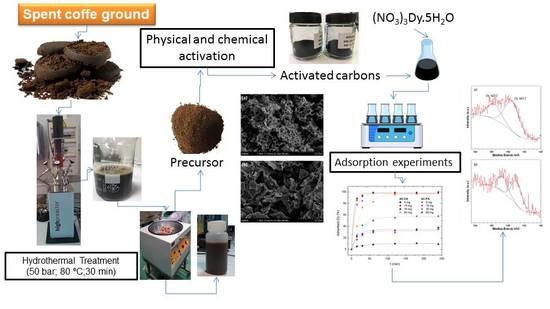
Dysprosium Removal from Water Using Active Carbons Obtained from Spent Coffee Ground
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Activated Carbons
Chemical Activation
Physical Activation
2.2. Characterization of the Activated Carbons
2.3. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of the Activated Carbons
3.1.1. Textural Properties
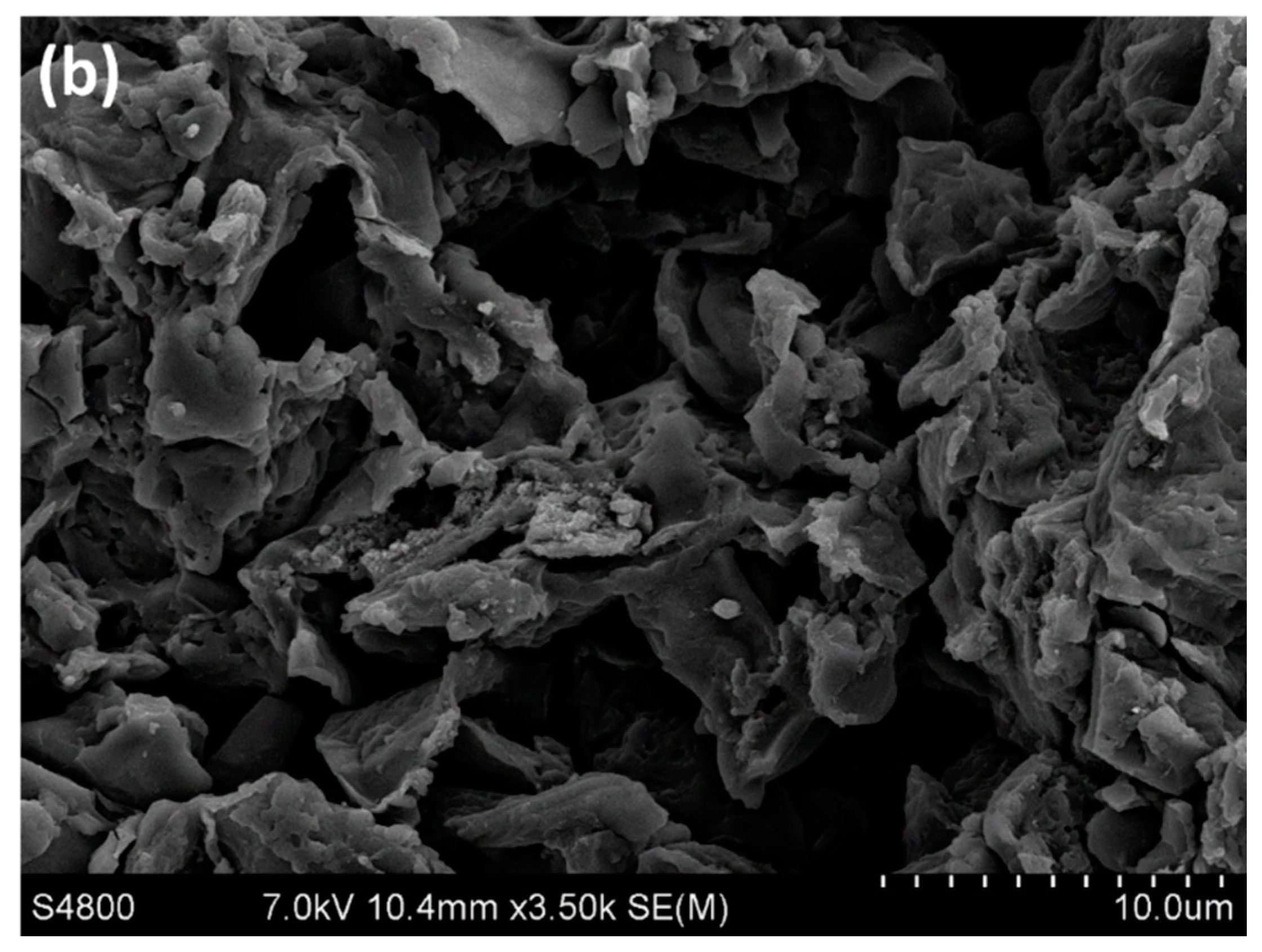
3.1.2. Scanning Electron Microscopy (SEM)
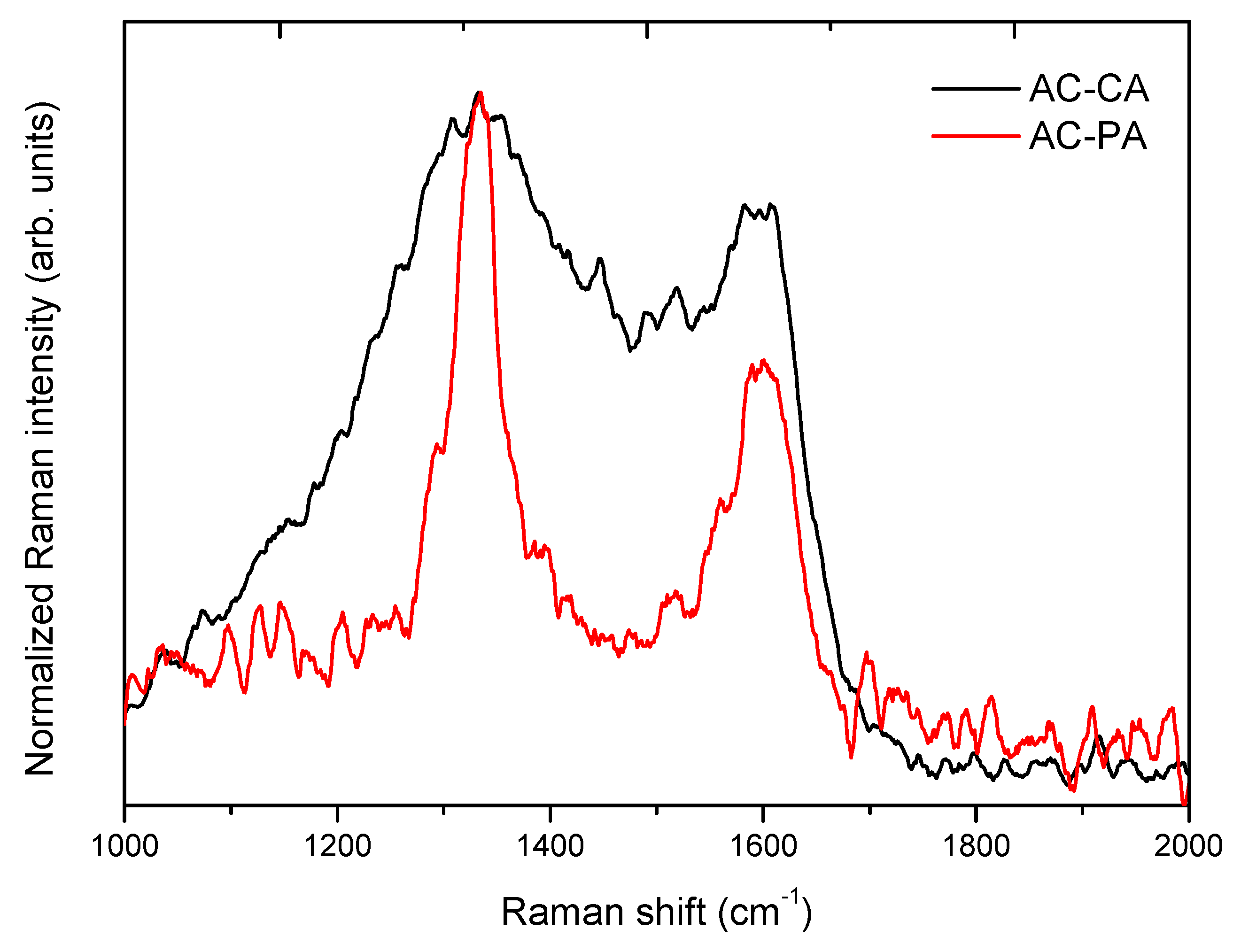
3.1.3. Raman Spectroscopy
3.2. Adsorption Experiments
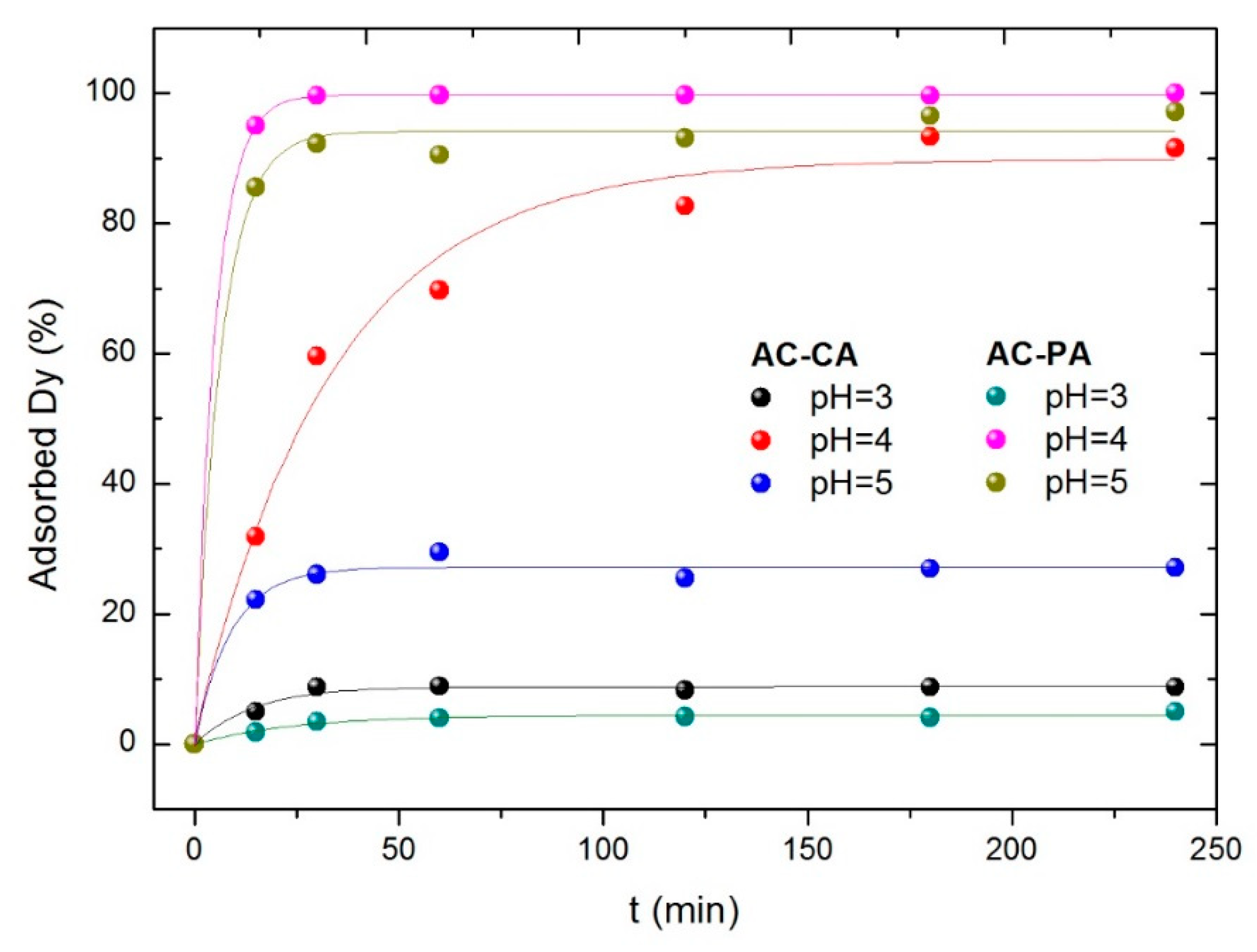
3.2.1. Influence of the Solution pH
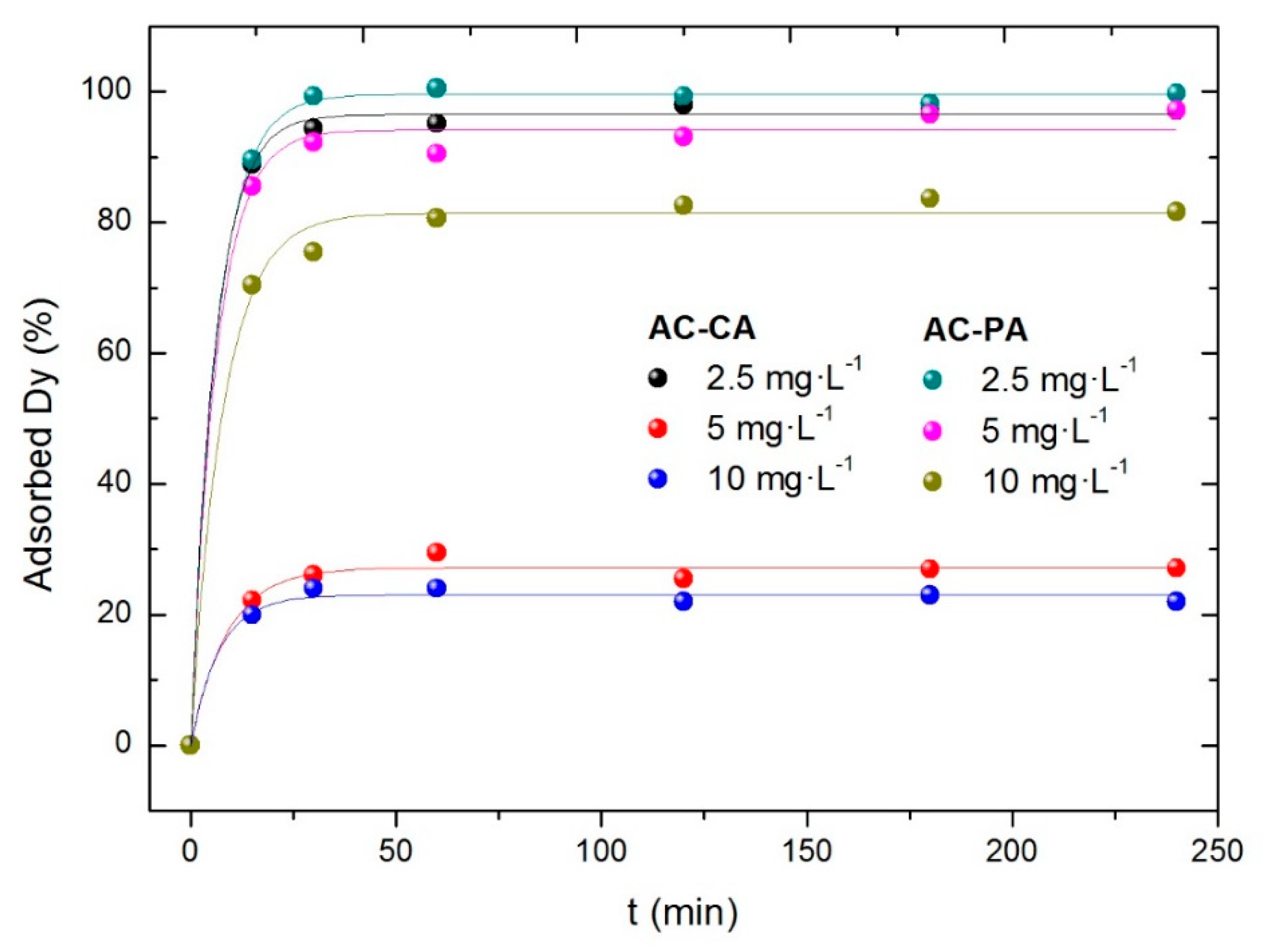
3.2.2. Influence of the Dysprosium Concentration
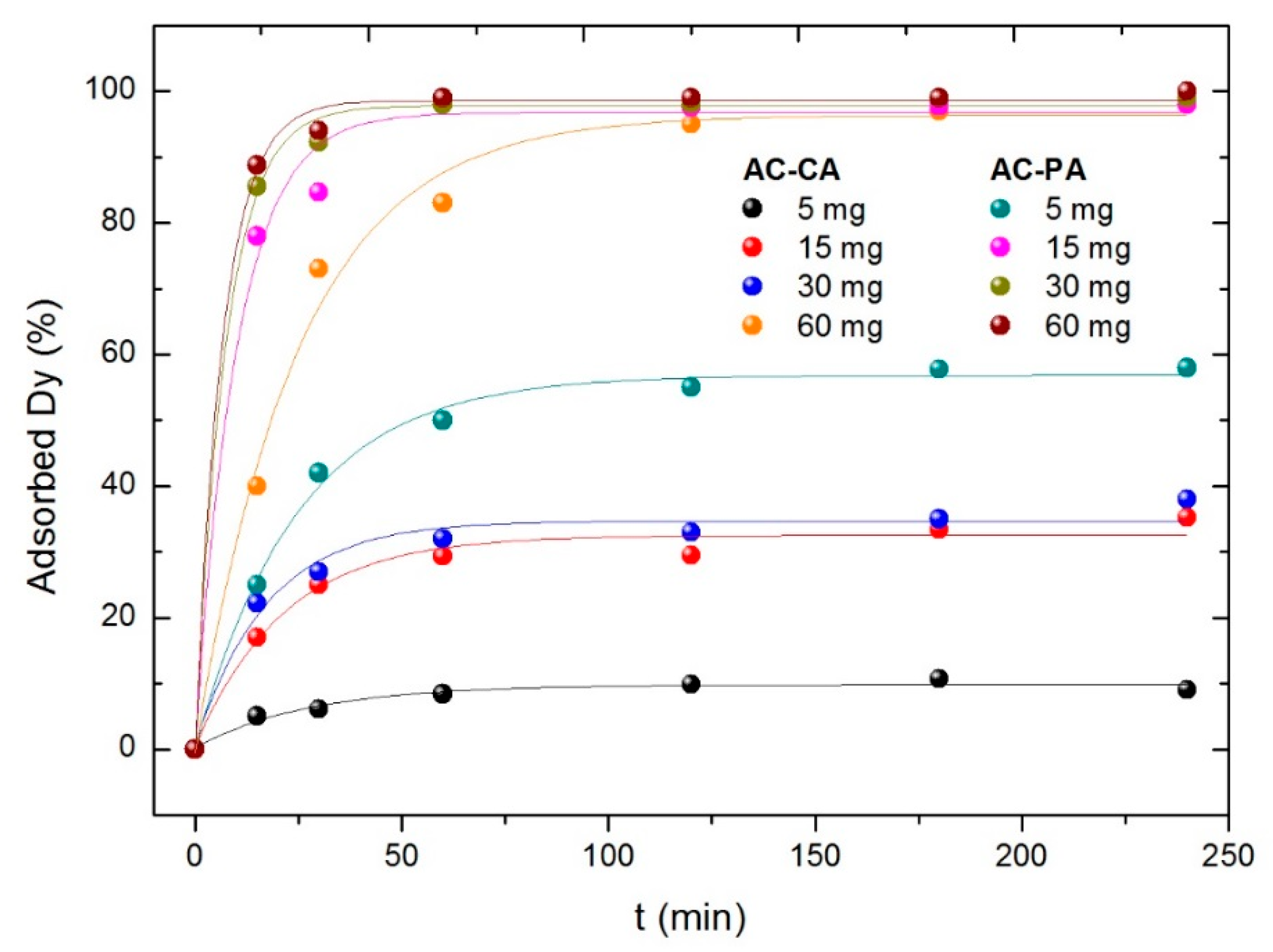
3.2.3. Influence of the Activated Carbon Amount
3.2.4. Equilibrium Isotherms
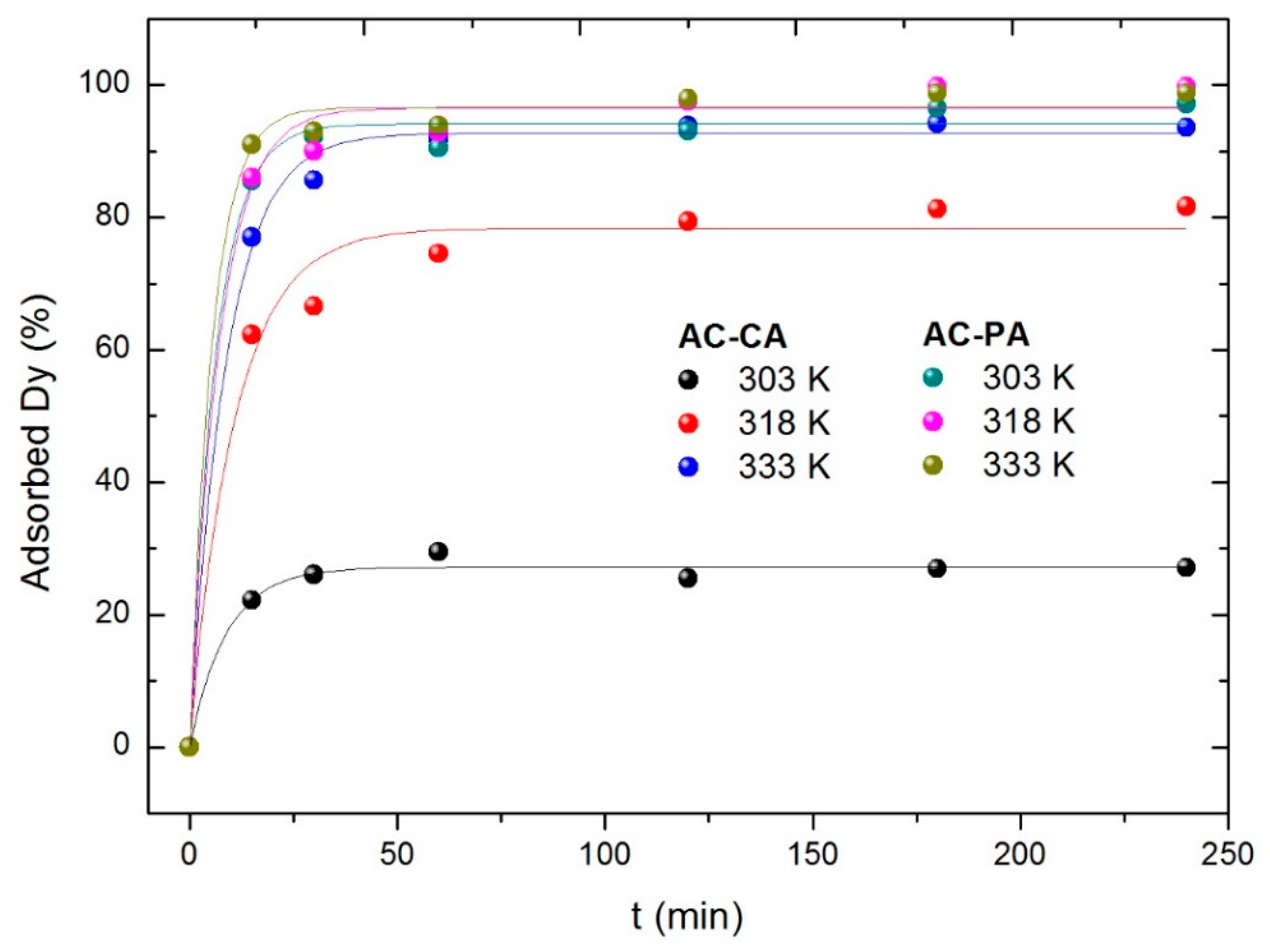
3.2.5. Effect of the Temperature, Kinetic and Thermodynamic Study
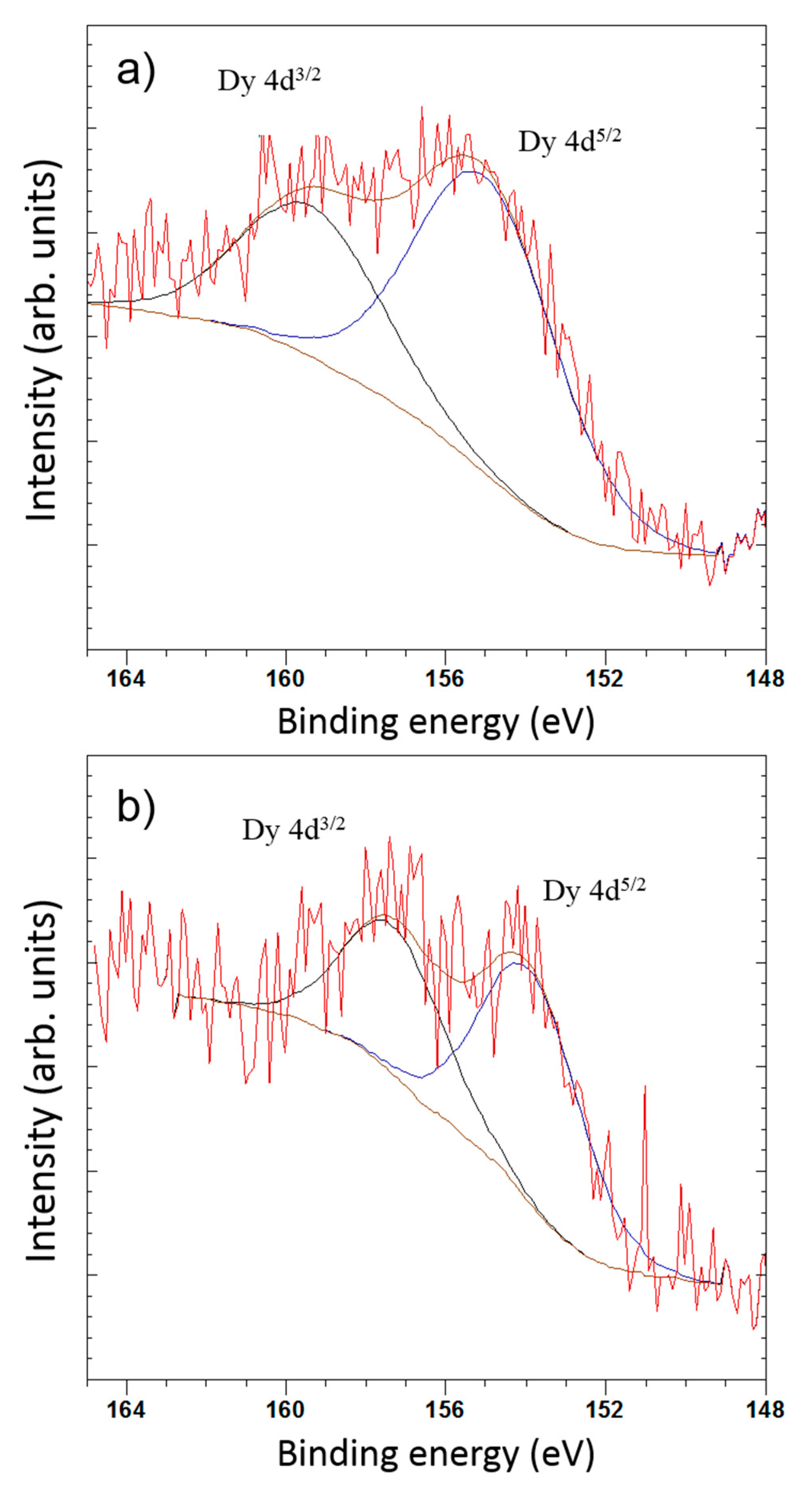
3.3. X-Ray Photoelectron Spectroscopy (XPS)
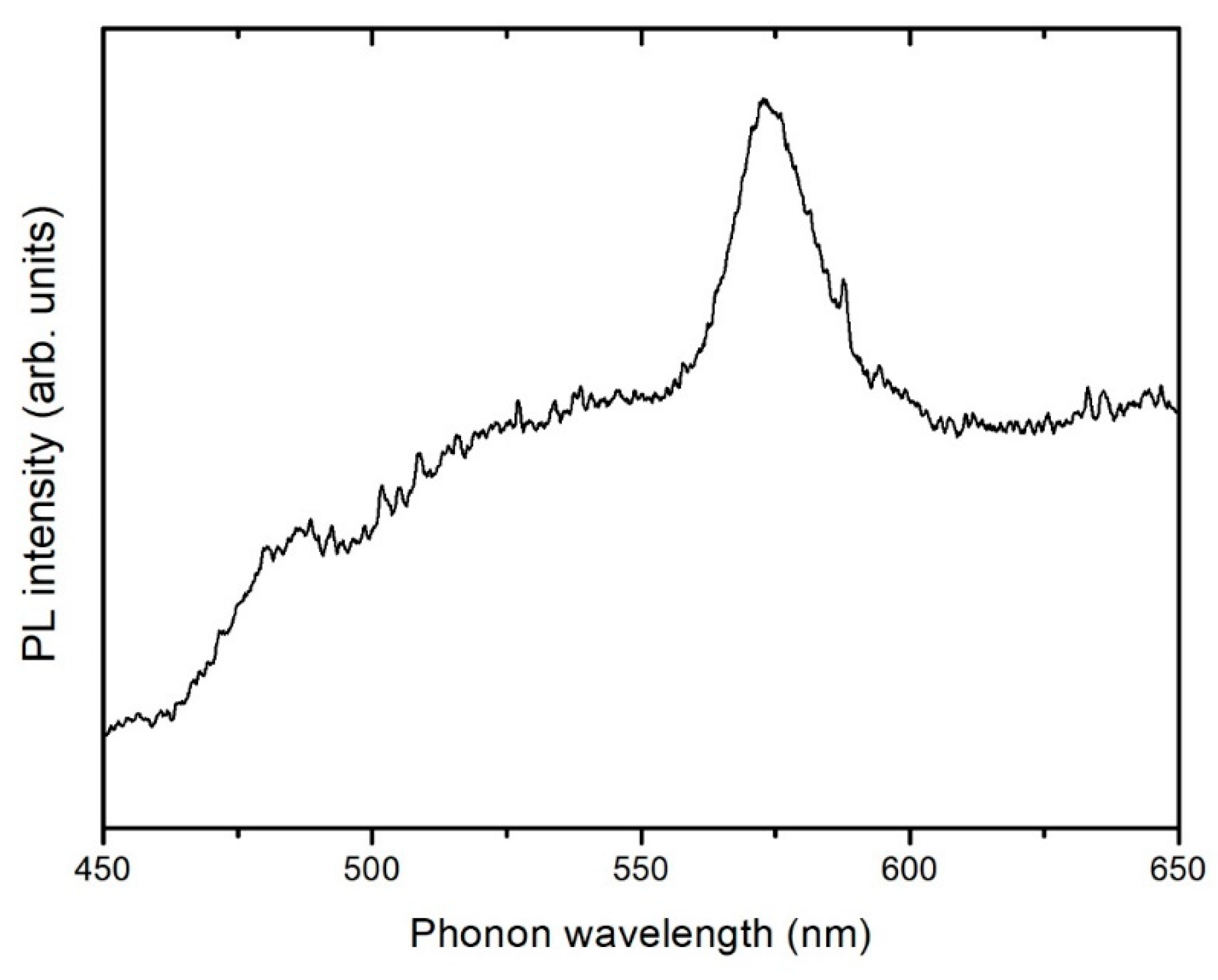
3.4. Photoluminescence Spectroscopy (PL)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| C0 (mg·L−1) | initial concentrations of the RE in solution |
| Ce (mg·L−1) | equilibrium concentrations of the RE in solution |
