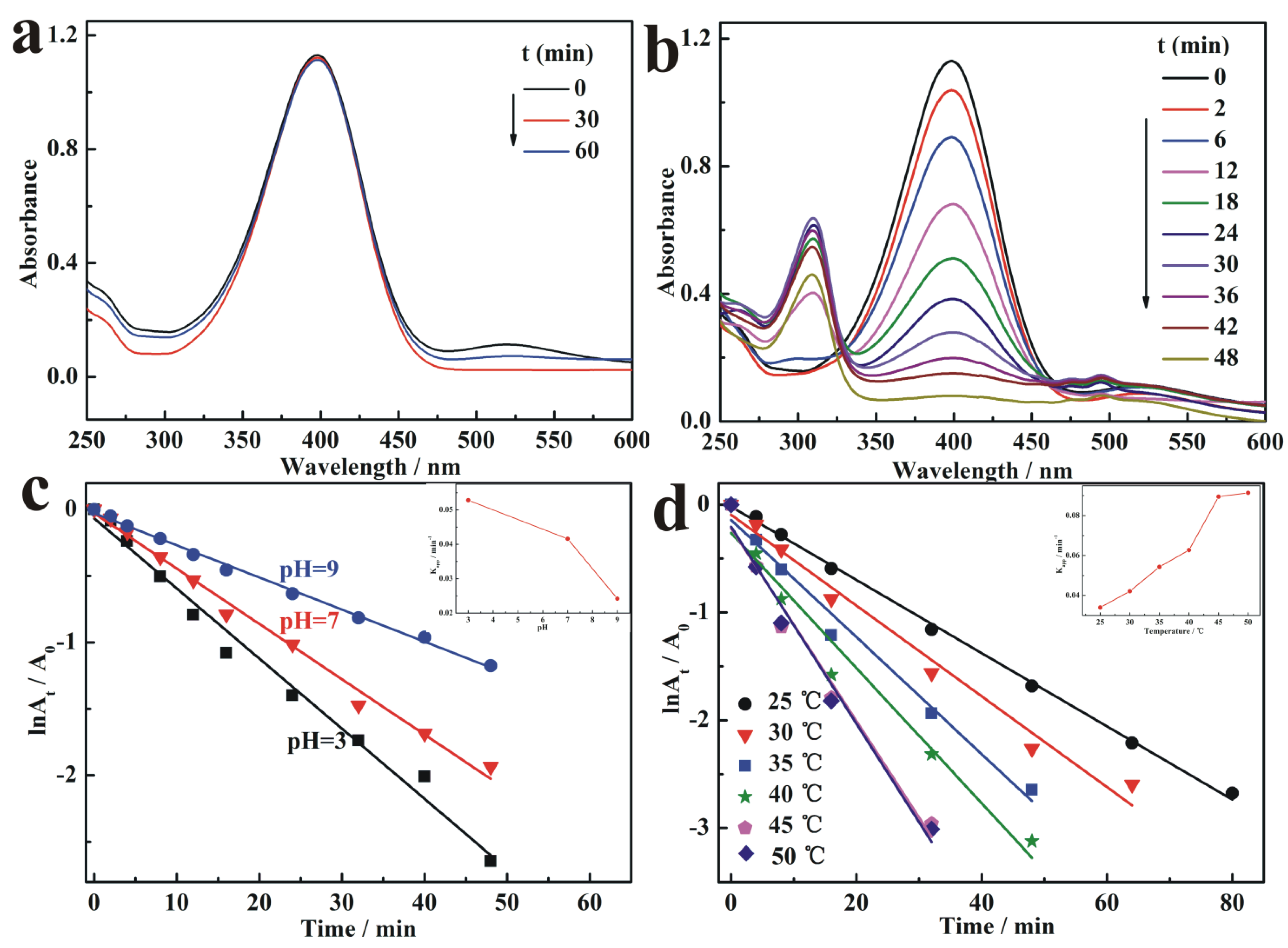
3.4. Catalytic Activity of AuNPs/BC-Poly(HEMA)
3.5. Reusable Stability of AuNPs/BC-Poly(HEMA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chia, X.; Sofer, Z.; Luxa, J.; Pumera, M. Layered Noble Metal Dichalcogenides: Tailoring Electrochemical and Catalytic Properties. ACS Appl. Mater. Inter. 2017, 9, 25587–25599. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Dias, M.R.S.; Wessler, G.C.; Taillon, J.A.; Salamanca-Riba, L.G.; Leite, M.S. Near-Field Optical Properties of Fully Alloyed Noble Metal Nanoparticles. Adv. Opt. Mater. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmuller, N. Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Gupta, I.; Brandelli, A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Inter. J. Pharmaceut. 2015, 496, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, P.; Velayudham, M.; Lu, K.L.; Rajagopal, S. Polyelectrolyte encapsulated gold nanoparticles as efficient active catalyst for reduction of nitro compounds by kinetic method. Appl. Catal. A Gen. 2012, 439, 197–205. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, X.; Feng, C.; Li, B.; Chen, T.; Lu, W.; Lei, X.; Xu, S. Crystal-Face-Selective Supporting of Gold Nanoparticles on Layered Double Hydroxide as Efficient Catalyst for Epoxidation of Styrene. ACS Catal. 2011, 1, 232–237. [Google Scholar] [CrossRef]
- Chen, M.; Kang, H.; Gong, Y.; Guo, J.; Zhang, H.; Liu, R. Bacterial Cellulose Supported Gold Nanoparticles with Excellent Catalytic Properties. ACS Appl. Mater. Inter. 2015, 7, 21717–21726. [Google Scholar] [CrossRef]
- Matos, I.D.O.; Alves W., A. Electrochemical Determination of Dopamine Based on Self-Assembled Peptide Nanostructure. ACS Appl. Mater. Inter. 2011, 3, 4437–4443. [Google Scholar] [CrossRef]
- Zhang, Z.; Sèbe, G.; Wang, X.; Tam, K.C. Gold nanoparticles stabilized by poly(4-vinylpyridine) grafted cellulose nanocrystals as efficient and recyclable catalysts. Carbohyd. Polym. 2017, 182, 61–68. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coordin. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Jin, L.; He, G.; Xue, J.; Xu, T.; Chen, H. Cu/graphene with high catalytic activity prepared by glucose blowing for reduction of p -nitrophenol. J. Clean. Prod. 2017, 161, 655–662. [Google Scholar] [CrossRef]
