Recent Advances in the Processing and Properties of Alumina–CNT/SiC Nanocomposites
Abstract
1. Introduction
2. Processing of SiC–Carbon Nanotube (CNT)/Alumina Nanocomposites
2.1. Powder Processing
2.2. Mechanical Alloying
2.3. Ultrasonication
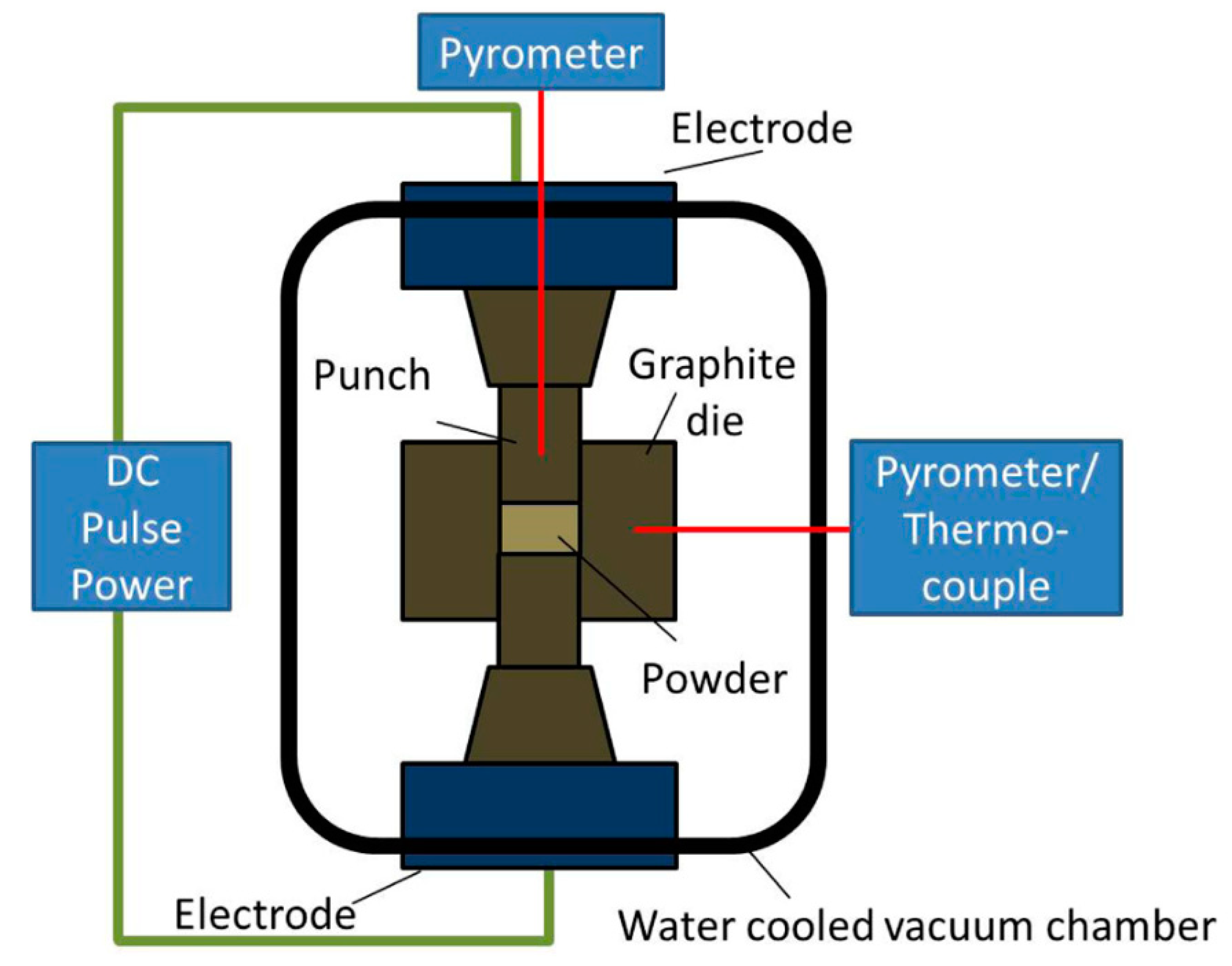
2.4. Powder Consolidation
Spark Plasma Sintering (SPS)
2.5. Processing of Alumina–SiC Nanocomposites
2.6. Processing of Alumina–CNT Nanocomposites
Functionalization of CNTs
2.7. Consolidation of Alumina/SiC and Alumina–CNT Nanopowders
2.8. Densification of Al2O3–SiC Nanocomposites
2.9. Densification of Al2O3-CNT Nanocomposites
3. Mechanical Properties of Alumina-Based Nanocomposites
3.1. Mechanical Properties of Alumina–SiC Nanocomposites
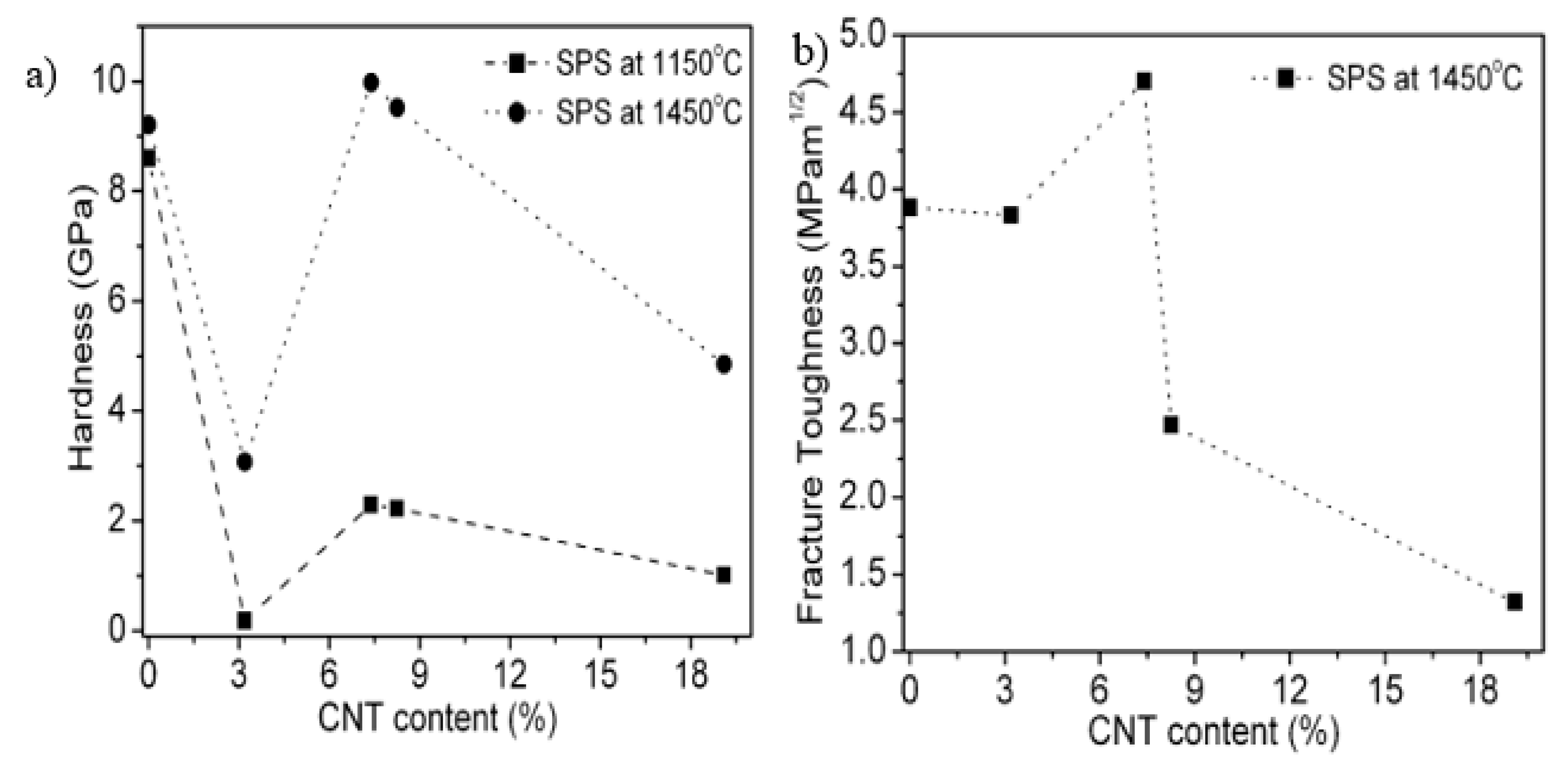
3.2. Mechanical Properties of Alumina–CNT Nanocomposites
4. Microstructures of Alumina-Based Nanocomposites
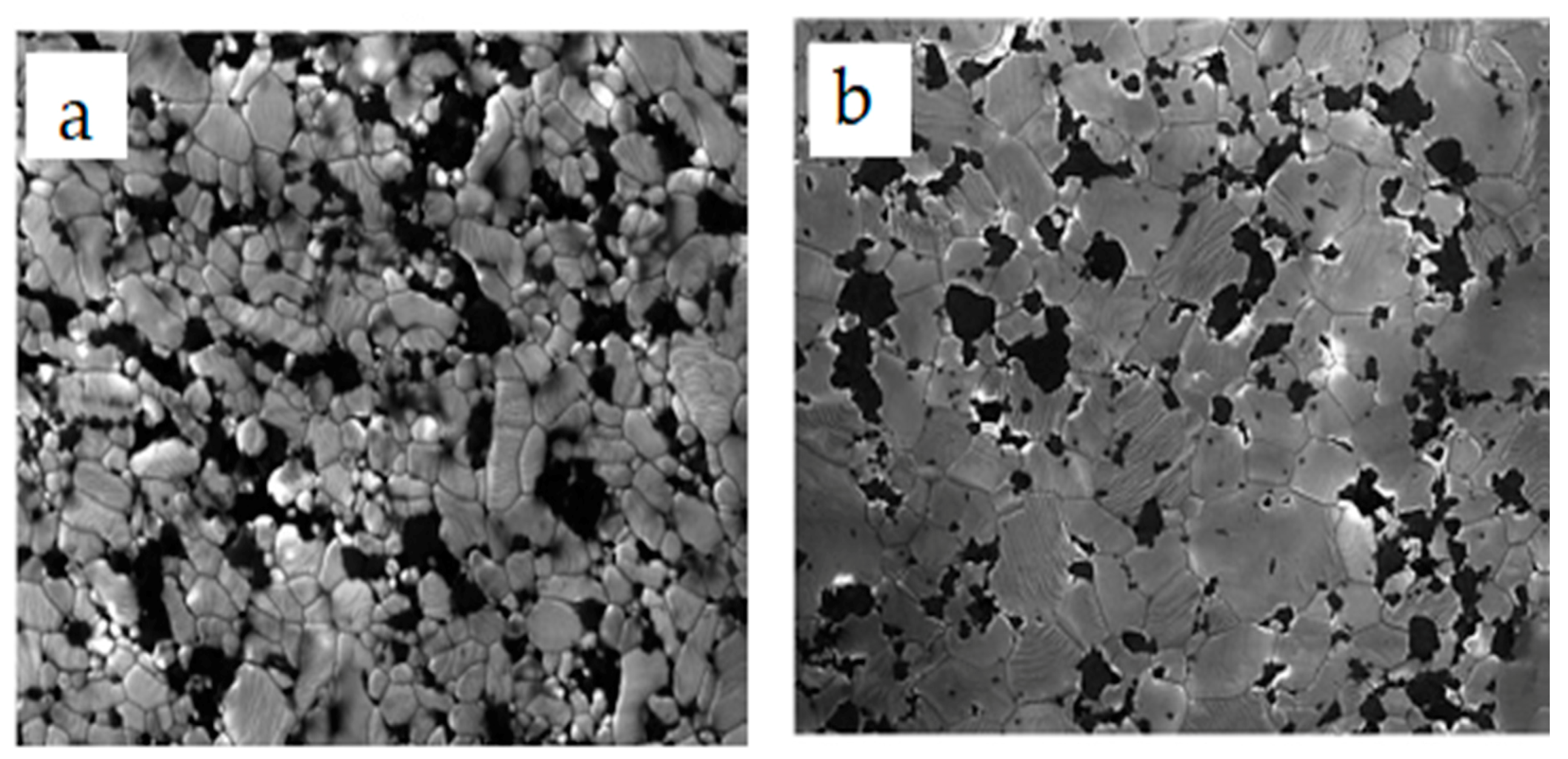