| qt (mg·g−1) | adsorption capacity at each time t |
| V (L) | volume of the solution |
| m (g) | mass of the activated carbon |
| qe (mg·g−1) | RE adsorbed amount by mass of the AC at the equilibrium |
| qm (mg·g−1) | maximum adsorption capacity of the adsorbent per unit mass of adsorbate |
| b (L·mg−1) | Langmuir constant |
| KF (L·g−1) | Freundlich constant |
| 1/n (adimensional) | adsorption intensity |
| AT (L·g−1) | Temkin isotherm equilibrium binding constant |
| T (K) | absolute temperature |
| B (J·mol−1) | ((R·T)/b_T) |
| bT | Temkin isotherm constant |
| R (kJ·K−1·mol−1) | universal gas constant |
| RL (adimensional) | Langmuir constant or equilibrium parameter. This parameter indicates if the isotherm is reversible (RL=0), favorable (0<RL<1), lineal (RL=1) or unfavorable (RL>1) where RL=1/(1+b·Co) |
| k1 (min−1) | pseudo-first-order adsorption constant |
| k2 (g·mg−1·min−1) | pseudo-second-order adsorption constant |
| A (g·mg−1·min−1) | temperature-independent factor |
| ΔH0 (kJ·mol−1) | standard enthalpy change |
| ΔS0 (J·mol−1·K−1) | standard entropy change |
| ΔS0 (J·mol−1·K−1) | standard Gibbs free energy |
| VT | total pore volume |
| Vμp | micropore volume |
| Dp | average micropore diameter (BJH desorption) |
| Sμs | microporous surface |
| NSμs | non-microporous surface |