- Zawada, K.; Tomaszewski, W.; Megiel, E. A smart synthesis of gold/polystyrene core-shell nanohybrids using TEMPO coated nanoparticles. RSC Adv. 2014, 4, 23876–23885. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Cui, Y.; Gao, D.; Chen, S. Highly Stable and Biocompatible Dendrimer-Encapsulated Gold Nanoparticles Catalysts for the Reduction of 4-Nitrophenol. New J. Chem. 2017, 41, 8399–8406. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]
- Zhang, M.; Bi, C.; Lin, H.; Cao, J.; Chen, S. Construction of novel Au/Bi12O17C12 composite with intensive visible light activity enhancement for contaminants removal. Mater. Lett. 2017, 191, 132–135. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R.A. Enhanced catalytic reduction of nitrophenols by sodium borohydride over highly recyclable Au@graphitic carbon nitride nanocomposites. Appl. Catal. B Environ. 2019, 240, 337–347. [Google Scholar] [CrossRef]
- Dayal, M.S.; Catchmark, J.M. Mechanical and structural property analysis of bacterial cellulose composites. Carbohyd. Polym. 2016, 144, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohyd. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhang, D.; Zhang, X.; Ma, Y.; Zhou, Y.; Qi, L. Biotemplated Synthesis of Gold Nanoparticle-Bacteria Cellulose Nanofiber Nanocomposites and Their Application in Biosensing. Adv. Funct. Mater. 2010, 20, 1152–1160. [Google Scholar] [CrossRef]
- Li, W.; Liu, R.; Kang, H.; Sun, Y.; Dong, F.; Huang, Y. Synthesis of amidoxime functionalized cellulose derivatives as a reducing agent and stabilizer for preparing gold nanoparticles. Polym. Chem. 2013, 4, 2556–2563. [Google Scholar] [CrossRef]
- Li, X.; Lv, P.; Yao, Y.; Feng, Q.; Mensah, A.; Li, D.; Wei, Q. A novel single-enzymatic biofuel cell based on highly flexible conductive bacterial cellulose electrode utilizing pollutants as fuel. Chem. Eng. J. 2020, 379, 122316. [Google Scholar] [CrossRef]
- Hirotaka, K.; Eriko, T.; Mami, H.; Yuuka, U.; Tsuguyuki, S.; Akira, I.; Takuya, K. Topochemical synthesis and catalysis of metal nanoparticles exposed on crystalline cellulose nanofibers. Chem. Commun. 2010, 46, 8567–8569. [Google Scholar]
- Mahmoud, K.A.; Male, K.B.; Hrapovic, S.; Luong, J.H. Cellulose nanocrystal/gold nanoparticle composite as a matrix for enzyme immobilization. ACS Appl. Mater. Inter. 2009, 1, 1383. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Fei, M.; Qi, L. Facile Synthesis and One-Dimensional Assembly of Cyclodextrin-Capped Gold Nanoparticles and Their Applications in Catalysis and Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar]