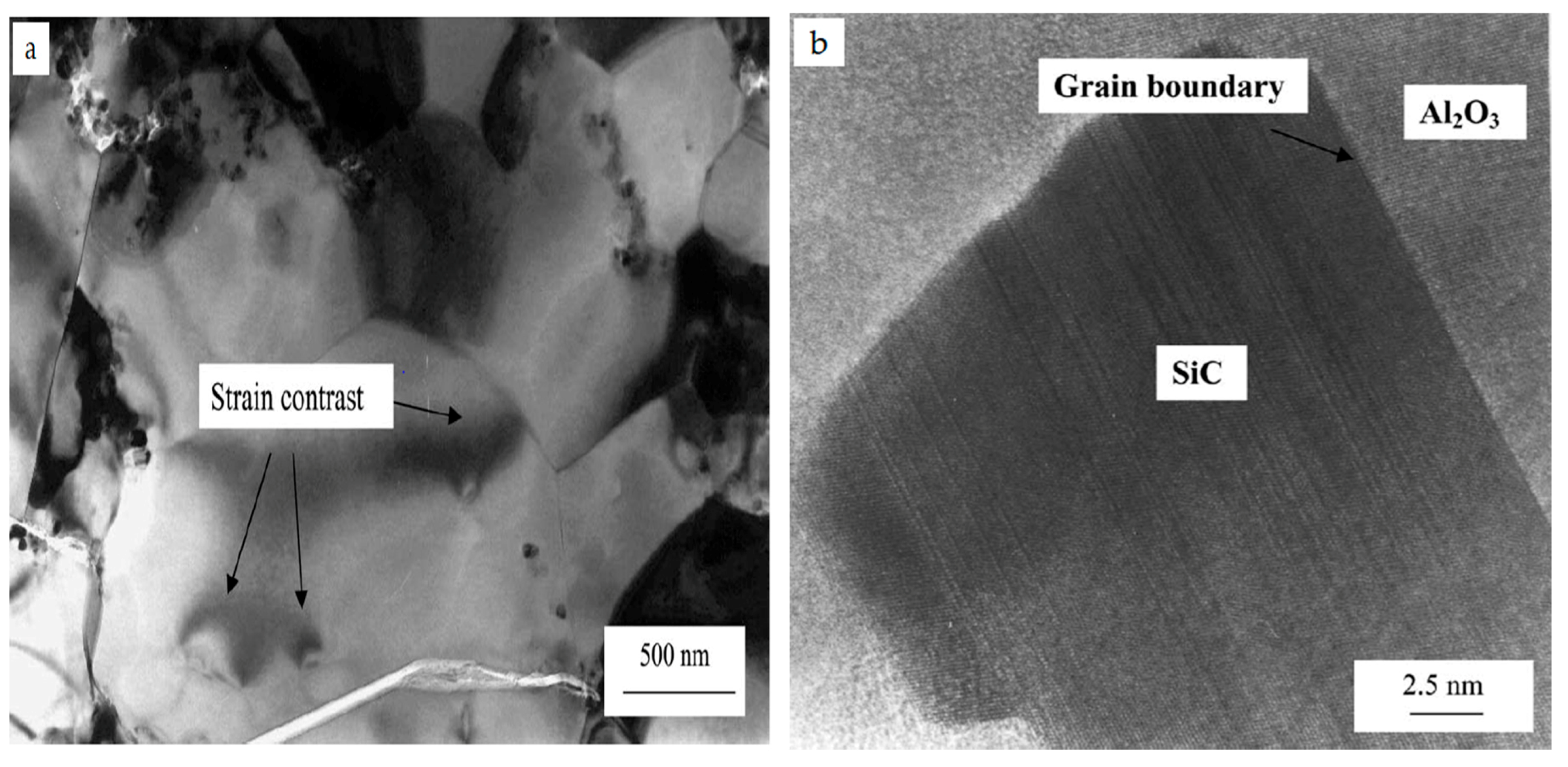
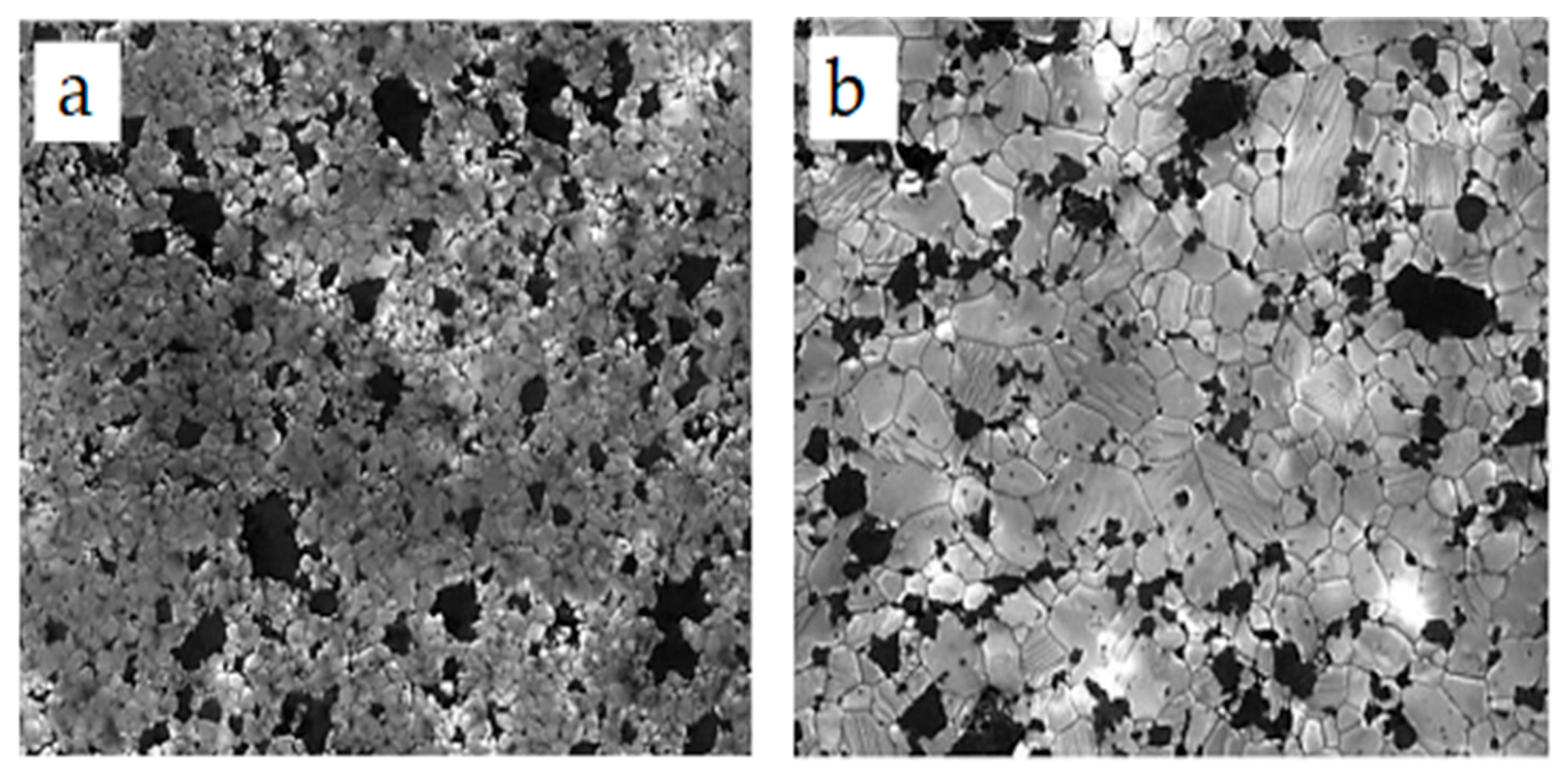
4.1. Microstructures of Alumina-SiC Nanocomposites
4.2. Microstructure of Alumina–CNT Nanocomposites
5. Thermal Properties of Alumina-Based Nanocomposites
5.1. Thermal Properties of Alumina–SiC Nanocomposites
5.1.1. Heat Capacity
5.1.2. Thermal Diffusivity
5.1.3. Thermal Conductivity
5.1.4. Models for Predicting the Thermal Conductivity of Nanocomposites
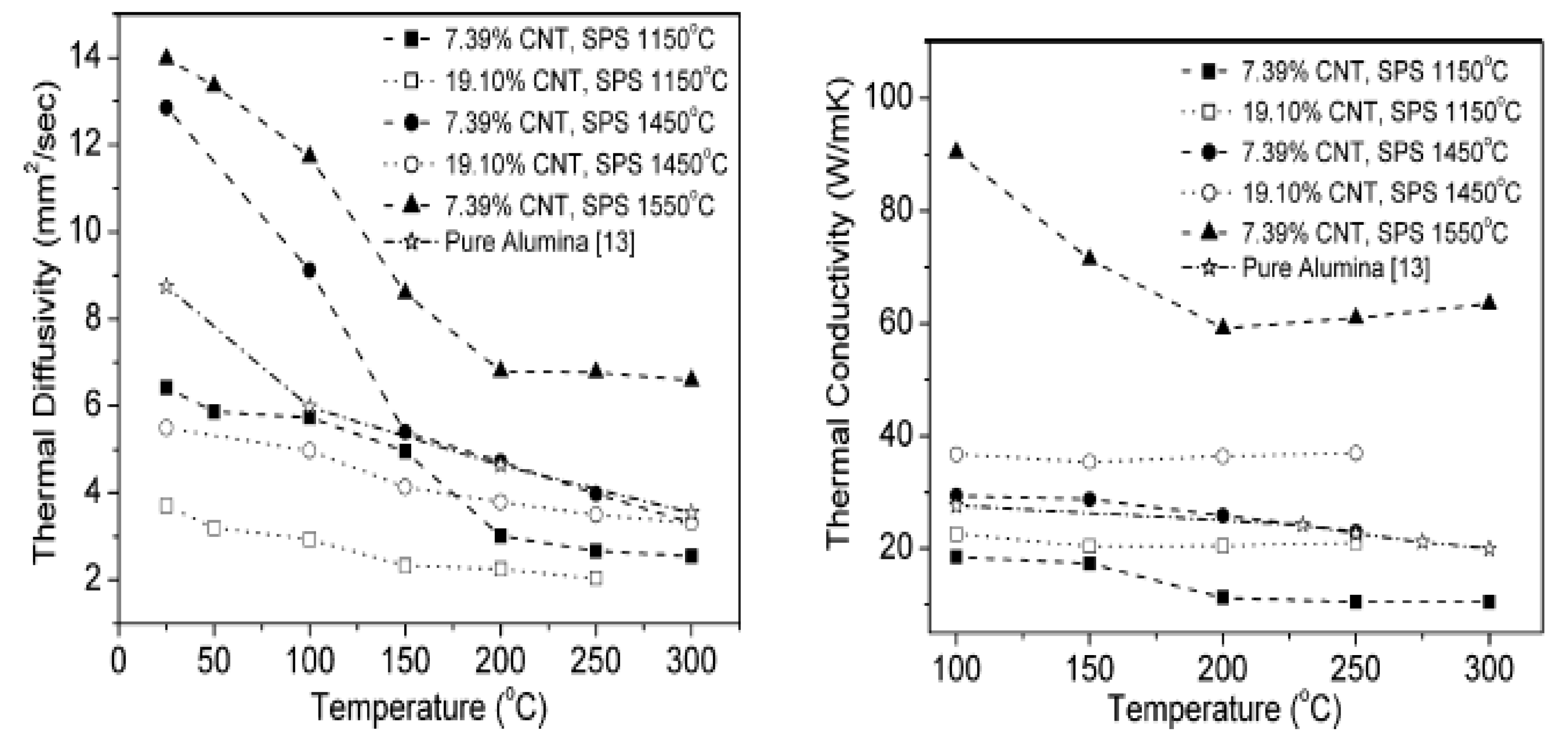
5.2. Thermal Properties of Alumina–CNT Nanocomposites
6. Electrical Properties of Alumina-Based Nanocomposites
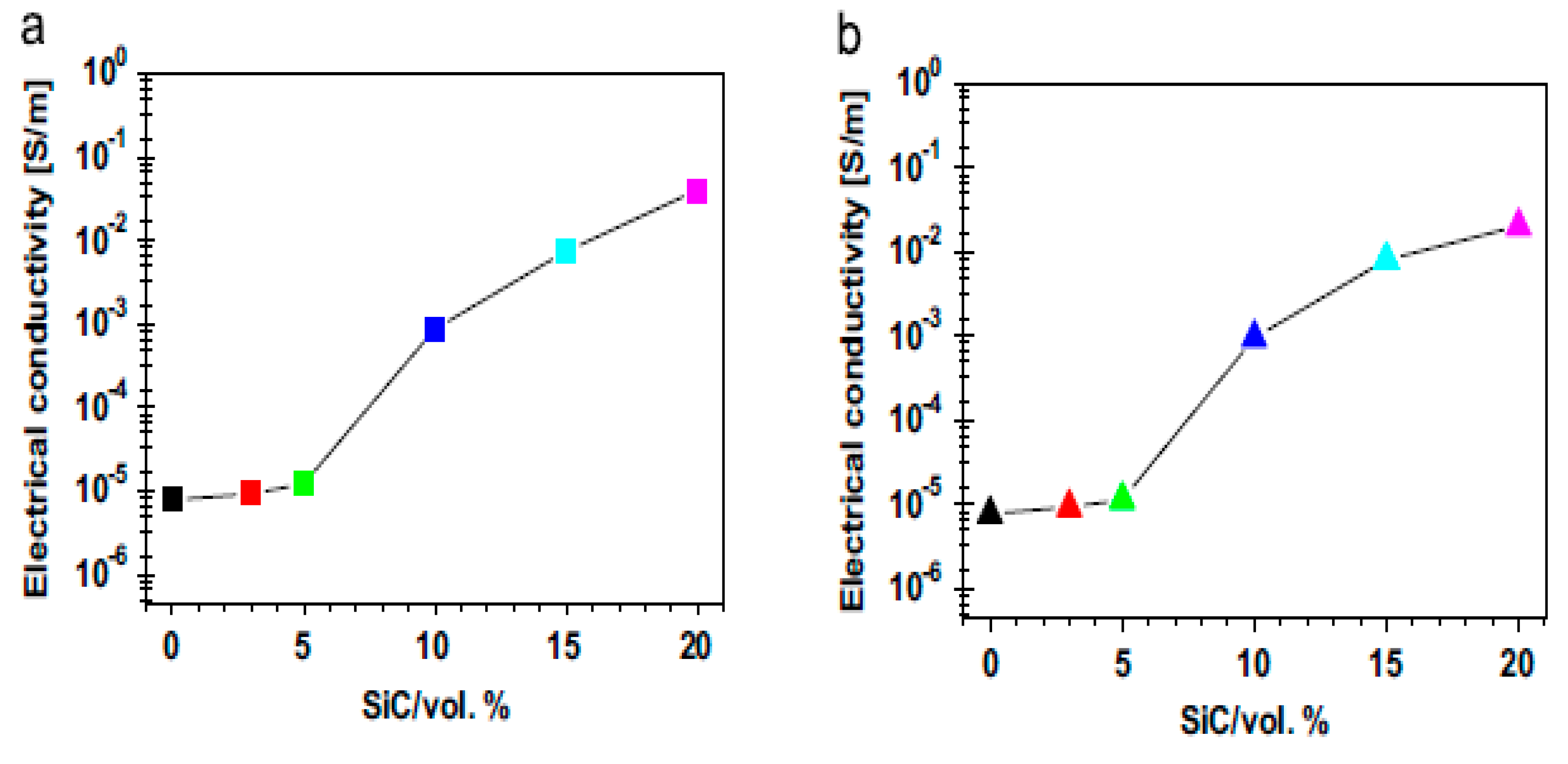
6.1. Electrical Properties of Al2O3–SiC Nanocomposites
6.2. Electrical Conductivity of Alumina–CNT Nanocomposites
7. Applications of Alumina-Based Nanocomposites
8. Conclusions
- (1)
- SiC and CNT must be effectively distributed in alumina to avoid agglomeration.