References
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Gradin, K.T.; Poulikidou, S.; Björklund, A.; Luttropp, C. Scrutinising the electric vehicle material backpack. J. Clean. Prod. 2018, 172, 1699–1710. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Pokrovsky, O.S.; Santana-Casiano, J.M.; González-Dávila, M. Bioadsorption of heavy metals. Prospect. Chall. Algal Biotechnol. 2017, 4, 233–255. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Tahir, W.; Choudhry, S. Production of activated carbon from tea waste and its application in water treatment. J. Biol. Environ. Sci. 2017, 11, 37–44. [Google Scholar]
- Malhotra, M.; Suresh, S.; Garg, A. Tea waste derived activated carbon for the adsorption of sodium diclofenac from wastewater: Adsorbent characteristics, adsorption isotherms, kinetics, and thermodynamics. Environ. Sci. Pollut. Res. 2018, 25, 32210–32220. [Google Scholar] [CrossRef]
- Suganya, S.; Kumar, P.S. Influence of ultrasonic waves on preparation of active carbon from coffee waste for the reclamation of effluents containing Cr(VI) ions. J. Ind. Eng. Chem. 2018, 60, 418–430. [Google Scholar]
- Laksaci, H.; Khelifi, A.; Trari, M.; Addoun, A. Synthesis and characterization of microporous activated carbon from coffee grounds using potassium hydroxides. J. Clean. Prod. 2017, 147, 254–262. [Google Scholar] [CrossRef]
