Interaction of Immune Cells and Tumor Cells in Gold Nanorod–Gelatin Composite Porous Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication and Characterization of AuNRs–Gelatin Composite Porous Scaffold
2.2. Photothermal Ablation Ability of Tumor Cells Cultured in AuNRs–Gelatin Composite Scaffold
2.3. Co-Culture of Immature DCs and Photothermally Ablated Tumor Cells in AuNRs–Gelatin Composite Scaffold
2.4. Statistical Analysis
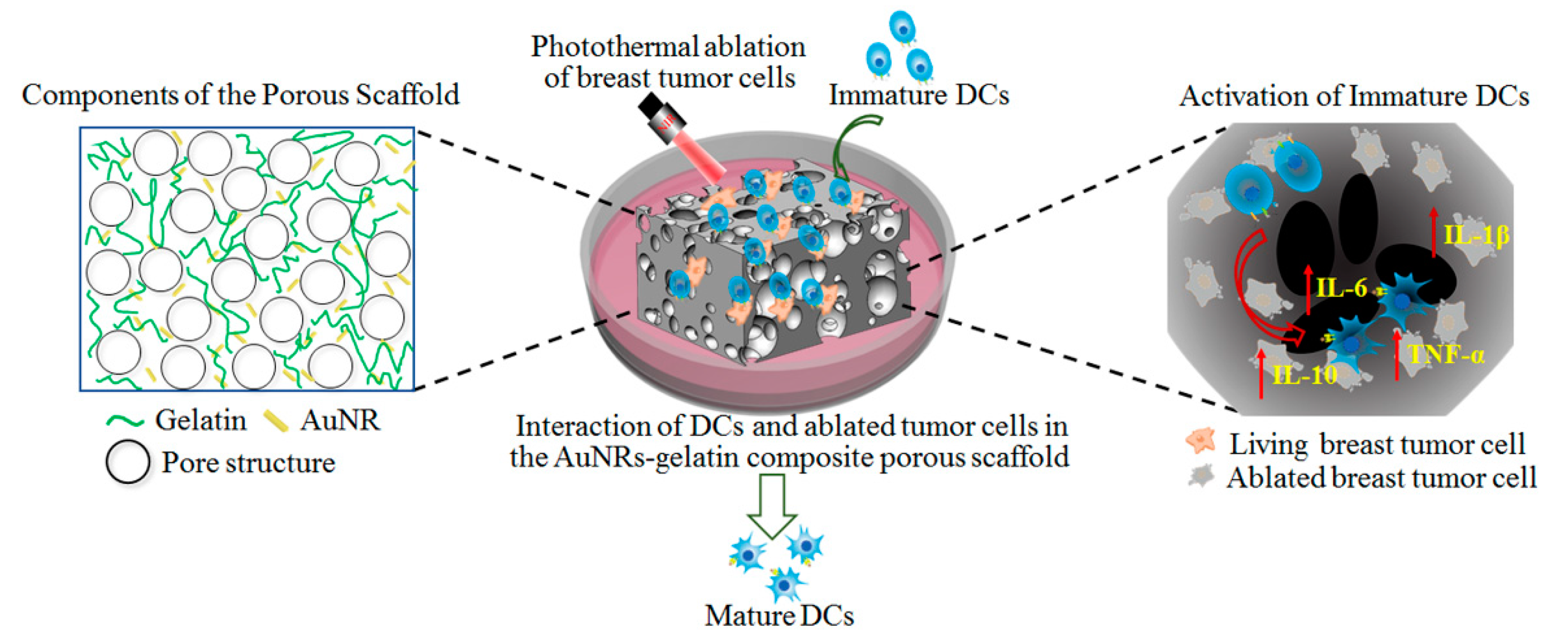
3. Results and Discussion
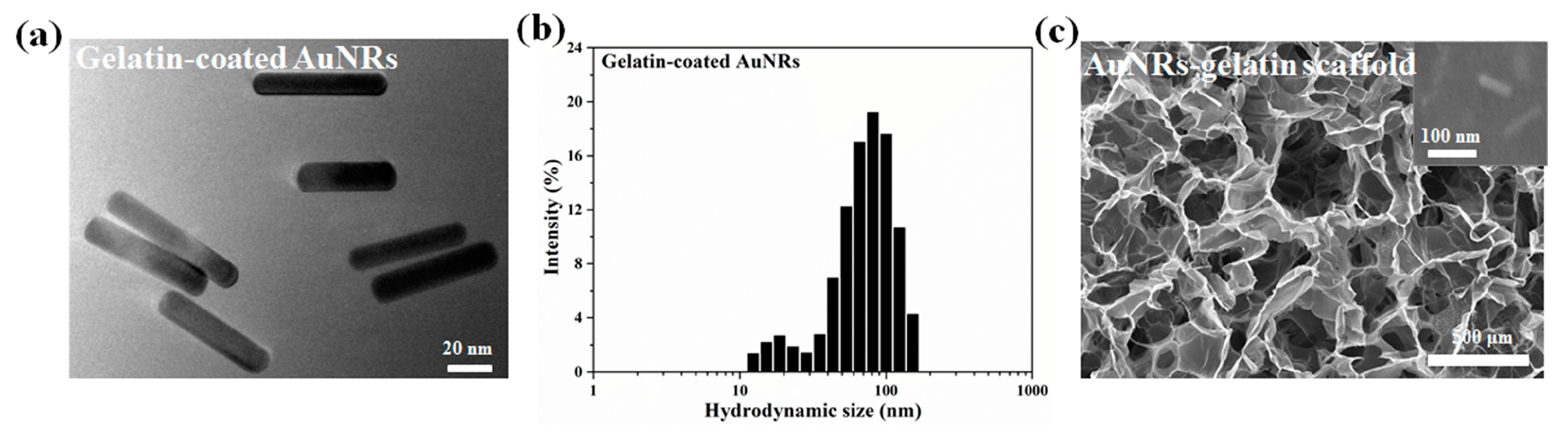
3.1. Characterization of AuNRs and AuNRs–Gelatin Composite Porous Scaffold
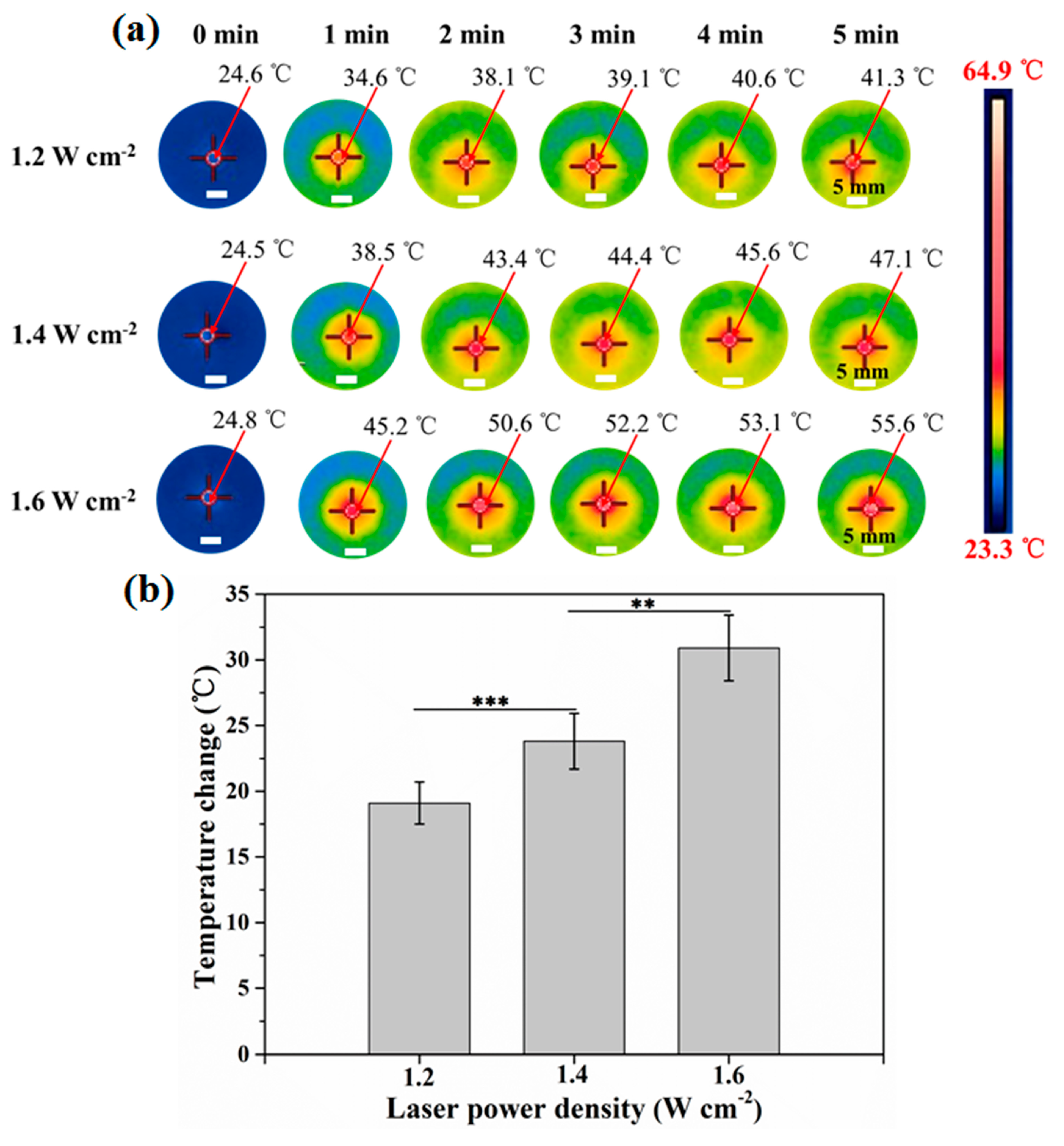
3.2. Photothermal Property of AuNRs–Gelatin Composite Scaffold
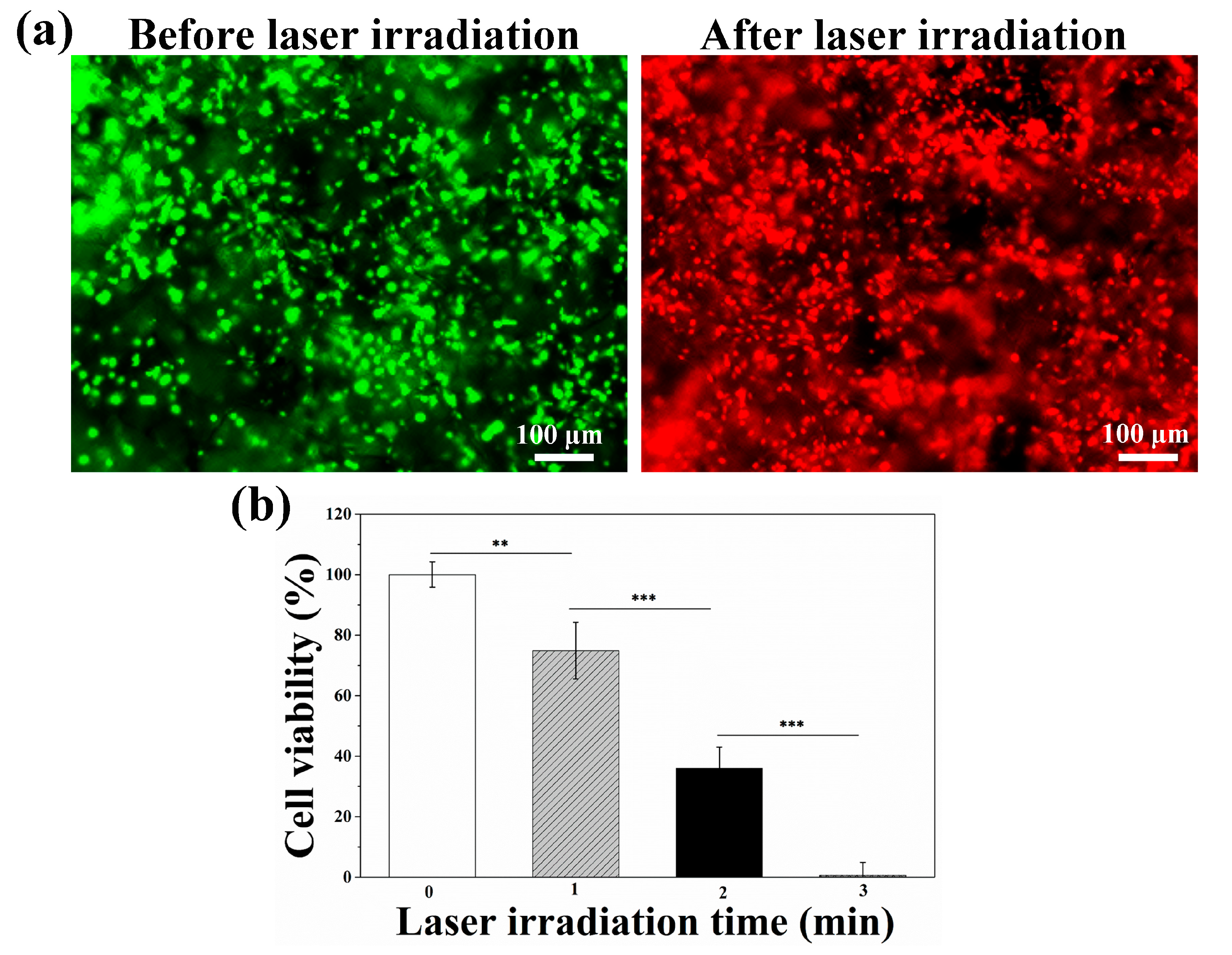
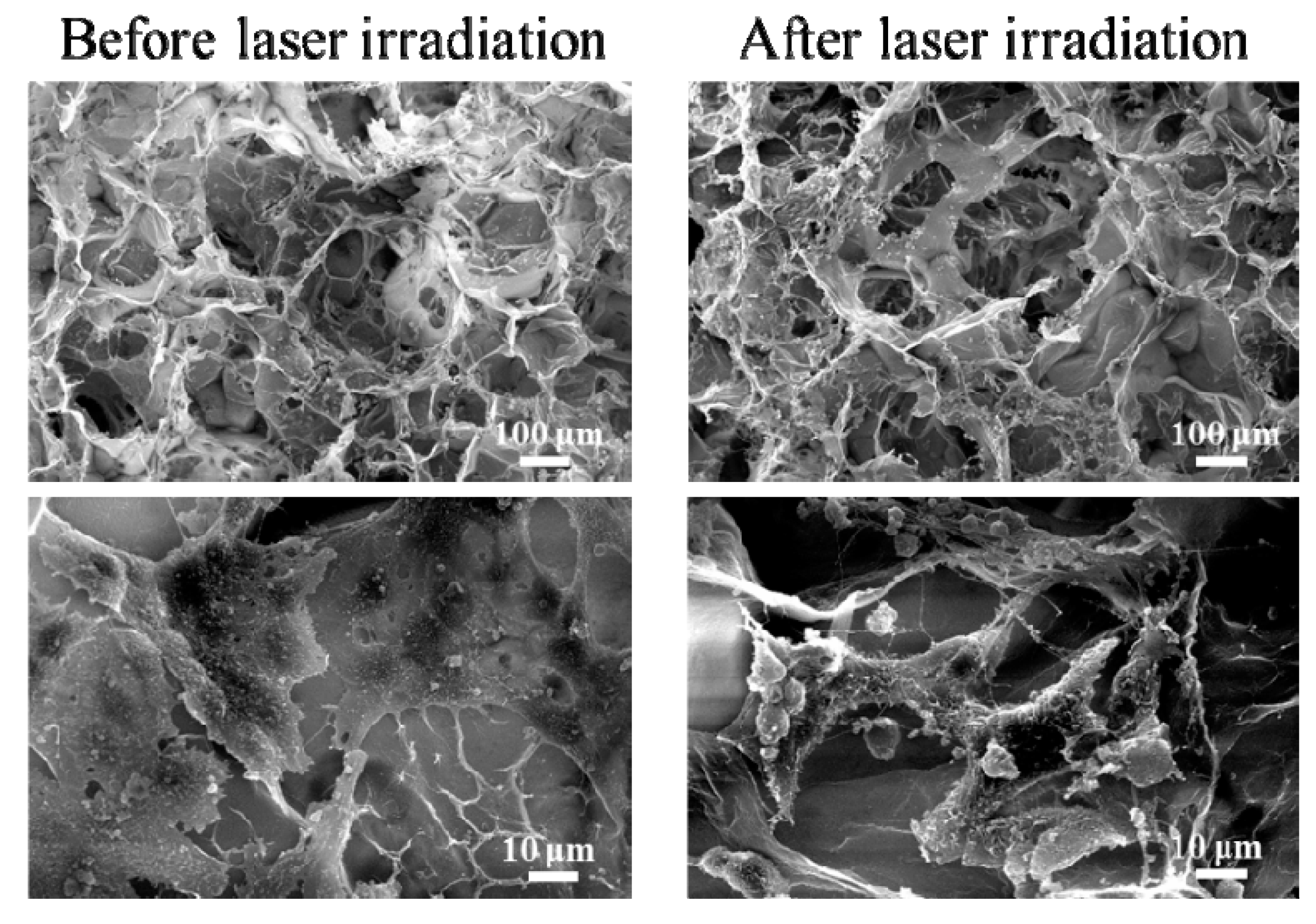
3.3. Photothermal Ablation of Tumor Cells Cultured in AuNRs–Gelatin Composite Porous Scaffold
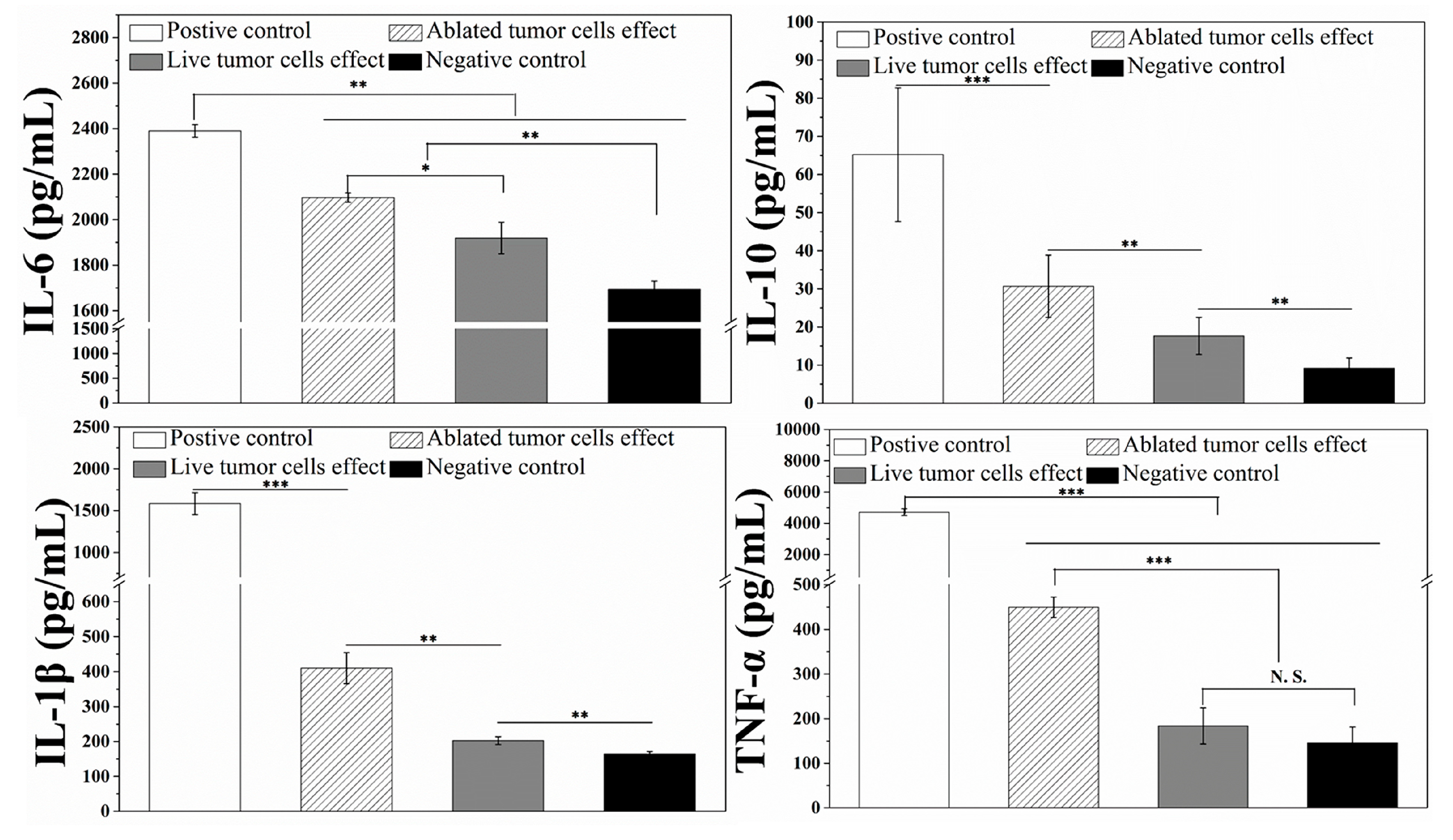
3.4. Interaction and Activation of Immature DCs with Photopthermally Ablated Tumor Cells in AuNRs–Gelatin Composite Porous Scaffold
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rheinbay, E.; Parasuraman, P.; Grimsby, J.; Tiao, G.; Engreitz, J.M.; Kim, J.; Lawrence, M.S.; Taylor-Weiner, A.; Rodriguez-Cuevas, S.; Rosenberg, M.; et al. Recurrent and functional regulatory mutations in breast cancer. Nature 2017, 547, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Libson, S.; Lippman, M. A