- (2)
- A combination of techniques, such as sonication and ball milling, is effective in the homogenous distribution of SiC in alumina matrices, especially at low nanophase concentrations.
- (3)
- Ultra-sonication, ball milling and molecular level mixing are suitable for dispersing CNTs in ceramic matrices at low concentrations of CNTs. However, for higher volume fractions of CNTs in alumina matrices, colloidal heterocoagulation and flocculation are highly recommended as means to facilitate homogenous distribution.
- (4)
- SiC and CNT concentrations must be optimized to enhance the mechanical and functional properties.
- (5)
- Appropriate consolidation techniques must be employed to prevent grain growth and the formation of inhomogeneous microstructures that lead to poor mechanical and functional performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, X.L.; Xu, F.M.; Zhang, Z.J.; Dong, Y.L.; Tan, Y.; Wang, L.; Yang, J.M. Mechanical properties of hot-pressed Al2O3/SiC composites. Mater. Sci. Eng. A 2010, 527, 4646–4649. [Google Scholar] [CrossRef]
- Niihara, K. New Design Concept of Structural Ceramics. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Ashby, M.F.; Ferreira, P.J.; Schodek, D.L. Nanomaterials: Classes and Fundamentals. Nanomater. Nanotechnol. Des. 2009, 177–197. [Google Scholar] [CrossRef]
- Chen, F.; Yang, S.; Wu, J.; Perez, J.A.G.; Shen, Q.; Schoenung, J.M.; Lavernia, E.J.; Zhang, L. Spark Plasma Sintering and Densification Mechanisms of Conductive Ceramics under Coupled Thermal/Electric Fields. J. Am. Ceram. Soc. 2015, 98, 732–740. [Google Scholar] [CrossRef]
- Kim, S.W.; Khalil, K.A.-R. High-Frequency Induction Heat Sintering of Mechanically Alloyed Alumina-Yttria-Stabilized Zirconia Nano-Bioceramics. J. Am. Ceram. Soc. 2006, 89, 1280–1285. [Google Scholar] [CrossRef]
- Gazawi, A.A. Microstructure and Mechanical Properties of Aluminum Based Nanocomposites Strengthened with Alumina and Silicon Carbide. Ph.D. Thesis, University of Waikato, Hamilton, New Zealand, June 2014. [Google Scholar]
- Ohji, T.; Jeong, Y.-K.; Choa, Y.-H.; Niihara, K. Strengthening and Toughening Mechanisms of Ceramic Nanocomposites. J. Am. Ceram. Soc. 1998, 60, 1453–1460. [Google Scholar] [CrossRef]
- Wang, D.; Xue, C.; Cao, Y.; Zhao, J. Microstructure design and preparation of Al2O3/TiC/TiN micro-nano-composite ceramic tool materials based on properties prediction with finite element method. Ceram. Int. 2018, 44, 5093–5101. [Google Scholar] [CrossRef]
- Sternitzke, M. Structural ceramic nanocomposites. J. Eur. Ceram. Soc. 1997, 17, 1061–1082. [Google Scholar] [CrossRef]
- Parchovianský, M.; Galusek, D.; Michálek, M.; Švančárek, P.; Kašiarová, M.; Dusza, J.; Hnatko, M. Effect of the volume fraction of SiC on the microstructure and creep behavior of hot pressed Al2O3/SiC composites. Ceram. Int. 2014, 40, 1807–1814. [Google Scholar] [CrossRef]
- Zhao, J.; Steams, L.C.; Martin, I.; Chan, H.M.; Miller, G.A.; Cook, R.E. Mechanical Behavior of Alumina-Silicon Carbide “Nanocomposites”. J. Am. Cream. Soc. 1993. [Google Scholar] [CrossRef]
- Sternitzke, M.; Derby, B.; Brook, R.J. Alumina/Silicon Carbide Nanocomposites by Hybrid Polymer/Powder Processing: Microstructures and Mechanical Properties. Scanning 1998, 48, 41–48. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- van Lier, G.; van Alsenoy, C.; van Doren, V.; Geerlings, P. Ab initio study of the elastic properties of single-walled carbon nanotubes and graphene. Chem. Phys. Lett. 2000, 326, 181–185. [Google Scholar] [CrossRef]
- Yu, M. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Shimoyama, H.; Sakai, G.; Kaneto, K. Physical properties of multiwalled carbon nanotubes. Int. J. Inorg. Mater. 1999, 1, 77–82. [Google Scholar] [CrossRef]
- De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Biercuk, M.J.; Llaguno, M.C.; Radosavljevic, M.; Hyun, J.K.; Johnson, A.T.; Fischer, J.E. Carbon nanotube composites for thermal management. Appl. Phys. Lett. 2002, 80, 2767–2769. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Rousset, A. Carbon nanotubes in novel ceramic matrix nanocomposites. Cream. Int. 2000, 26, 677–683. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Li, W. Colloidal Processing of Carbon Nanotube/Alumina Composites. Chem. Mater. 2002, 14, 5169–5172. [Google Scholar] [CrossRef]
- Rul, S.; Lefèvre-Schlick, F.; Capria, E.; Laurent, C.; Peigney, A. Percolation of single-walled carbon nanotubes in ceramic matrix nanocomposites. Acta Mater. 2004, 52, 1061–1067. [Google Scholar] [CrossRef]
- Shi, S.L.; Liang, J. Effect of multiwall carbon nanotubes on electrical and dielectric properties of yttria-stabilized zirconia ceramic. J. Am. Ceram. Soc. 2006, 89, 3533–3535. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Karandikar, P.G.; Chou, T.-W. Fabrication and characterization of reaction bonded silicon carbide/carbon nanotube composites. J. Phys. D Appl. Phys. 2005, 38, 3962–3965. [Google Scholar] [CrossRef]
- Tatami, J.; Katashima, T.; Komeya, K.; Meguro, T.; Wakihara, T. Electrically conductive CNT-dispersed silicon nitride ceramics. J. Am. Ceram. Soc. 2005, 88, 2889–2893. [Google Scholar] [CrossRef]
- Ighodaro, O.L.; Okoli, O.I. Fracture toughness enhancement for alumina systems: A review. Int. J. Appl. Ceram. Technol. 2008, 5, 313–323. [Google Scholar] [CrossRef]
- Materials, H.; Moradkhani, A.; Baharvandi, H. International Journal of Refractory Metals Microstructural analysis of fracture surfaces and determination of mechanical properties of Al2O3–SiC–MgO nanocomposites. Int. J. Refract. Met. Hard Mater. 2017, 67, 40–55. [Google Scholar] [CrossRef]
- Hasselman, D.P.H.; Johnson, L.F. Effective Thermal Conductivity of Composites with Interfacial Thermal Barrier Resistance. J. Compos. Mater. 1987, 21, 508–515. [Google Scholar] [CrossRef]
- Hasselman, D.P.H. Comment on “Inverse Problem for Composites with Imperfect Interface: Determination of Interfacial Thermal Resistance, Thermal Conductivity of Constituents, and Microstructural Parameters”. J. Am. Ceram. Soc. 2002, 85, 1643–1645. [Google Scholar] [CrossRef]
- Aaoulli, A.; Bai, S.; Cheng, H.M.; Bai, J.B. Mechanical and electrical properties of a MWNT/epoxy composite. Compos. Sci. Technol. 2002, 62, 1993–1998. [Google Scholar] [CrossRef]
- Zheng, G.; Sano, H.; Suzuki, K.; Kobayashi, K.; Uchiyama, Y.; Cheng, H.-M. A TEM study of microstructure of carbon fiber/polycarbosilane-derived SiC composites. Carbon N. Y. 1999, 37, 2057–2062. [Google Scholar] [CrossRef]
- Wei, T.; Fan, Z.; Luo, G.; Wei, F. A new structure for multi-walled carbon nanotubes reinforced alumina nanocomposite with high strength and toughness. Mater. Lett. 2008, 62, 641–644. [Google Scholar] [CrossRef]
- Salvetat, J.-P.; Briggs, G.; Bonard, J.-M.; Bacsa, R.; Kulik, A.; Stöckli, T.; Burnham, N.; Forró, L. Elastic and Shear Moduli of Single-Walled Carbon Nanotube Ropes. Phys. Rev. Lett. 1999, 82, 944–947. [Google Scholar] [CrossRef]
- Prakash, T.; Sivasankaran, S.; Sasikumar, P. Mechanical and Tribological Behaviour of Friction-Stir-Processed Al 6061 Aluminium Sheet Metal Reinforced with Al2O3/0.5Gr Hybrid Surface Nanocomposite. Arab. J. Sci. Eng. 2015, 40, 559–569. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, L. Manufacture and electrical properties of multiwalled carbon nanotube/BaTiO3 nanocomposite ceramics. J. Mater. Chem. 2004, 14, 2536. [Google Scholar] [CrossRef]
- Bala’zsi, C.; Shen, Z.; Ka’nya, Z.; Kasztovszky, Z.; We’ber, F.; Va’rtesy, Z.; Bira’, L.P.; Kiricsi, I.; Arat’a, P. Processing of carbon nanotube reinforced silicon nitride composites by spark plasma sintering. Compos. Sci. Technol. 2005, 65, 727–733. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L. Development of a dispersion process for carbon nanotubes in ceramic matrix by heterocoagulation. Carbon N. Y. 2003, 41, 1063–1068. [Google Scholar] [CrossRef]
- Poyato, R.; Vasiliev, A.L.; Padture, N.P.; Tanaka, H.; Nishimura, T. Aqueous colloidal processing of single-wall carbon nanotubes and their composites with ceramics. Nanotechnology 2006, 17, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.B.; Cha, S.I.; Kim, K.T.; Lee, K.H.; Hong, S.H. Fabrication of carbon nanotube reinforced alumina matrix nanocomposite by sol-gel process. Mater. Sci. Eng. A 2005, 395, 124–128. [Google Scholar] [CrossRef]
- Flahaut, E.; Peigney, A.; Laurent, C.; Marlière, C.; Chastel, F.; Rousset, A. Carbon nanotube–metal–oxide nanocomposites: Microstructure, electrical conductivity and mechanical properties. Acta Mater. 2000, 48, 3803–3812. [Google Scholar] [CrossRef]
- Bala’zsi, C.; Sedla’kov’, K.; Czig’ny, Z. Structural characterization of Si3N4-carbon nanotube interfaces by transmission electron microscopy. Compos. Sci. Technol. 2008, 68, 1596–1599. [Google Scholar] [CrossRef]
- Balani, K.; Bakshi, S.R.; Lahiri, D.; Agarwal, A. Grain growth behavior of aluminum oxide reinforced with carbon nanotube during plasma spraying and PostSpray consolidation. Int. J. Appl. Ceram. Technol. 2010, 7, 846–855. [Google Scholar] [CrossRef]
- An, J.-W.; You, D.-H.; Lim, D.-S. Tribological properties of hot-pressed alumina–CNT composites. Wear 2003, 255, 677–681. [Google Scholar] [CrossRef]
- Venkateswaran, T.; Basu, B.; Raju, G.B.; Kim, D.Y. Densification and properties of transition metal borides-based cermets via spark plasma sintering. J. Eur. Ceram. Soc. 2006, 26, 2431–2440. [Google Scholar] [CrossRef]