- Alcaraz, L.; López Fernández, A.; García-Díaz, I.; López, F.A. Preparation and characterization of activated carbons from winemaking wastes and their adsorption of methylene blue. Adsorpt. Sci. Technol. 2018, 36, 1331–1351. [Google Scholar] [CrossRef]
- Alguacil, F.; Alcaraz, L.; García-Díaz, I.; López, F. Removal of Pb2+ in Wastewater via Adsorption onto an Activated Carbon Produced from Winemaking Waste. Metals 2018, 8, 697. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A.; Fiol, N.; Villaescusa, I. Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. C. R. Chim. 2015, 18, 88–99. [Google Scholar] [CrossRef]
- Alslaibi, T.M.; Abustan, I.; Ahmad, M.A.; Abu Foul, A. Preparation of Activated Carbon from Olive Stone Waste: Optimization Study on the Removal of Cu2+, Cd2+, Ni2+, Pb 2+, Fe 2+, and Zn2+ from Aqueous Solution Using Response Surface Methodology. J. Dispers. Sci. Technol. 2014, 35, 913–925. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Fouodjouo, M.; Fotouo-Nkaffo, H.; Laminsi, S.; Cassini, F.A.; de Brito-Benetoli, L.O.; Debacher, N.A. Adsorption of copper (II) onto cameroonian clay modified by non-thermal plasma: Characterization, chemical equilibrium and thermodynamic studies. Appl. Clay Sci. 2017, 142, 136–144. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A.; Takada, A.; Tomita, N. Structural Information from the Raman Spectra of Activated Carbon Materials; Carbon: New York, NY, USA, 2000. [Google Scholar]
- Sfyris, D.; Sfyris, G.I.; Galiotis, C. Stress intrepretation of graphene E-2g and A-1g vibrational modes: Theoretical analysis. arXiv 2017, arXiv:1706.04465. [Google Scholar]
- Wei, Z.; Pan, R.; Hou, Y.; Yang, Y.; Liu, Y. Graphene-supported Pd catalyst for highly selective hydrogenation of resorcinol to 1, 3-cyclohexanedione through giant π-conjugate interactions. Sci. Rep. 2015, 5, 15664. [Google Scholar] [CrossRef] [PubMed]
- Ettehadi Gargari, J.; Sid Kalal, H.; Shakeri, A.; Khanchi, A. Synthesis and characterization of Silica/polyvinyl imidazole/H2PO4-core-shell nanoparticles as recyclable adsorbent for efficient scavenging of Sm(III) and Dy(III) from water. J. Colloid Interface Sci. 2017, 505, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, R.; Hanif, J. Adsorption of dysprosium ions on activated charcoal from aqueous solutions. Carbon 1995, 33, 215–220. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Koochaki-Mohammadpour, S.M.A.; Torab-Mostaedi, M.; Talebizadeh-Rafsanjani, A.; Naderi-Behdani, F. Adsorption Isotherm, Kinetic, Thermodynamic, and Desorption Studies of Lanthanum and Dysprosium on Oxidized Multiwalled Carbon Nanotubes. J. Dispers. Sci. Technol. 