review of clinical aspects of breast cancer. Int. Rev. Psychiatry 2014, 26, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Diestra, D.; Thapa, B.; Badillo-Diaz, D.; Beltran-Huarac, J. Graphene Oxide/ZnS:Mn Nanocomposite Functionalized with Folic Acid as a Nontoxic and Effective Theranostic Platform for Breast Cancer Treatment. Nanomaterials 2018, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, C.; Zhang, Y.; Wang, H.; Chen, G.; Wang, M.; Wang, Q. Programmable Chemotherapy and Immunotherapy against Breast Cancer Guided by Multiplexed Fluorescence Imaging in the Second Near-Infrared Window. Adv. Mater. 2018, 30, e1804437. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Madni, A.; Correia, A.; Rehman, M.; Balasubramanian, V.; Khan, M.M.; Santos, H.A. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int. J. Nanomed. 2019, 14, 4961–4974. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mao, J.-H.; Zhu, W.; Jain, A.K.; Liu, K.; Brown, J.B.; Karpen, G.H. Centromere and kinetochore gene misexpression predicts cancer patient survival and response to radiotherapy and chemotherapy. Nat. Commun. 2016, 7, 12619. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Su, M. Polydopamine Nanoparticles for Combined Chemo- and Photothermal Cancer Therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Lee, J.E.; Jin, S.M.; Lee, J.H.; Lee, I.S.; Yang, I.; Kim, J.S.; Kim, S.K.; Cho, M.H.; et al. Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angew. Chem. 2006, 45, 7754–7758. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, C.; Li, H.; Xiao, A.; Guo, T.; Guan, B.-O. Insight into the local near-infrared photothermal dynamics of graphene oxide functionalized polymers through optical microfibers. Phys. Chem. Chem. Phys. 2018, 20, 5256–5263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Obiweluozor, F.O.; Emechebe, G.A.; Tiwari, A.P.; Kim, J.Y.; Park, C.H.; Kim, C.S. Short duration cancer treatment: Inspired by a fast bio-resorbable smart nano-fiber device containing NIR lethal polydopamine nanospheres for effective chemo-photothermal cancer therapy. Int. J. Nanomed. 2018, 13, 6375–6390. [Google Scholar] [CrossRef]
- Moon, H.; Kumar, D.; Kim, H.; Sim, C.; Chang, J.H.; Kim, J.M.; Kim, H.; Lim, D.K. Amplified photoacoustic performance and enhanced photothermal stability of reduced graphene oxide coated gold nanorods for sensitive photoacoustic imaging. ACS Nano 2015, 9, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chang, J.; Shi, M.; Pan, W.; Li, N.; Tang, B. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem. Int. Ed. 2019, 58, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Tian, Y.; Zhang, L.; Han, X.; Wang, Q.; Cheng, W. Targeted delivery of reduced graphene oxide nanosheets using multifunctional ultrasound nanobubbles for visualization and enhanced photothermal therapy. Int. J. Nanomed. 2018, 13, 7859–7872. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Lin, P.Y.; Huang, P.H.; Kuo, C.Y.; Shalumon, K.T.; Chen, M.Y.; Chen, J.P. Magnetic Graphene Oxide for Dual Targeted Delivery of Doxorubicin and Photothermal Therapy. Nanomaterials 2018, 8, 193. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Yang, Y.; Kawazoe, N.; Chen, G. Sub-10 nm gold nanoparticles promote adipogenesis and inhibit osteogenesis of mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 1353–1362. [Google Scholar] [CrossRef]
- Cheng, Y.; Chang, Y.; Feng, Y.; Jian, H.; Tang, Z.; Zhang, H. Deep-Level Defect Enhanced Photothermal Performance of Bismuth Sulfide-Gold Heterojunction Nanorods for Photothermal Therapy of Cancer Guided by Computed Tomography Imaging. Angew. Chem. 2018, 57, 246–251. [Google Scholar] [CrossRef]
- Liu, L.; Xie, H.J.; Mu, L.M.; Liu, R.; Su, Z.B.; Cui, Y.N.; Xie, Y.; Lu, W.L. Functional chlorin gold nanorods enable to treat breast cancer by photothermal/photodynamic therapy. Int. J. Nanomed. 2018, 13, 8119–8135. [Google Scholar] [CrossRef]
- Gan, R.; Fan, H.; Wei, Z. Photothermal Response of Hollow Gold Nanorods under Femtosecond Laser Irradiation. Nanomaterials 2019, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.W.; Chuang, E.Y.; Chen, H.L.; Wan, D.; Korupalli, C.; Liao, Z.X.; Chiu, Y.L.; Chia, W.T.; Lin, K.J.; Sung, H.W. Photothermal tumor ablation in mice with repeated therapy sessions using NIR-absorbing micellar hydrogels