- Yamanaka, S.; Gonda, R.; Kawasaki, A.; Sakamoto, H.; Mekuchi, Y.; Kun, M.; Tsukada, T. Fabrication and thermal properties of carbon nanotube/nickel composite by spark plasma sintering method. Mater. Trans. 2007, 48, 2506–2512. [Google Scholar] [CrossRef]
- Balko, J.; Svanˇ, P.; Sedláˇ, J.; Dusza, J.; Lofaj, F.; Galusek, D. Mechanical properties and sliding wear behaviour of Al2O3-SiC nanocomposites with 3–20 vol % SiC. J. Eur. Ceram. Soc. 2017, 37, 4297–4306. [Google Scholar] [CrossRef]
- Chae, J.H.; Kim, K.H.; Choa, Y.H.; Matsushita, J.I.; Yoon, J.W.; Shim, K.B. Microstructural evolution of Al2O3-SiC nanocomposites during spark plasma sintering. J. Alloys Compd. 2006, 413, 259–264. [Google Scholar] [CrossRef]
- Palmero, P. Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods. Nanomaterials 2015, 5, 656–696. [Google Scholar] [CrossRef]
- Lee, K.; Mo, C.B.; Park, S.B.; Hong, S.H. Mechanical and electrical properties of multiwalled CNT-alumina nanocomposites prepared by a sequential two-step processing of ultrasonic spray pyrolysis and spark plasma sintering. J. Am. Ceram. Soc. 2011, 94, 3774–3779. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of Sheet Resistivities with the Four-Point Probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Zulkarnain, M.; Husaini, A.B.M.; Mariatti, M.; Azid, I.A. Particle Dispersion Model for Predicting the Percolation Threshold of Nano-Silver Composite. Arab. J. Sci. Eng. 2015. [Google Scholar] [CrossRef]
- Barzegar-Bafrooei, H.; Ebadzadeh, T. Synthesis of nanocomposite powders of γ-alumina-carbon nanotube by sol-gel method. Adv. Powder Technol. 2011, 22, 366–369. [Google Scholar] [CrossRef]
- Cammarata, R.C. Nanomaterials: Synthesis, Properties and Applications Edited by A S Edelstein Institute of Physics Publishing Bristol and Philadelphia. Mater. Sci. 1996, 27, 1145. [Google Scholar]
- Abdullahi, K.; Al-Aqeeli, N. Mechanical Alloying and Spark Plasma Sintering of Nano-SiC Reinforced Al–12Si–0.3Mg Alloy. Arab. J. Sci. Eng. 2014, 39, 3161–3168. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, Y.; Hu, W. Preparation of alumina/carbon nanotubes composites by chemical precipitation. Ceram. Int. 2009, 35, 1305–1310. [Google Scholar] [CrossRef]
- Skiba, T.; Haušild, P.; Karlík, M.; Vanmeensel, K.; Vleugels, J. Mechanical properties of spark plasma sintered FeAl intermetallics. Intermetallics 2010, 18, 1410–1414. [Google Scholar] [CrossRef]
- Saheb, N. Sintering Behavior of CNT Reinforced Al6061 and Al2124 Nanocomposites. Adv. Mater. Sci. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Nawaz, A.; Aneela, K.; Chuang, H.W. Mechanism of Intercalation Extent in Polymer/Clay Nanocomposites. Arab. J. Sci. Eng. 2015, 40, 3373–3377. [Google Scholar] [CrossRef]
- Mohammad, K.; Saheb, N. Molecular level mixing: An approach for synthesis of homogenous hybrid ceramic nanocomposite powders. Powder Technol. 2015, 291, 121–130. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Kamrani, S.; Riedel, R.; Reihani, S.M.S.; Kleebe, H.J. Effect of Reinforcement Volume Fraction on the Mechanical Properties of Al--SiC Nanocomposites Produced by Mechanical Alloying and Consolidation. J. Compos. Mater. 2010, 44, 313–326. [Google Scholar] [CrossRef]
- Naeem, H.T.; Mohammad, K.S.; Ahmad, K.R.; Rahmat, A. Characteristics of Al–Zn–Mg–Cu Alloys with Nickel Additives Synthesized via Mechanical Alloying, Cold Compaction, and Heat Treatment. Arab. J. Sci. Eng. 2014, 39, 9039–9048. [Google Scholar] [CrossRef]
- Sanders, P.G.; Eastman, J.A.; Weertman, J.R. Elastic and tensile behavior of nanocrystalline copper and palladium. Acta Mater. 1997, 45, 4019–4025. [Google Scholar] [CrossRef]
- Malow, T.R.; Koch, C.C. Mechanical properties in tension of mechanically attrited nanocrystalline iron by the use of the miniaturized disk bend test. Acta Mater. 1998, 46, 6459–6473. [Google Scholar] [CrossRef]
- Gustafsson, S.; Falk, L.K.L.; Lidén, E.; Carlström, E. Pressureless sintered Al2O3-SiC nanocomposites. Ceram. Int. 2008, 34, 1609–1615. [Google Scholar] [CrossRef]
- Qu, H.; Zhu, S.; Li, Q.; Ouyang, C. Microstructure and mechanical properties of hot-pressing sintered WC—x vol .% Al2O3 composites. Mater. Sci. Eng. A 2012, 543, 96–103. [Google Scholar] [CrossRef]
- Pettersson, A.; Magnusson, P.; Lundberg, P.; Nygren, M. Titanium-titanium diboride composites as part of a gradient armour material. Int. J. Impact Eng. 2006, 32, 387–399. [Google Scholar] [CrossRef]
- Dirras, G.; Gubicza, J.; Tingaud, D.; Billard, S. Microstructure of Al-Al2O3 nanocomposite formed by in situ phase transformation during Al nanopowder consolidation. Mater. Chem. Phys. 2011, 129, 846–852. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Bykov, Y.V.; Rybakov, K.I.; Semenov, V.E. Microwave sintering of nanostructured ceramic materials. Nanotechnol. Russ. 2011, 6, 647–661. [Google Scholar] [CrossRef]
- Al, O.; Ghobadi, H.; Ebadzadeh, T.; Sadeghian, Z.; Barzegar-bafrooei, H.; Nemati, A. Microwave-assisted sintering of Al2O3-MWCNT nanocomposites. Ceram. Int. 2017, 43, 6105–6109. [Google Scholar] [CrossRef]
- Viswanathan, V.; Laha, T.; Balani, K.; Agarwal, A.; Seal, S. Challenges and advances in nanocomposite processing techniques. Mater. Sci. Eng. R Rep. 2006, 54, 121–285. [Google Scholar] [CrossRef]
- Ghasali, E.; Alizadeh, M.; Ebadzadeh, T. Mechanical and microstructure comparison between microwave and spark plasma sintering of Al-B4C composite. J. Alloys Compd. 2016, 655, 93–98. [Google Scholar] [CrossRef]
- DHulbert, M.; Jiang, D.; Dudina, D.V.; Mukherjee, A.K. The synthesis and consolidation of hard materials by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2009, 27, 367–375. [Google Scholar] [CrossRef]
- Al-Aqeeli, N.; Abdullahi, K.; Hakeem, A.S.; Suryanarayana, C.; Laoui, T.; Nouari, S. Synthesis, characterisation and mechanical properties of SiC reinforced Al based nanocomposites processed by MA and SPS. Powder Metall. 2013, 56, 149–157. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y.; Nagaoka, T.; Sugioka, M.; Tanaka, M.; Takeuchi, T.; Tani, J.I.; Kawahara, M.; Makino, Y.; et al. Processing of Al/SiC composites in continuous solid-liquid co-existent state by SPS and their thermal properties. Compos. Part B Eng. 2012, 43, 2012–2019. [Google Scholar] [CrossRef]
- Kessel, H.U.; Hennicke, J.; Schmidt, J.; Weißgärber, T.; Kieback, B.F.; Herrmann, M.; Räthel, J. “FAST” field assisted sintering technology—A new process for the production of metallic and ceramic sintering materials. In Pulvermetallurgie in Wissenschaft und Praxis 22—Pulvermetallurgie—Kompetenz und Perspektive; Heimdall Verlag: Witten, Germay, 2008. [Google Scholar]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-Assisted Sintering Technology/Spark Plasma Sintering: Mechanisms, Materials, and Technology Developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Vanmeensel, K.; Laptev, A.; Hennicke, J.; Vleugels, J.; Vanderbiest, O. Modelling of the temperature distribution during field assisted sintering. Acta Mater. 2005, 53, 4379–4388. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Hong, J.; Miyamoto, H.; Miyamoto, K.; Nishikawa, Y.; Torre, S.D.D. Mechanical Properties and Microstructure of Nano-SiC–Al2O3 Composites Densified by Spark Plasma Sintering. J. Eur. Ceram. Soc. 1999, 19, 609–613. [Google Scholar] [CrossRef]
- Wang, H.Z.; Gao, L.; Guo, J.K. Effect of nanoscale SiC particles on the microstructure of Al2O3 ceramics. Ceram. Int. 2000, 26, 391–396. [Google Scholar] [CrossRef]
- Maensiri, S.; Roberts, S.G. Thermal Shock Resistance of Sintered Alumina/Silicon Carbide Nanocomposites Evaluated by Indentation Techniques. J. Am. Ceram. Soc. 2002, 85, 1971–1978. [Google Scholar] [CrossRef]
- Kara, H.; Roberts, S.G. Polishing behavior and surface quality of alumina and alumina/silicon carbide nanocomposites. J. Am. Ceram. Soc. 2000, 83, 1219–1225. [Google Scholar] [CrossRef]
- Parchovianský, M.; Galusek, D.; Švančárek, P.; Sedláček, J.; Šajgalík, P. Thermal behavior, electrical conductivity and microstructure of hot pressed Al2O3/SiC nanocomposites. Ceram. Int. 2014, 40, 14421–14429. [Google Scholar] [CrossRef]
- Borrell, A.; Álvarez, I.; Torrecillas, R.; Rocha, V.G.; Fernández, A. Microstructural design for mechanical and electrical properties of spark plasma sintered Al2O3–SiC nanocomposites. Mater. Sci. Eng. A 2012, 534, 693–698. [Google Scholar] [CrossRef]
- Takahashi, K.; Yokouchi, M.; Lee, S.; Ando, K. Crack-Healing Behavior of Al2O3 Toughened by SiC Whiskers. Ceramics 2003, 47, 2143–2147. [Google Scholar] [CrossRef]
- Sglavo, V.M.; de Genua, F.; Molinari, A.; Casari, F. Alumina/silicon carbide laminated composites by spark plasma sintering. J. Am. Ceram. Soc. 2009, 92, 2693–2697. [Google Scholar] [CrossRef]
- Nieto, M.I.; Miranzo, P.; Aza, D.; Moya, J.S. Effect of Atmosphere on Microstructural Evolution of Pressureless Sintered Sintered Al2O3/SiC Composites. J. Ceram. Soc. Jpn. 1992, 100, 459–462. [Google Scholar] [CrossRef]
- Oh, S.T.; Tajima, K.I.; Ando, M.; Ohji, T. Strengthening of porous alumina by pulse electric current sintering and nanocomposite processing. J. Am. Ceram. Soc. 2000, 83, 1314–1316. [Google Scholar] [CrossRef]