2014, 35, 244–254. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Sahraei, R.; Daneshfar, A. Characterization of zinc oxide nanorods loaded on activated carbon as cheap and efficient adsorbent for removal of methylene blue. J. Ind. Eng. Chem. 2015, 21, 986–993. [Google Scholar] [CrossRef]
- Barreca, D.; Gasparotto, A.; Milanov, A.; Tondello, E.; Devi, A.; Fischer, R.A. Nanostructured Dy2O3 films: An XPS Investigation. Surf. Sci. Spectra 2007, 14, 52–59. [Google Scholar] [CrossRef]
- Chemingui, S.; Ferhi, M.; Horchani-Naifer, K.; Férid, M. Synthesis and luminescence characteristics of Dy3+ doped KLa(PO3)4. J. Lumin. 2015, 166, 82–87. [Google Scholar] [CrossRef]
- Grobelna, B.; Synak, A.; Bojarski, P.; Szczodrowski, K.; Kukliński, B.; Raut, S.; Gryczyński, I. Synthesis and luminescence characteristics of Dy3+ ions in silica xerogels doped with Ln2-xDyx(WO4)3. Opt. Mater. 2013, 35, 456–461. [Google Scholar] [CrossRef]
- Alcaraz, L.; Isasi, J.; Díaz-Guerra, C. Influence of the synthesis conditions of Y0.9Dy0.1VO4 and silica-coated Y0.9Dy0.1VO4 nanophosphors on the powder morphology and luminescence emission intensity. J. Nanopart. Res. 2019, 21, 81. [Google Scholar] [CrossRef]