formed in situ. Biomaterials 2015, 56, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Encinas-Basurto, D.; Ibarra, J.; Juarez, J.; Pardo, A.; Barbosa, S.; Taboada, P.; Valdez, M.A. Hybrid folic acid-conjugated gold nanorods-loaded human serum albumin nanoparticles for simultaneous photothermal and chemotherapeutic therapy. Mater. Sci. Eng. C 2018, 91, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded calcium phosphate nanoparticles for the osteogenic differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 6801–6810. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, C.; Zhai, D.; Luo, Y.; Chen, Y.; Lv, F.; Yi, Z.; Deng, Y.; Wang, J.; Chang, J.; et al. A Bifunctional Biomaterial with Photothermal Effect for Tumor Therapy and Bone Regeneration. Adv. Funct. Mater. 2016, 26, 1197–1208. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Li, J.; Chen, Y.; Chen, Y.; Kawazoe, N.; Chen, G. Bifunctional scaffolds for the photothermal therapy of breast tumor cells and adipose tissue regeneration. J. Mater. Chem. B 2018, 6, 7728–7736. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Kawazoe, N.; Chen, G. Composite scaffolds of gelatin and gold nanoparticles with tunable size and shape for photothermal cancer therapy. J. Mater. Chem. B 2017, 5, 245–253. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Chen, S.; Kawazoe, N.; Chen, G. Preparation of gelatin/Fe3O4 composite scaffolds for enhanced and repeatable cancer cell ablation. J. Mater. Chem. B 2016, 4, 5664–5672. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Ng, C.W.; Li, J.; Pu, K. Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Wang, D.; Hao, M.; Kong, C.; Zhao, X.; Gao, Y.; Li, J.; Liu, B.; Yang, B.; et al. Inhibition of Autophagy Promoted Apoptosis and Suppressed Growth of Hepatocellular Carcinoma upon Photothermal Exposure. J. Biomed. Nanotechnol. 2019, 15, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.S.C.; Gouvea, A.L.; de Moura, L.D.; Paterno, L.G.; de Souza, P.E.N.; Bastos, A.P.; Damasceno, E.A.M.; Veiga-Souza, F.H.; de Azevedo, R.B.; Bao, S.N. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J. Nanobiotechnol. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Long, J.-T.; Cheang, T.-Y.; Zhuo, S.-Y.; Zeng, R.-F.; Dai, Q.-S.; Li, H.-P.; Fang, S. Anticancer drug-loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in lung cancer metastasis. J. Nanobiotechnol. 2014, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Larmonier, N.; Merino, D.; Nicolas, A.; Cathelin, D.; Besson, A.; Bateman, A.; Solary, E.; Martin, F.; Katsanis, E.; Bonnotte, B. Apoptotic, necrotic, or fused tumor cells: An equivalent source of antigen for dendritic cell loading. Apoptosis 2006, 11, 1513–1524. [Google Scholar] [CrossRef]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations-boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zeng, Y.; Whitesell, L.; Katsanis, E. Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood 2001, 97, 3505–3512. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Kawazoe, N.; Chen, G. Photothermal Ablation of Cancer Cells by Albumin-Modified Gold Nanorods and Activation of Dendritic Cells. Materials 2018, 12, 31. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Liang, C.; Xiang, J.; Peng, R.; Liu, Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014, 26, 8154–8162. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhu, Y.; Zhang, M.; Xiao, Y.; Du, X.; Liu, H.; Wang, S. The induction of maturation on dendritic cells by TiO2 and Fe3O4@ TiO2 nanoparticles via NF-kappaB signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 39, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Serda, R.E. Particle platforms for cancer immunotherapy. Int. J. Nanomed. 2013, 8, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hu, Q.; Bellotti, A.; Gu, Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017, 29, 1606036. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Li, J.; Li, J.E.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef]
- Imhof, M.; Karas, I.; Gomez, I.; Eger, A.; Imhof, M. Interaction of tumor cells with the immune system: Implications for dendritic cell therapy and cancer progression. Drug Discov. Today 2013, 18, 35–42. [Google Scholar] [CrossRef]
- Friedl, P.; den Boer, A.T.; Gunzer, M. Tuning immune responses: Diversity and adaptation of the immunological synapse. Nat. Rev. Immunol. 2005, 5, 532–545. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Goldberg, M.S. Immunoengineering: How nanotechnology can enhance cancer immunotherapy. Cell 2015, 161, 201–204. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, Q.; Yang, J.; Ren, Q.; Cao, W.; Yang, J.; Yu, Z.; Yu, F.; Wu, Y.; Shi, H.; et al. Co-culture of apoptotic breast cancer cells with immature dendritic cells: A novel approach for DC-based vaccination in breast cancer. Braz. J. Med. Biol. Res. 2012, 45, 510–515. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Kawazoe, N.; Chen, G. Interaction of Immune Cells and Tumor Cells in Gold Nanorod–Gelatin Composite Porous Scaffolds. Nanomaterials 2019, 9, 1367. https://doi.org/10.3390/nano9101367
Wang X, Kawazoe N, Chen G. Interaction of Immune Cells and Tumor Cells in Gold Nanorod–Gelatin Composite Porous Scaffolds. Nanomaterials. 2019; 9(10):1367. https://doi.org/10.3390/nano9101367
Chicago/Turabian StyleWang, Xiuhui, Naoki Kawazoe, and Guoping Chen. 2019. "Interaction of Immune Cells and Tumor Cells in Gold Nanorod–Gelatin Composite Porous Scaffolds" Nanomaterials 9, no. 10: 1367. https://doi.org/10.3390/nano9101367
APA StyleWang, X., Kawazoe, N., & Chen, G. (2019). Interaction of Immune Cells and Tumor Cells in Gold Nanorod–Gelatin Composite Porous Scaffolds. Nanomaterials, 9(10), 1367. https://doi.org/10.3390/nano9101367