- Dong, H.; Fei, Z.; Hu, X.; Han, Z.; Koh, K.; Chen, H. Analyte induced AuNPs aggregation enhanced surface plasmon resonance for sensitive detection of paraquat. Biosens. Bioelectron. 2018, 117, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Kumar, R. Functionalized cellulose with hydroxyethyl methacrylate and glycidyl methacrylate for metal ions and dye adsorption applications. Int. J. Biol. Macromol. 2019, 134, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Beers, K.L.; Boo, S.; Gaynor, S.G.; Matyjaszewski, K. Atom Transfer Radical Polymerization of 2-Hydroxyethyl Methacrylate. Macromolecules 1999, 32, 695–699. [Google Scholar] [CrossRef]
- Dalton, P.D.; Flynn, L.; Shoichet, M.S. Manufacture of poly (2-hydroxyethyl methacrylate-co-methyl methacrylate) hydrogel tubes for use as nerve guidance channels. Biomaterial 2002, 23, 3843–3851. [Google Scholar] [CrossRef]
- Sadakbayeva, Z.; Dušková-Smrčková, M.; Šturcová, A.; Pfleger, J.; Dušek, K. Microstructured Poly (2-Hydroxyethyl Methacrylate)/Poly (Glycerol Monomethacrylate) Interpenetrating Network Hydrogels: UV-Scattering Induced Accelerated Formation and Tensile Behavior. Eur. Polym. J. 2018, 101, 304–313. [Google Scholar] [CrossRef]
- Yamada, K.; Ishiguro, Y.; Kimura, Y.; Asamoto, H.; Minamisawa, H. Two-step grafting of 2-hydroxyethyl methacrylate (HEMA) and 2-(dimethylamino) ethyl methacrylate (DMAEMA) onto a polyethylene plate for enhancement of Cr(VI) ion adsorption. Environ. Technol. 2017, 40, 855–869. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Fedorczyk, M.; Krzywicka, A.; Cieciórski, P.; Romański, J.; Megiel, E. A Novel Strategy for the Synthesis of Amphiphilic and Thermoresponsive Poly (N-isopropylacrylamide)-b-Polystyrene Block Copolymers via ATRP. Polymers 2019, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Gerelbaatar, K.; Tsogoo, A.; Dashzeveg, R.; Tsedev, N.; Ganbold, E.O. Reduction of 2,4-Dinitrophenol to 2,4-Diaminophenol Using AuNPs and AgNPs as Catalyst. Solid State Phenom. 2018, 271, 76–84. [Google Scholar] [CrossRef]
- Megiel, E. Surface modification using TEMPO and its derivatives. Adv. Colloid. Interf. 2017, 250, 158–184. [Google Scholar] [CrossRef]
- Lv, P.; Zhou, H.; Mensah, A.; Feng, Q.; Wang, D.; Hu, X.; Cai, Y.; Lucia, L.A.; Li, D.; Wei, Q. A highly flexible self-powered biosensor for glucose detection by epitaxial deposition of gold nanoparticles on conductive bacterial cellulose. Chem. Eng. J. 2018, 351, 177–188. [Google Scholar] [CrossRef]