- Grobelna, B.; Synak, A.; Bojarski, P. The luminescence properties of dysprosium ions in silica xerogel doped with Gd1.6Dy0.4(WO4)3. Opt. Appl. 2012, 42, 337–344. [Google Scholar]
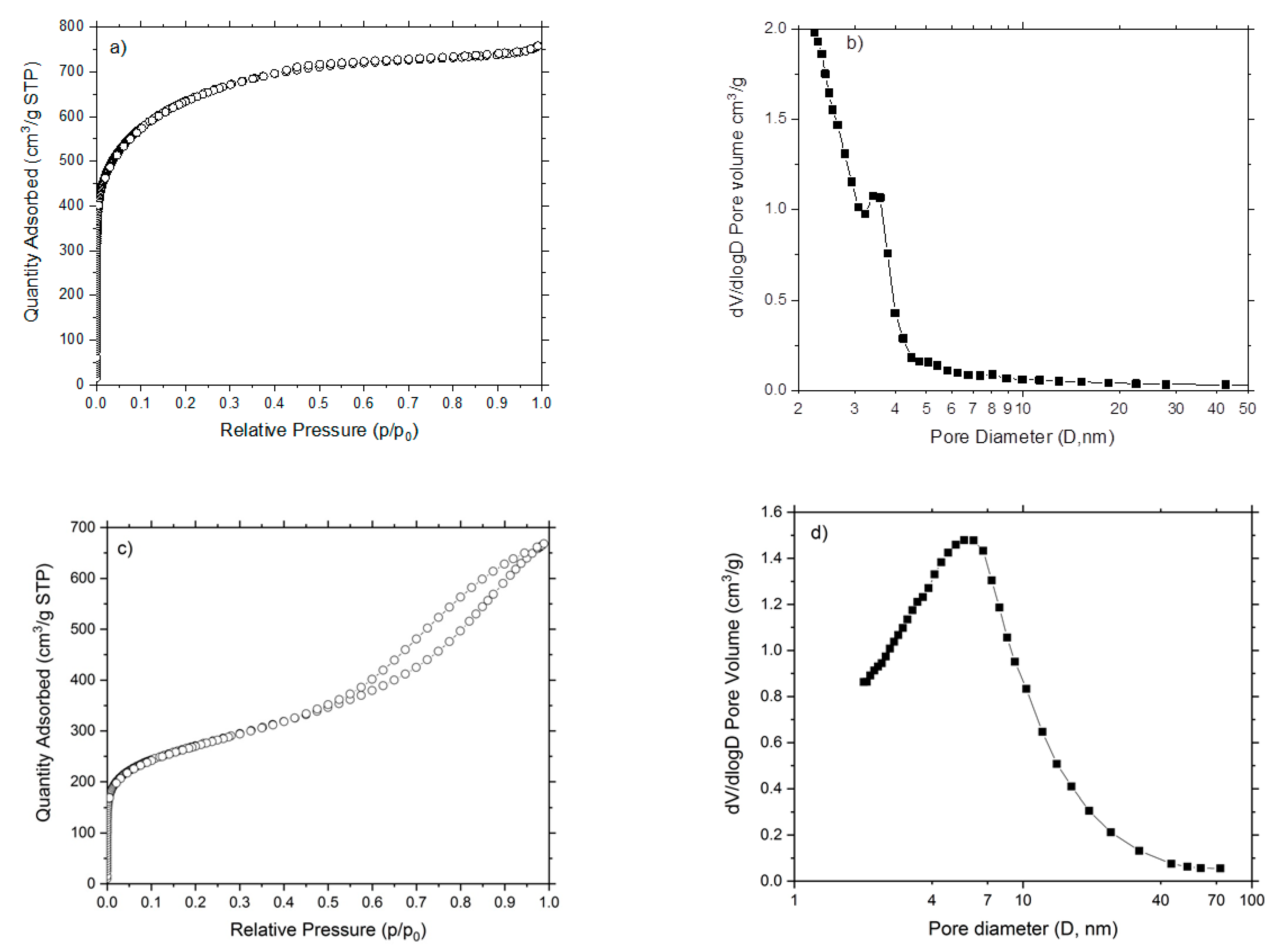

| Sample | VT (cm3·g−1) | Vμp (cm3·g−1) | Dp (nm) | SμS (m2·g−1) | NSμs (m2·g−1) | SBET (m2·g−1) |
|---|---|---|---|---|---|---|
| AC-QA | 1.17 | 1.07 | 3.29 | 2265.0 | 65.6 | 2330.6 |
| AC-PA | 1.03 | 0.03 | 5.68 | 244.8 | 736.8 | 981.6 |
| Sample | Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg·g−1) | b (L·mg−1) | RL | R2 | KF (L·g−1) | 1/n | R2 | AT | bT | R2 | |
| AC-CA | 28.11 | 6.42 | 0.02 | 0.996 | 24.82 | 0.08 | 0.713 | 0.23 | 0.16 | 0.582 |
| AC-PA | 29.05 | 10.5 | 0.03 | 0.998 | 81.44 | 0.17 | 0.722 | 0.79 | 0.10 | 0.800 |
| Sample | T (K) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 (·10-3) | qe | R2 | ||
| AC-CA | 303 | 0.016 | 17.693 | 0.784 | 0.795 | 32.362 | 0.997 |
| 318 | 0.017 | 19.470 | 0.830 | 1.012 | 32.467 | 0.995 | |
| 333 | 0.057 | 19.931 | 0.933 | 10172 | 33.670 | 0.999 | |
| AC-PA | 303 | 0.020 | 8.194 | 0.740 | 0.932 | 28.490 | 0.999 |
| 318 | 0.023 | 8.366 | 0.965 | 1.132 | 30.120 | 0.999 | |
| 333 | 0.047 | 21.270 | 0.785 | 1.232 | 32.362 | 0.999 | |
| Sample | T (K) | −ΔH0 (kJ·mol−1) | ΔS0 (J·mol−1·K−1) | −ΔG0 (kJ·mol−1) |