- NAKAHIRA, A. Sintering Behaviors and Consolidation for Al2O3/SiC Nanocomposites. J. Ceram. Soc. Jpn. 1992, 100, 448–453. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Yan, H.; Jiang, K. Spark plasma sintering of alumina composites with graphene platelets and silicon carbide nanoparticles. Adv. Eng. Mater. 2014, 1–8. [Google Scholar] [CrossRef]
- Watanabe, C.; Kimura, O. Sintering and Mechanical Properties of SiC-Whisker/Al2O3 Composites with Two-Layered Structure Society; Ashikaga of Technology of Materials Science and Ceramic Technology of Technology: Kanagawa, Japan, 1992; Volume 592. [Google Scholar]
- Sciti, D.; Vicens, J.; Bellosi, A. Microstructure and properties of alumina-SiC nanocomposites prepared from ultrafine powders. J. Mater. Sci. 2002, 37, 3747–3758. [Google Scholar] [CrossRef]
- Ko, Y.M.; Kwon, W.T.; Kim, Y.W. Development of Al2O3-SiC composite tool for machining application. Ceram. Int. 2004, 30, 2081–2086. [Google Scholar] [CrossRef]
- Sun, X.; Bao, W.; Lv, Y.; Deng, J.; Wang, X. Synthesis of high quality single-walled carbon nanotubes by arc discharge method in large scale. Mater. Lett. 2007, 61, 3956–3958. [Google Scholar] [CrossRef]
- Hou, P.-X.; Liu, C.; Cheng, H.-M. Purification of carbon nanotubes. Carbon N. Y. 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Ayala, P.; Grüneis, A.; Grimm, D.; Kramberger, C.; Engelhard, R.; Rümmeli, M.; Schumann, J.; Kaltofen, R.; Büchner, B.; Schaman, C.; et al. Cyclohexane triggers staged growth of pure and vertically aligned single wall carbon nanotubes. Chem. Phys. Lett. 2008, 454, 332–336. [Google Scholar] [CrossRef]
- Meng, L.; Fu, C.; Lu, Q. Advanced technology for functionalization of carbon nanotubes. Prog. Nat. Sci. 2009, 19, 801–810. [Google Scholar] [CrossRef]
- Rubio, N.; Fabbro, C.; Herrero, M.A.; de la Hoz, A.; Meneghetti, M.; Fierro, J.L.G.; Prato, M.; Vazquez, E. Ball-milling modification of single-walled carbon nanotubes: Purification, cutting, and functionalization. Small 2011, 7, 665–674. [Google Scholar] [CrossRef]
- Saito, T.; Matsushige, K.; Tanaka, K. Chemical treatment and modification of multi-walled carbon nanotubes. Phys. B Condens. Matter. 2002, 323, 280–283. [Google Scholar] [CrossRef]
- Mansoor, M.; Shahid, M.; Habib, A. Strengthening of Bisphenol-A Epoxy Resin by the Addition of Multi-Wall Carbon Nanotubes. Arab. J. Sci. Eng. 2014, 39, 6411–6420. [Google Scholar] [CrossRef]
- Ma, P.C.; Wang, S.Q.; Kim, J.-K.; Tang, B.Z. In-Situ Amino Functionalization of Carbon Nanotubes Using Ball Milling. J. Nanosci. Nanotechnol. 2009, 9, 749–753. [Google Scholar] [CrossRef]
- Inam, F.; Heaton, A.; Brown, P.; Peijs, T.; Reece, M.J. Effects of dispersion surfactants on the properties of ceramic-carbon nanotube (CNT) nanocomposites. Ceram. Int. 2014, 40, 511–516. [Google Scholar] [CrossRef]
- Hanzel, O.; Sedláček, J.; Šajgalík, P. New approach for distribution of carbon nanotubes in alumina matrix. J. Eur. Ceram. Soc. 2014, 34, 1845–1851. [Google Scholar] [CrossRef]
- Reinert, L.; Zeiger, M.; Suárez, S.; Presser, V.; Mücklich, F. Dispersion analysis of carbon nanotubes, carbon onions, and nanodiamonds for their application as reinforcement phase in nickel metal matrix composites. RSC Adv. 2015, 5, 95149–95159. [Google Scholar] [CrossRef]
- Bakhsh, N.; Khalid, F.A.; Hakeem, A.S. Effect of sintering temperature on densification and mechanical properties of pressureless sintered CNT-alumina nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2014, 60, 012059. [Google Scholar] [CrossRef]
- Tonello, K.P.d.S.; Trombini, V.; Bressiani, A.H.d.; Bressiani, J.C. Ceramic Processing of NBC Nanometric Powders Obtained by High Energy Milling and by Reactive Milling. Mater. Sci. Forum. 2012, 727–728, 909–913. [Google Scholar] [CrossRef]
- Aminzare, M.; Eskandari, A.; Baroonian, M.H.; Berenov, A.; Hesabi, Z.R. Hydroxyapatite nanocomposites: Synthesis, sintering and mechanical properties. Ceram. Int. 2013, 39, 2197–2206. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, P. Processing and properties of carbon nanotube/alumina nanocomposites: A review. Rev. Adv. Mater. Sci. 2014, 37, 53–82. [Google Scholar]
- Zhang, T.; Kumari, L.; Du, G.H.; Li, W.Z.; Wang, Q.W.; Balani, K.; Agarwal, A. Mechanical properties of carbon nanotube-alumina nanocomposites synthesized by chemical vapor deposition and spark plasma sintering. Compos. Part A Appl. Sci. Manuf. 2009, 40, 86–93. [Google Scholar] [CrossRef]
- Kumari, L.; Zhang, T.; Du, G.; Li, W.; Wang, Q.; Datye, A.; Wu, K. Thermal properties of CNT-Alumina nanocomposites. Compos. Sci. Technol. 2008, 68, 2178–2183. [Google Scholar] [CrossRef]
- Inam, F.; Yan, H.; Peijs, T.; Reece, M.J. The sintering and grain growth behaviour of ceramic-carbon nanotube nanocomposites. Compos. Sci. Technol. 2010, 70, 947–952. [Google Scholar] [CrossRef]
- Inam, F.; Yan, H.; Jayaseelan, D.D.; Peijs, T.; Reece, M.J. Electrically conductive alumina-carbon nanocomposites prepared by Spark Plasma Sintering. J. Eur. Ceram. Soc. 2010, 30, 153–157. [Google Scholar] [CrossRef]
- Zhan, G.-D.; Mukherjee, A.K. Carbon nanotube reinforced alumina-based ceramics with novel mechanical, electrical, and thermal properties. Int. J. Appl. Ceram. Technol. 2004, 1, 161–171. [Google Scholar] [CrossRef]
- Saheb, N.; Mohammad, K. Hard and Tough Al2O3-SiC-CNT Hybrid Nanocomposite Produced by Molecular Level Mixing and Spark Plasma Sintering. J. Ceram. Int. 2018, 54, 401–410. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures and Nanomaterials—Synthesis, Properties and Applications; World Scientific Publishers: Singapore, 2004. [Google Scholar]
- De Genua, F.; Sglavo, V.M. High Strength Engineered Alumina-Silicon Carbide Laminated Composites by Spark Plasma Sintering. Procedia Eng. 2011, 10, 2621–2626. [Google Scholar] [CrossRef]
- Unlu, M.D.; Goller, G.; Yucel, O.; Sahin, F.C. The Spark Plasma Sintering of Silicon Carbide Ceramics Using Alumina. Acta Phys. Pol. A 2014, 125, 257–259. [Google Scholar] [CrossRef]
- Johnson, O.T.; Rokebrand, P.; Sigalas, I. Microstructure and Properties of Al2O3–SiC. In Proceedings of the World Congress on Engineering, London, UK, 2–4 July 2014; Volume II, pp. 3–6. [Google Scholar]
- Sikder, P.; Sarkar, S.; Biswas, K.G.; Das, S.; Basu, S.; Das, P.K. Improved densification and mechanical properties of spark plasma sintered carbon nanotube reinforced alumina ceramics. Mater. Chem. Phys. 2016, 170, 99–107. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.G.; Guo, S.; Xiu, Z.; Duan, K.; Hu, X.Z. Intragranular particle residual stress strengthening of Al2O3-SiC nanocomposites. J. Am. Ceram. Soc. 2005, 88, 1536–1543. [Google Scholar] [CrossRef]
- Ohji, T.; Nakahira, A.; Hirano, T.; Niihara, K. Tensile creep behavior of alumina-silicon carbide nanocomposite. J. Eur. Ceram. Soc. 1994, 77, 3259–3262. [Google Scholar] [CrossRef]
- Belmonte, M.; Nieto, M.I.; Osendi, M.I.; Miranzo, P. Influence of the SiC grain size on the wear behaviour of Al2O3/SiC composites. J. Eur. Ceram. Soc. 2006, 26, 1273–1279. [Google Scholar] [CrossRef]
- Sakka, Y.; Suzuki, T.S.; Uchikoshi, T. Fabrication and some properties of textured alumina-related compounds by colloidal processing in high-magnetic field and sintering. J. Eur. Ceram. Soc. 2008, 28, 935–942. [Google Scholar] [CrossRef]
- Echeberria, J.; Ollo, J.; Bocanegra-Bernal, M.H.; Garcia-Reyes, A.; Dominguez-Rios, C.; Aguilar-Elguezabal, A.; Reyes-Rojas, A. Sinter and hot isostatic pressing (HIP) of multi-walled carbon nanotubes (MWCNTs) reinforced ZTA nanocomposites: Microstructure and fracture toughness. Int. J. Refract. Metals Hard Mater. 2010, 28, 399–406. [Google Scholar] [CrossRef]
- Yazdani, B.; Porwal, H.; Xia, Y.; Yan, H.; Reece, M.J.; Zhu, Y. Role of synthesis method on microstructure and mechanical properties of graphene/carbon nanotube toughened Al2O3 nanocomposites. Ceram. Int. 2015, 41, 9813–9822. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Lee, K.H.; Mo, C.B.; Hong, S.H. Strengthening and toughening of carbon nanotube reinforced alumina nanocomposite fabricated by molecular level mixing process. Scr. Mater. 2005, 53, 793–797. [Google Scholar] [CrossRef]
- Ahmad, I.; Cao, H.; Chen, H.; Zhao, H.; Kennedy, A.; Zhu, Y.Q. Carbon nanotube toughened aluminium oxide nanocomposite. J. Eur. Ceram. Soc. 2010, 30, 865–873. [Google Scholar] [CrossRef]
- Aguilar-Elguézabal, A.; Bocanegra-Bernal, M.H. Fracture behaviour of α-Al2O3 ceramics reinforced with a mixture of single-wall and multi-wall carbon nanotubes. Compos. Part B Eng. 2014, 60, 463–470. [Google Scholar] [CrossRef]
- Zhang, S.C.; Fahrenholtz, W.G.; Hilmas, G.E.; Yadlowsky, E.J. Pressureless sintering of carbon nanotube-Al2O3 composites. J. Eur. Ceram. Soc. 2010, 30, 1373–1380. [Google Scholar] [CrossRef]
- Reveron, H.; Zaafrani, O.; Fantozzi, G. Microstructure development, hardness, toughness and creep behaviour of pressureless sintered alumina/SiC micro-nanocomposites obtained by slip-casting. J. Eur. Ceram. Soc. 2010, 30, 1351–1357. [Google Scholar] [CrossRef]
- Saheb, N.; Mohammad, K. Microstructure and mechanical properties of spark plasma sintered Al2O3-SiC-CNTs hybrid nanocomposites. Ceram. Int. 2016, 42, 12330–12340. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, J.F.; Yao, K.F.; Chen, L.D. Spark plasma sintering and thermal conductivity of carbon nanotube bulk materials. J. Appl. Phys. 2005, 97. [Google Scholar] [CrossRef]