- Trovatti, E.; Fernandes, S.C.M.; Rubatat, L.; Freire, C.S.R.; Neto, C.P. Sustainable nanocomposite films based on bacterial cellulose and pullulan. Cellulose 2012, 19, 729–737. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, J. Bacterial cellulose/poly (ethylene glycol) composite: Characterization and first evaluation of biocompatibility. Cellulose 2010, 17, 83–91. [Google Scholar] [CrossRef]
- Kaim, A.; Szydlowska, J.; Piotrowski, P.; Megiel, E. One-pot synthesis of gold nanoparticles densely coated with nitroxide spins. Polyhedron 2012, 46, 119–123. [Google Scholar] [CrossRef]
- Gozdziewska, M.; Cichowicz, G.; Markowska, K.; Zawada, K.; Megiel, E. Nitroxide-coated silver nanoparticles: Synthesis, surface physicochemistry and antibacterial activity. RSC Adv. 2015, 5, 58403–58415. [Google Scholar] [CrossRef]
- Audichon, T.; Guenot, B.; Baranton, S.; Cretin, M.; Lamy, C.; Coutanceau, C. Preparation and characterization of supported Ru x Ir (1-x) O 2 nano-oxides using a modified polyol synthesis assisted by microwave activation for energy storage applications. Appl. Catal. B Environ. 2017, 200, 493–502. [Google Scholar] [CrossRef]
- Blanco, E.; Atienzar, P.; Hernández, P.; Quintana, C. The Langmuir-Hinshelwood approach for kinetic evaluation of cucurbit [7] uril-capped gold nanoparticles in the reduction of the antimicrobial nitrofurantoin. Phys. Chem. Chem. Phys. 2017, 19, 18913–18923. [Google Scholar] [CrossRef] [PubMed]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 Reduction: A Classification Problem. Chemphyschem 2017, 18, 3266–3273. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Feng, Q.; Li, D.; Christopher, N.; Ke, H.; Wei, Q. Reusable Surface-Modified Bacterial Cellulose Based on Atom Transfer Radical Polymerization Technology with Excellent Catalytic Properties. Nanomaterials 2019, 9, 1443. https://doi.org/10.3390/nano9101443
Li X, Feng Q, Li D, Christopher N, Ke H, Wei Q. Reusable Surface-Modified Bacterial Cellulose Based on Atom Transfer Radical Polymerization Technology with Excellent Catalytic Properties. Nanomaterials. 2019; 9(10):1443. https://doi.org/10.3390/nano9101443
Chicago/Turabian StyleLi, Xin, Quan Feng, Dawei Li, Narh Christopher, Huizhen Ke, and Qufu Wei. 2019. "Reusable Surface-Modified Bacterial Cellulose Based on Atom Transfer Radical Polymerization Technology with Excellent Catalytic Properties" Nanomaterials 9, no. 10: 1443. https://doi.org/10.3390/nano9101443
APA StyleLi, X., Feng, Q., Li, D., Christopher, N., Ke, H., & Wei, Q. (2019). Reusable Surface-Modified Bacterial Cellulose Based on Atom Transfer Radical Polymerization Technology with Excellent Catalytic Properties. Nanomaterials, 9(10), 1443. https://doi.org/10.3390/nano9101443