|---|---|---|---|---|
| AC-CA | 303 | 79.18 | 327.64 | 178.45 |
| 318 | 183.37 | |||
| 333 | 188.29 | |||
| AC-PA | 303 | 159.65 | 628.29 | 350.02 |
| 318 | 359.44 | |||
| 333 | 368.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaraz, L.; Escudero, M.E.; Alguacil, F.J.; Llorente, I.; Urbieta, A.; Fernández, P.; López, F.A. Dysprosium Removal from Water Using Active Carbons Obtained from Spent Coffee Ground. Nanomaterials 2019, 9, 1372. https://doi.org/10.3390/nano9101372
Alcaraz L, Escudero ME, Alguacil FJ, Llorente I, Urbieta A, Fernández P, López FA. Dysprosium Removal from Water Using Active Carbons Obtained from Spent Coffee Ground. Nanomaterials. 2019; 9(10):1372. https://doi.org/10.3390/nano9101372
Chicago/Turabian StyleAlcaraz, Lorena, María Esther Escudero, Francisco José Alguacil, Irene Llorente, Ana Urbieta, Paloma Fernández, and Félix Antonio López. 2019. "Dysprosium Removal from Water Using Active Carbons Obtained from Spent Coffee Ground" Nanomaterials 9, no. 10: 1372. https://doi.org/10.3390/nano9101372
APA StyleAlcaraz, L., Escudero, M. E., Alguacil, F. J., Llorente, I., Urbieta, A., Fernández, P., & López, F. A. (2019). Dysprosium Removal from Water Using Active Carbons Obtained from Spent Coffee Ground. Nanomaterials, 9(10), 1372. https://doi.org/10.3390/nano9101372