- Saheb, N.; Hayat, U. Electrical conductivity and thermal properties of spark plasma sintered Al2O3-SiC-CNT hybrid nanocomposites. Ceram. Int. 2017, 43, 5715–5722. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Subhani, T.; Saeed, K.; Islam, M.; Wang, N.; Zhu, Y. Synergic in fl uence of MWCNTs and SiC nanoparticles on the microstructure and properties of Al2O3 ceramic hybrid nanocomposites. Curr. Appl. Phys. 2016, 16, 1649–1658. [Google Scholar] [CrossRef]
- Dong, Y.L.; Xu, F.M.; Shi, X.L.; Zhang, C.; Zhang, Z.J.; Yang, J.M.; Tan, Y. Fabrication and mechanical properties of nano-/micro-sized Al2O3/SiC composites. Mater. Sci. Eng. A 2009, 504, 49–54. [Google Scholar] [CrossRef]
- Rodriguez, J.; Martın, A.; Pastor, J.Y.; Llorca, J.; Bartolome, J.F.; Moya, S. Sliding Wear of Alumina/Silicon Carbide Nanocomposites. J. Am. Ceram. Soc. 1999, 82, 2252–2254. [Google Scholar] [CrossRef]
- Yazdi, A.R.; Baharvandi, H.; Abdizadeh, H.; Purasad, J.; Fathi, A.; Ahmadi, H. Effect of sintering temperature and siliconcarbide fraction on density, mechanical properties and fracture mode of alumina-silicon carbide micro/nanocomposites. Mater. Des. 2012, 37, 251–255. [Google Scholar] [CrossRef]
- Yamamoto, G.; Omori, M.; Hashida, T.; Kimura, H. A novel structure for carbon nanotube reinforced alumina composites with improved mechanical properties. Nanotechnology 2008, 19, 315708. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; You, D.H.; Choi, H.J.; Lim, S.H.; Jang, H. Effect of CNT distribution on tribological behavior of alumina-CNT composites. Wear 2005, 259, 539–544. [Google Scholar] [CrossRef]
- Hanzel, O.; Sedláˇ, J.; Tatarková, M.; Sajgalík, P. Mechanical and tribological properties of alumina-MWCNTs composites sintered by rapid hot-pressing. J. Eur. Ceram. Soc. 2017, 37, 4821–4831. [Google Scholar] [CrossRef]
- Rahman, O.S.A.; Sribalaji, M.; Mukherjee, B.; Laha, T.; Kumar, A. Synergistic eff ect of hybrid carbon nanotube and graphene nanoplatelets reinforcement on processing, microstructure, interfacial stress and mechanical properties of Al2O3 nanocomposites. Ceram. Int. 2018, 44, 2109–2122. [Google Scholar] [CrossRef]
- Mudimela, P.R.; Nasibulina, L.I.; Nasibulin, A.G.; Cwirzen, A.; Valkeapaa, M.; Habermehl-Cwirzen, K.; Malm, J.E.M.; Karppinen, M.J.; Penttala, V.; Koltsova, T.S.; et al. Synthesis of carbon nanotubes and nanofibers on silica and cement matrix materials. J. Nanomater. 2009, 2009, 526128. [Google Scholar] [CrossRef]
- Liu, Y.; Ramirez, C.; Zhang, L.; Wu, W.; Padture, N.P. Acta Materialia In situ direct observation of toughening in isotropic nanocomposites of alumina ceramic and multiwall carbon nanotubes. Acta Mater. 2017, 127, 203–210. [Google Scholar] [CrossRef]
- Ahmad, I.; Unwin, M.; Cao, H.; Chen, H.; Zhao, H.; Kennedy, A.; Zhu, Y.Q. Multi-walled carbon nanotubes reinforced Al2O3 nanocomposites: Mechanical properties and interfacial investigations. Compos. Sci. Technol. 2010, 70, 1199–1206. [Google Scholar] [CrossRef]
- Ghobadi, H.; Nemati, A.; Ebadzadeh, T.; Sadeghian, Z.; Barzegar-Bafrooei, H. Improving CNT distribution and mechanical properties of MWCNT reinforced alumina matrix. Mater. Sci. Eng. A 2014, 617, 110–114. [Google Scholar] [CrossRef]
- Yamamoto, G.; Shirasu, K.; Nozaka, Y.; Wang, W.; Hashida, T. Microstructure-property relationships in pressureless-sintered carbon nanotube/alumina composites. Mater. Sci. Eng. A 2014, 617, 179–186. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, P.K. Effect of sintering temperature and nanotube concentration on microstructure and properties of carbon nanotube/alumina nanocomposites. Ceram. Int. 2014, 40, 7449–7458. [Google Scholar] [CrossRef]
- Winarto, W.; Priadi, D.; Sofyan, N.; Wicaksono, A. Wear Resistance and Surface Hardness of Carbon Nanotube Reinforced Alumina Matrix Nanocomposite by Cold Sprayed Process. Procedia Eng. 2017, 170, 108–112. [Google Scholar] [CrossRef]
- Michálek, M.; Sedláček, J.; Parchoviansky, M.; Michálková, M.; Galusek, D. Mechanical properties and electrical conductivity of alumina/MWCNT and alumina/zirconia/MWCNT composites. Ceram. Int. 2014, 40, 1289–1295. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, P.K. Statistical analysis of mechanical properties of pressureless sintered multiwalled carbon nanotube/alumina nanocomposites. Mater. Chem. Phys. 2012, 137, 511–518. [Google Scholar] [CrossRef]
- Zakeri, M.; Shademan, M. Effect of Ceramic Particulate on the Mechanical Properties of PVP–HA–Alumina Nanocomposite. Arab. J. Sci. Eng. 2013, 39, 2227–2233. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Chen, X.; Wen, Y.; Jia, Q. Evolution of phase composition and microstructure of commercial Al2O3gel in different heat treatment condition. Ceram. Int. 2018, 44, 7883–7890. [Google Scholar] [CrossRef]
- Varo, T.; Canakci, A. Effect of the CNT Content on Microstructure, Physical and Mechanical Properties of Cu-Based Electrical Contact Materials Produced by Flake Powder Metallurgy. Arab. J. Sci. Eng. 2015, 40, 2711–2720. [Google Scholar] [CrossRef]
- Kumari, L.; Zhang, T.; Du, G.H.; Li, W.Z.; Wang, Q.W.; Datye, A.; Wu, K.H. Synthesis, microstructure and electrical conductivity of carbon nanotube-alumina nanocomposites. Ceram. Int. 2009, 35, 1775–1781. [Google Scholar] [CrossRef]
- Fabbri, L.; Scafè, E.; Dinelli, G. Thermal and elastic properties of alumina-silicon carbide whisker composites. J. Eur. Ceram. Soc. 1994, 14, 441–446. [Google Scholar] [CrossRef]
- Krukowski, S.; Witek, A.; Adamczyk, J.; Jun, J.; Bockowski, M.; Grzegory, I.; Lucznik, B.; Nowak, G.; Wróblewski, M.; Presz, A.; et al. Thermal properties of indium nitride. J. Phys. Chem. Solids 1998, 59, 289–295. [Google Scholar] [CrossRef]
- Castle, N. Thermal Conductivity and Thermal Diffusivity. Available online: www.tainstruments.com (accessed on 25 November 2014).
- Wang, W.; Yamamoto, G.; Shirasu, K.; Nozaka, Y.; Hashida, T. Effects of processing conditions on microstructure, electrical conductivity and mechanical properties of MWCNT/alumina composites prepared by flocculation. J. Eur. Ceram. Soc. 2015, 35, 3903–3908. [Google Scholar] [CrossRef]
- Callister, W.D. Fundamentals of Materials Science and Engineering: An Interactive e. Text; John Wiely: New York, NY, USA, 2001. [Google Scholar]
- Callister, W.D. Materials Science and Engineering an Introduction, 6th ed.; John Wiely: New York, NY, USA, 2003; pp. 481–526. [Google Scholar]
- McCullough, R.L. Generalized combining rules for predicting transport properties of composite materials. Compos. Sci. Technol. 1985, 22, 3–21. [Google Scholar] [CrossRef]
- Progelhof, R.C.; Throne, J.L.; Ruetsch, R.R. Methods for predicting the thermal conductivity of composite systems: A review. Polym. Eng. Sci. 1976, 16, 615–625. [Google Scholar] [CrossRef]
- Cheng, S.C.; Vachon, R.I. The prediction of the thermal conductivity of two and three phase solid heterogeneous mixtures. Int. J. Heat Mass Transf. 1969, 12, 249–264. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Two-Com Ponent Systems. I EC Fundument. 1959, 1, 187–191. [Google Scholar] [CrossRef]
- Nielsen, L.E. Thermal conductivity of particulate-filled polymers. J. Appl. Polym. Sci. 1973, 17, 3819–3820. [Google Scholar] [CrossRef]
- Nielsen, L.E. The Thermal and Electrical Conductivity of Two-Phase Systems. Ind. Eng. Chem. Fundam. 1974, 13, 17–20. [Google Scholar] [CrossRef]
- Barea, R.; Belmonte, M.; Osendi, M.I.; Miranzo, P. Thermal conductivity of Al2O3/SiC platelet composites. J. Eur. Ceram. Soc. 2003, 23, 1773–1778. [Google Scholar] [CrossRef]
- Mccluskey, P.H.; Williams, R.K.; Graves, R.S.; Tiegs, T.N. Thermal Diffusivity/Conductivity of Alumina-Silicon Carbide Composites. J. Am. Cream. Soc. 1990, 64, 461–464. [Google Scholar] [CrossRef]
- Haselman, D.P.H.; Donaldson, K.Y.; Geiger, A.L. Effect of Reinforcement Particle Size on the Thermal Conductivity of a Particulate-Silicon Carbide-Reinforced Aluminum Matrix Composite. J. Am. Ceram. Soc. 1992, 75, 3137–3140. [Google Scholar] [CrossRef]
- Kawai, C. Effect of Interfacial Reaction on the Thermal Conductivity of Al–SiC Composites with SiC Dispersions. J. Am. Ceram. Soc. 2001, 84, 896–898. [Google Scholar] [CrossRef]
- Park, T.; Banerjee, S.; Hemraj-Benny, T.; Wang, S.S. Purification Strategies and Purity Visualization techniques for Single-walled Carbon Canotubes. J. Mater. Chem. 2006, 16, 141–154. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic crystals with high thermal. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Watari, K. High thermal conductivity Non-oxide Ceramics J. Ceram. Soc. Jpn. 2001, 1091, 7–16. [Google Scholar] [CrossRef]
- Shin, J.H.; Choi, J.; Kim, M.; Hong, S.H. Comparative study on carbon nanotube- and reduced graphene oxide-reinforced alumina ceramic composites. Ceram. Int. 2018, 44, 8350–8357. [Google Scholar] [CrossRef]
- Duong, H.M.; Papavassiliou, D.V.; Mullen, K.J.; Wardle, B.L. The Prediction of Thermal Properties of Single Walled Carbon Nanotube Suspensions. 2007. Semantic Scholar Web Site. Available online: https://www.semanticscholar.org/paper/Prediction-of-thermal-properties-of-single-walled-Duong-Papavassiliou/94d5c886cb7113b463038d49028de05320aa4172 (accessed on 14 June 2007).
- Hone, J.; Llaguno, M.C.; Nemes, N.M.; Johnson, A.T.; Fischer, J.E.; Walters, D.A.; Casavant, M.J.; Schmidt, J.; Smalley, R.E. Electrical and thermal transport properties of magnetically aligned single wall carbon nanotube films. Appl. Phys. Lett. 2000, 77, 666–668. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Farooq, U.; Subhani, T. Effect of Multiwall Carbon Nanotubes on the Ablative Properties of Carbon Fiber-Reinforced Epoxy Matrix Composites. Arab. J. Sci. Eng. 2015, 40, 1529–1538. [Google Scholar] [CrossRef]
- Sivakumar, R.; Guo, S.; Nishimura, T.; Kagawa, Y. Thermal conductivity in multi-wall carbon nanotube/silica-based nanocomposites. Scr. Mater. 2007, 56, 265–268. [Google Scholar] [CrossRef]
- Ho, K.H.; Newman, S.T. State of the art electrical discharge machining (EDM). Int. J. Mach. Tools Manuf. 2003, 43, 1287–1300. [Google Scholar] [CrossRef]
- Berkowitz, B. Analysis of Fracture Network Connectivity Using Percolation Theory. Math. Geol. 1995, 27, 467–483. [Google Scholar] [CrossRef]
- McLachlan, D.S.; Sauti, G. The AC and DC conductivity of nanocomposites. J. Nanomater. 2007, 2007. [Google Scholar] [CrossRef]
- Lux, F. Models proposed to explain the electrical conductivity of mixtures made of conductive and insulating materials. J. Mater. Sci. 1993, 28, 285–301. [Google Scholar] [CrossRef]
- Sawaguchi, A.; Toda, K.; Niihara, K. Mechanical and Electrical properties of silicon nitride-silicon carbide Nanocomposite Materials. J. Am. Ceram. Soc. 1991, 74, 1142–1144. [Google Scholar] [CrossRef]
- Sheng, P. Fluctuation-induced tunneling conduction in disordered materials. Phys. Rev. B 1980, 21, 2180–2195. [Google Scholar] [CrossRef]
- Ahmad, K.; Pan, W. Dramatic effect of multiwalled carbon nanotubes on the electrical properties of alumina based ceramic nanocomposites. Compos. Sci. Technol. 2009, 69, 1016–1021. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, P.K. Role of interface on electrical conductivity of carbon nanotube/alumina nanocomposite. Ceram. Int. 2014, 40, 2723–2729. [Google Scholar] [CrossRef]
- Guo, S.; Sivakumar, R.; Kitazawa, H.; Kagawa, Y. Electrical properties of silica-based nanocomposites with multiwall carbon nanotubes. J. Am. Ceram. Soc. 2007, 90, 1667–1670. [Google Scholar] [CrossRef]
- Poorteman, M.; Traianidis, M.; Bister, G.; Cambier, F. Colloidal processing, hot pressing and characterisation of electroconductive MWCNT-alumina composites with compositions near the percolation threshold. J. Eur. Ceram. Soc. 2009, 29, 669–675. [Google Scholar] [CrossRef]
- Ukai, T.; Sekino, T.; Hirvonen, A.T.; Tanaka, N.; Kusunose, T.; Nakayama, T.; Niihara, K. Preparation and Electrical Properties of Carbon Nanotubes Dispersed Zirconia Nanocomposites. Key Eng. Mater. 2006, 317–318, 661–664. [Google Scholar] [CrossRef]
- Abid, P.; Sehrawat, S.S.; Islam, P.; Gulati, M.; Talib, P.; Mishra, M. Khanuja: CNTs loading dependence sensor performance Analysis. Sens. Actuators A Phys. 2018, 269, 62–69. [Google Scholar] [CrossRef]
- Sehrawat, P.; Islam, S.S.; Mishra, P.; Khanuja, M. A multi-prong approach towards the development of high performance Temperature sensor using MWCNTs/Al2O3 composite fi lm. Mater. Res. Bull. 2018, 99, 1–9. [Google Scholar] [CrossRef]

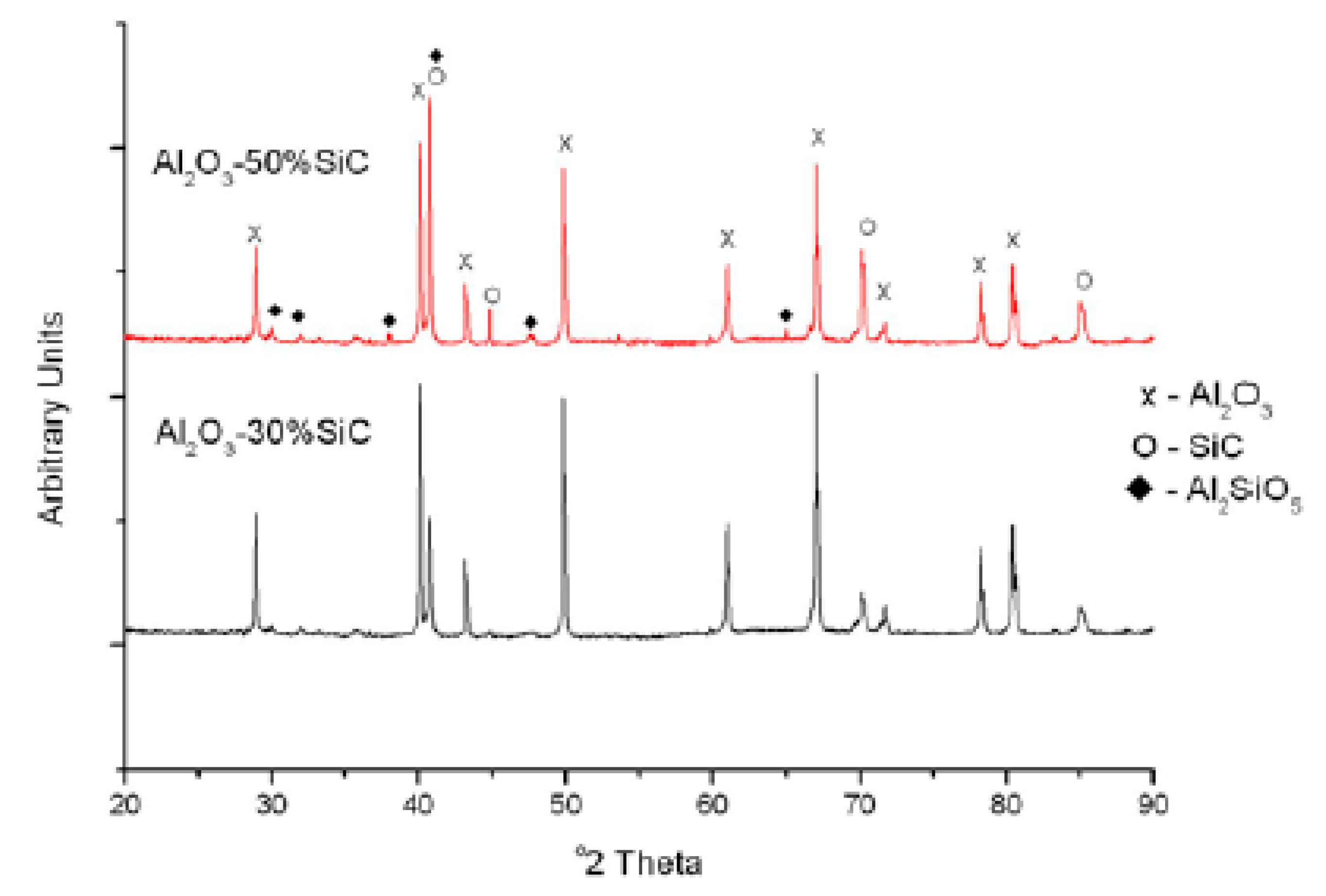
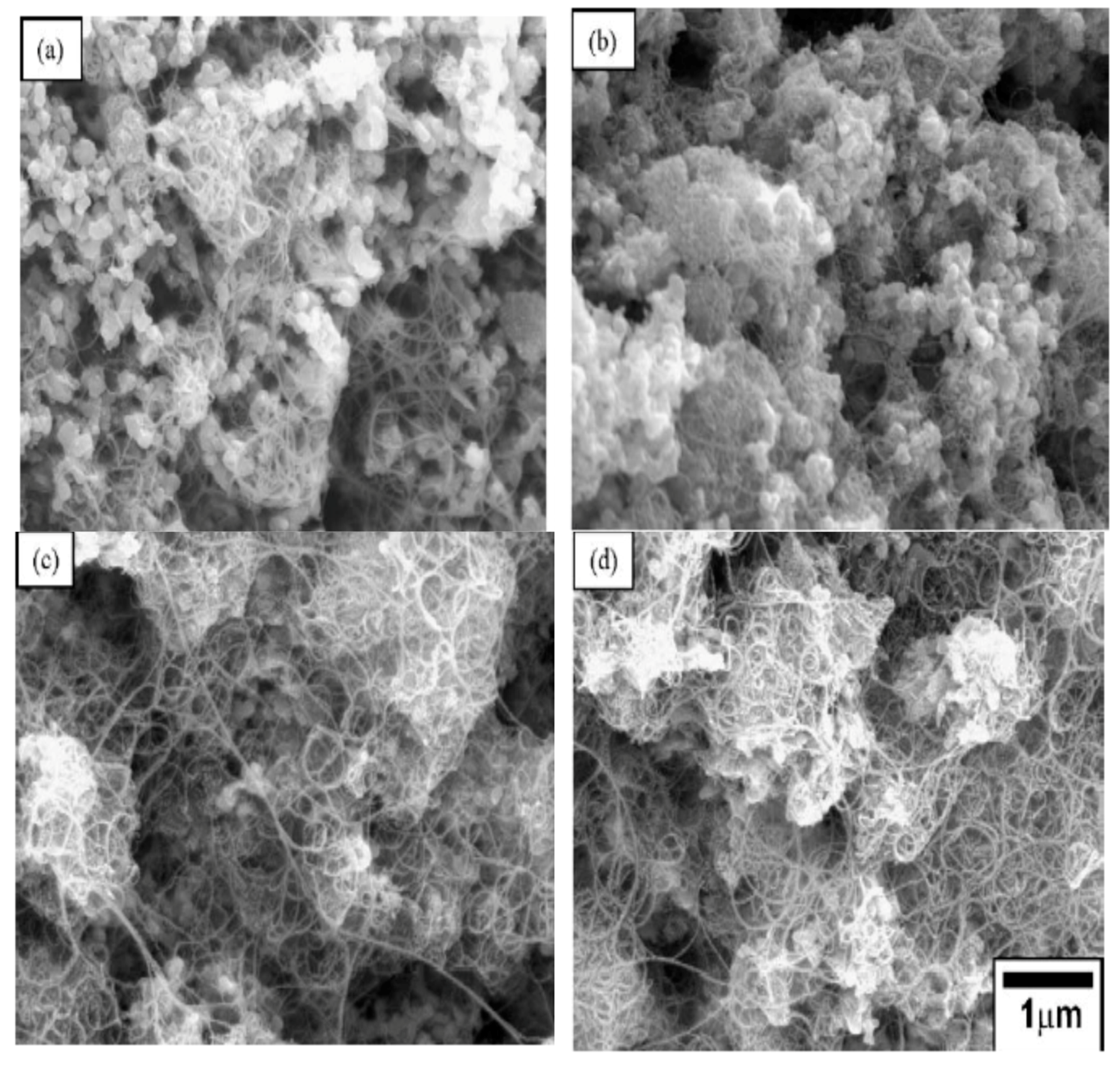

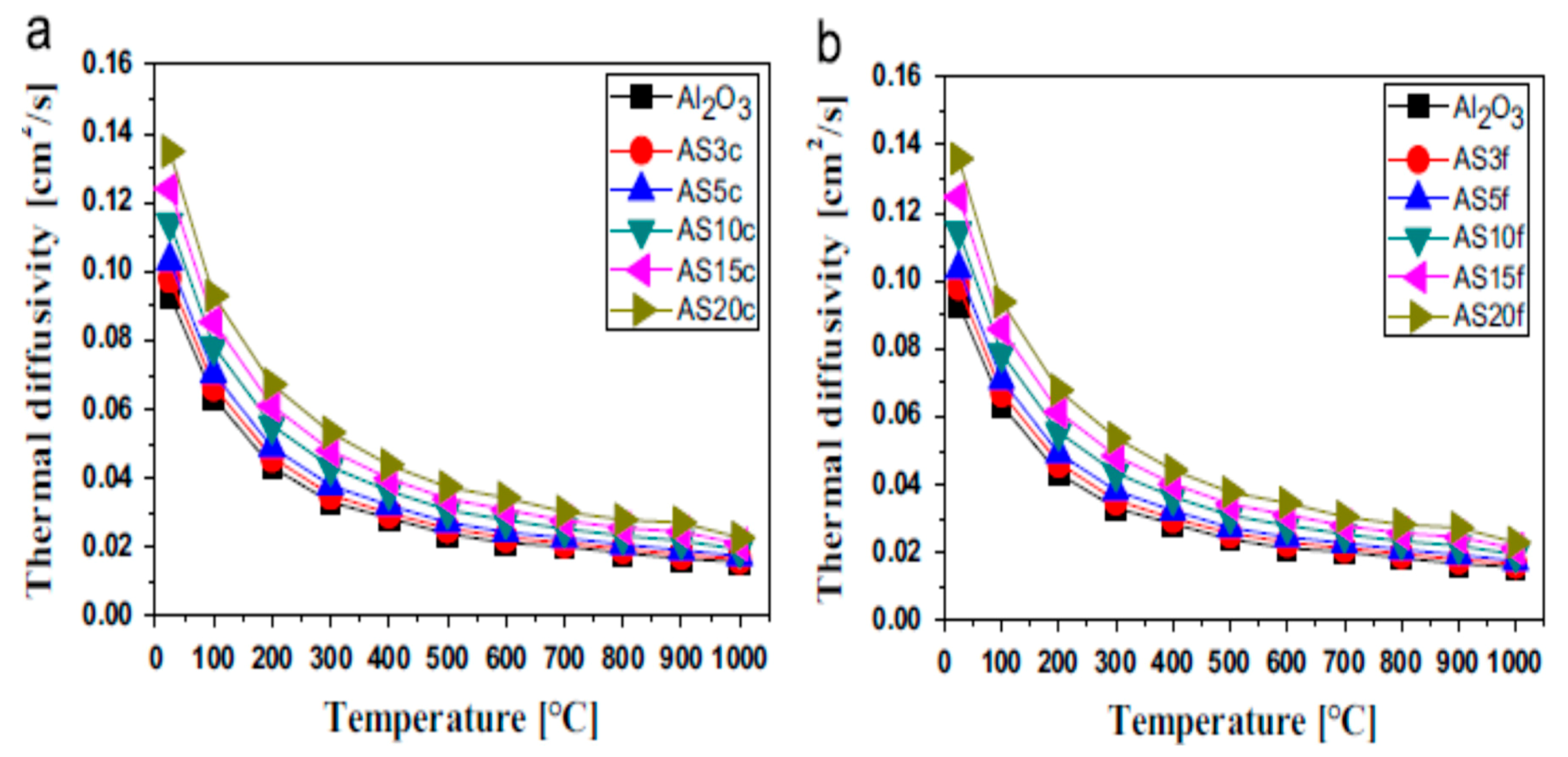
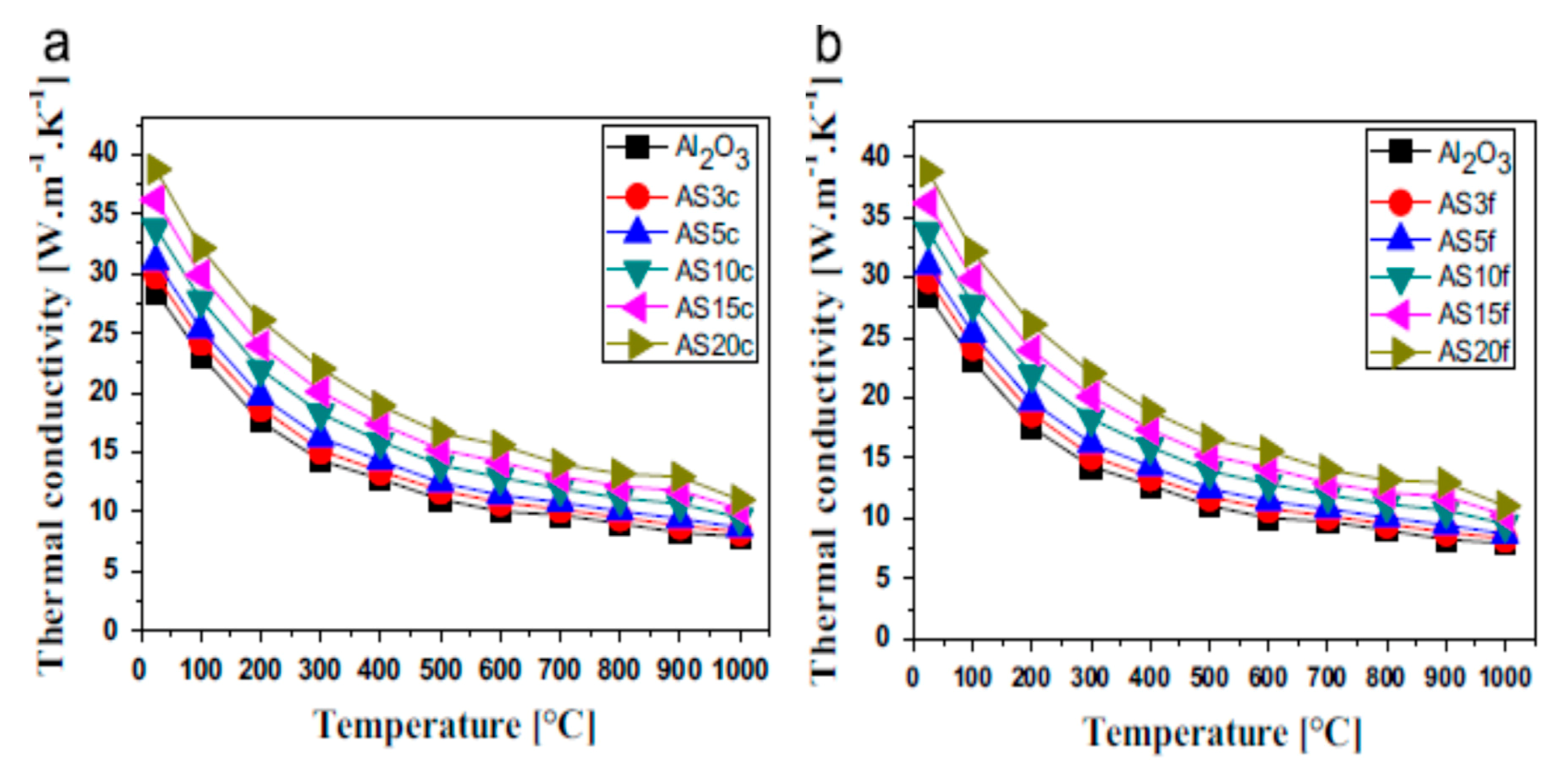
| Nanocomposites | Consolidation Type | Densification (%) | Hardness (GPa) | Fracture Toughness (MPam1/2) | Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|
| Al2O3–5SiC | SPS | 99.5 | 19 | 4.5 | 980 | [81] |
| Al2O3–5SiC | HP | ----- | ----- | 4.7 | 467 | [82] |
| Al2O3–17SiC | HP | 98 | 22 | 4.6 | 383 | [91] |
| Al2O3–5SiC | HP | 99.9 | 20.4 | 3.3 | 760 | [11] |
| Al2O3–5SiC | HP | 98 | 18.8 | ----- | 451 | [138] |
| Al2O3–5SiC | SPS | 98 | ----- | ----- | 380 | [90] |
| Al2O3–5SiC | CIP | 99.9 | 21.2 | ----- | ----- | [83] |
| Al2O3–5SiC | HP | 92 | 20.1 | 2.9 | ----- | [94] |
| Al2O3–5SiC | HP | 98.2 | 24 | 7.1 | 363.8 | [137] |
| Al2O3–10SiC | SPS | 98.7 | 17.2 | 4.4 | ----- | [120] |
| Al2O3–10SiC | HP | 99.1 | 22 | 5.4 | 550 | [139] |
| Al2O3–5SiC | HP | 99.67 | ----- | 4.7 | 641 | [122] |
| Al2O3–5SiC | SPS | 99.76 | 21.78 | 2.65 | ----- | [133] |
| Nanocomposites | Consolidation Type | Densification (%) | Hardness (GPa) | Fracture Toughness (MPam1/2) | Strength (MPa) | Ref. |
|---|---|---|---|---|---|---|
| Al2O3–7.39MWCNT | SPS | 79.1 | 9.98 | 4.7 | ----- | [111] |
| Al2O3–2MWCNT | HP | 99 | 18 | 4.2 | ----- | [146] |
| Al2O3–10SWCNT | SPS | 95.2 | 16.1 | 9.7 | ----- | [115] |
| Al2O3–4MWCNT | HP | 98 | 17 | 4.2 | 380 | [129] |
| Al2O3–0.1SWCNT | HP | 95.28 | 15.55 | 2.85 | ----- | [130] |
| Al2O3–1MWCNT | SPS | ----- | 17 | 3.7 | ----- | [128] |
| Al2O3–4MWCNT | HP | ----- | 20 | ----- | ----- | [43] |
| Al2O3–1MWCNT | HP | 99.5 | 15.5 | 6.0 | ----- | [151] |
| Al2O3–0.3MWCNT | CIP | 98.02 | 19.52 | 4.83 | 257.53 | [149] |
| Al2O3–1MWCNT | Pressureless | 99 | 17.1 | 4.1 | 543 | [131] |
| Al2O3–0.9MWCNT | Pressureless | 98.5 | ----- | 5.83 | 742.6 | [148] |
| Al2O3–3MWCNT | Pressureless | 96.4 | ----- | 4.7 | 363 | [147] |
| Al2O3–0.3MWCNT | Pressureless | 99.1 | 21 | 4.7 | 250 | [152] |
| Al2O3–0.1MWCNT | SPS | ----- | 17.6 | 4.9 | ----- | [21] |
| Al2O3–0.5MWCNT | SPS | ----- | 15.0 | 5.8 | 420 | [127] |
| Al2O3–1MWCNT | SPS | ----- | ----- | 5.0 | ----- | [104] |
| Nanocomposites | Consolidation Type | Densification (%) | Thermal Conductivity (W/mK) | Ref. |
|---|---|---|---|---|
| Al2O3–20SiC | HP | 99.3 | 38 | [85] |
| Al2O3–60SiCw | HP | ----- | 42.1 | [157] |
| Al2O3–30SiCp | HP | 99 | 49 | [169] |
| Al2O3–20SiCw | HP | 98.3 | 34.25 | [170] |
| Nanocomposites | Consolidation Type | Densification (%) | Thermal Conductivity (W/mK) | Ref. |
|---|---|---|---|---|
| Al2O3–10SWCNT | SPS | 95.2 | 11.4 | [115] |
| Al2O3–7.39MWCNT | SPS | 84.2 | 90.44 | [112] |
| Al2O3–0.15MWCNT | CIP | 98.45 | 47.14 | [149] |
| Nanocomposites | Consolidation Type | Densification (%) | Electrical Conductivity (S/m) | Ref. |
|---|---|---|---|---|
| Alumina–20SiC | HP | 99.3 | 4.05 × 10−2 | [85] |
| Alumina–17SiC | HP | 99.0 | 5.88 × 10−3 | [86] |
| Nanocomposites | Consolidation Type | Densification (%) | Electrical Conductivity (S/m) | Ref. |
|---|---|---|---|---|
| Alumina–3%MWCNT | SPS | ----- | 1.245 | [188] |
| Alumina–5%MWCNT | SPS | 99 | 576 | [114] |
| Alumina–19.1%MWCNT | SPS | 59.7 | 3336 | [156] |
| Alumina–1%MWCNT | SPS | ----- | 2.5 | [49] |
| Alumina–5.7%SWCNT | SPS | 100 | 1050 | [115] |
| Alumina–1%MWCNT | HP | 99.5 | 10−2 | [151] |
| Alumina–2.4%MWCNT | CIP | 83.96 | 0.1 | [149] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momohjimoh, I.; Hussein, M.A.; Al-Aqeeli, N. Recent Advances in the Processing and Properties of Alumina–CNT/SiC Nanocomposites. Nanomaterials 2019, 9, 86. https://doi.org/10.3390/nano9010086
Momohjimoh I, Hussein MA, Al-Aqeeli N. Recent Advances in the Processing and Properties of Alumina–CNT/SiC Nanocomposites. Nanomaterials. 2019; 9(1):86. https://doi.org/10.3390/nano9010086
Chicago/Turabian StyleMomohjimoh, Ibrahim, Mohamed A. Hussein, and Nasser Al-Aqeeli. 2019. "Recent Advances in the Processing and Properties of Alumina–CNT/SiC Nanocomposites" Nanomaterials 9, no. 1: 86. https://doi.org/10.3390/nano9010086
APA StyleMomohjimoh, I., Hussein, M. A., & Al-Aqeeli, N. (2019). Recent Advances in the Processing and Properties of Alumina–CNT/SiC Nanocomposites. Nanomaterials, 9(1), 86. https://doi.org/10.3390/nano9010086





