Manganese/Yttrium Codoped Strontium Nanohexaferrites: Evaluation of Magnetic Susceptibility and Mossbauer Spectra
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Structural Properties
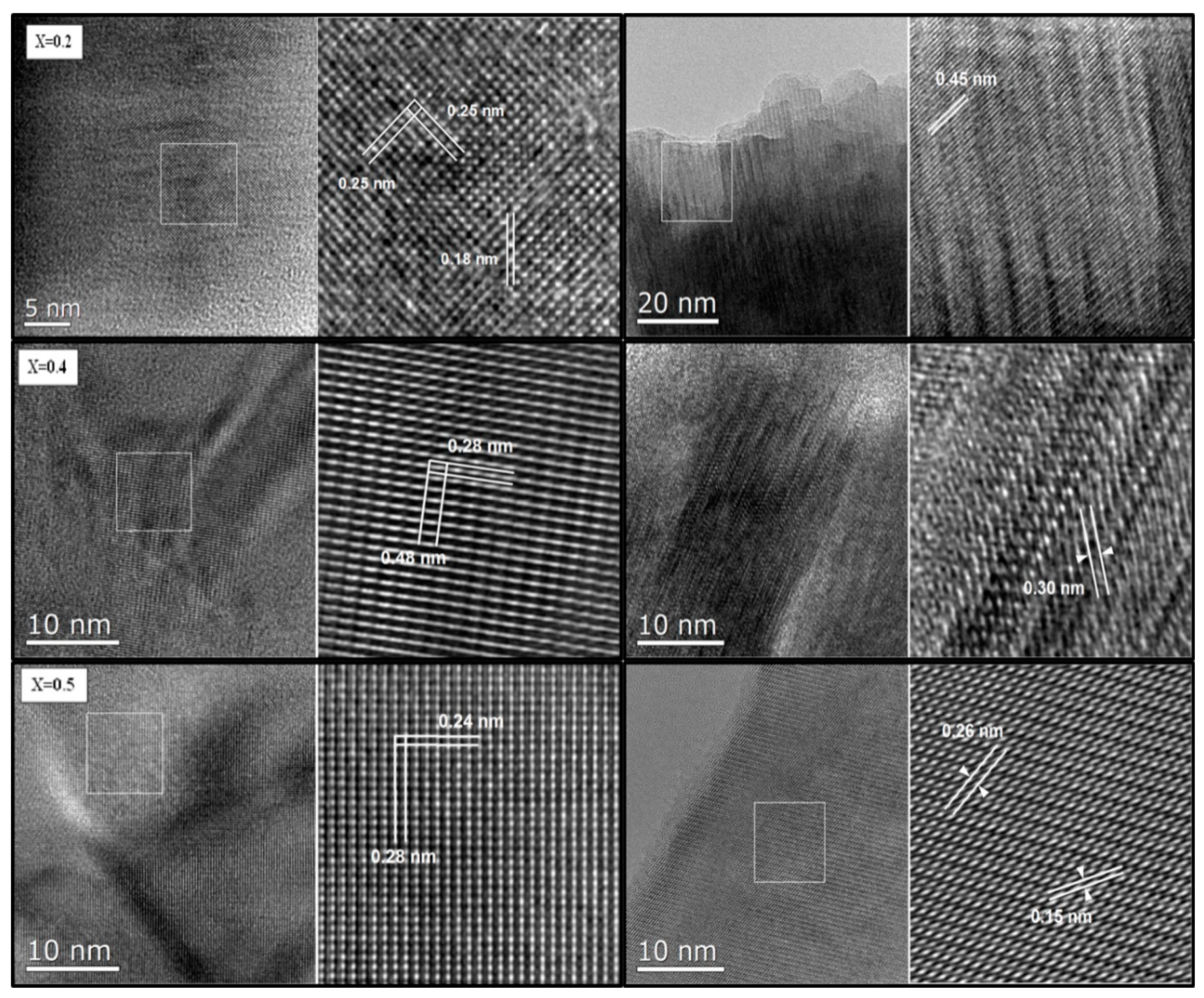
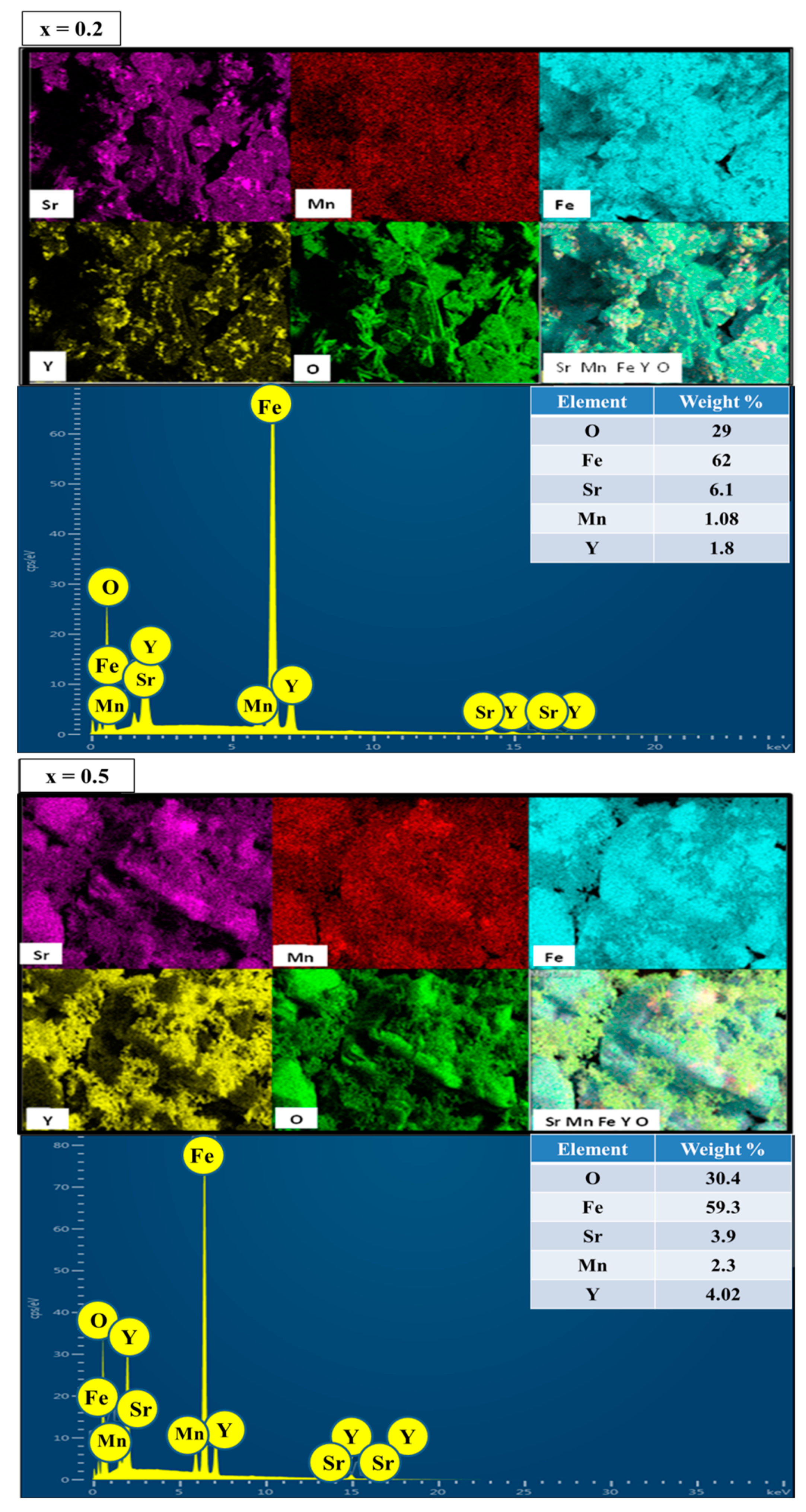
3.2. Morphology
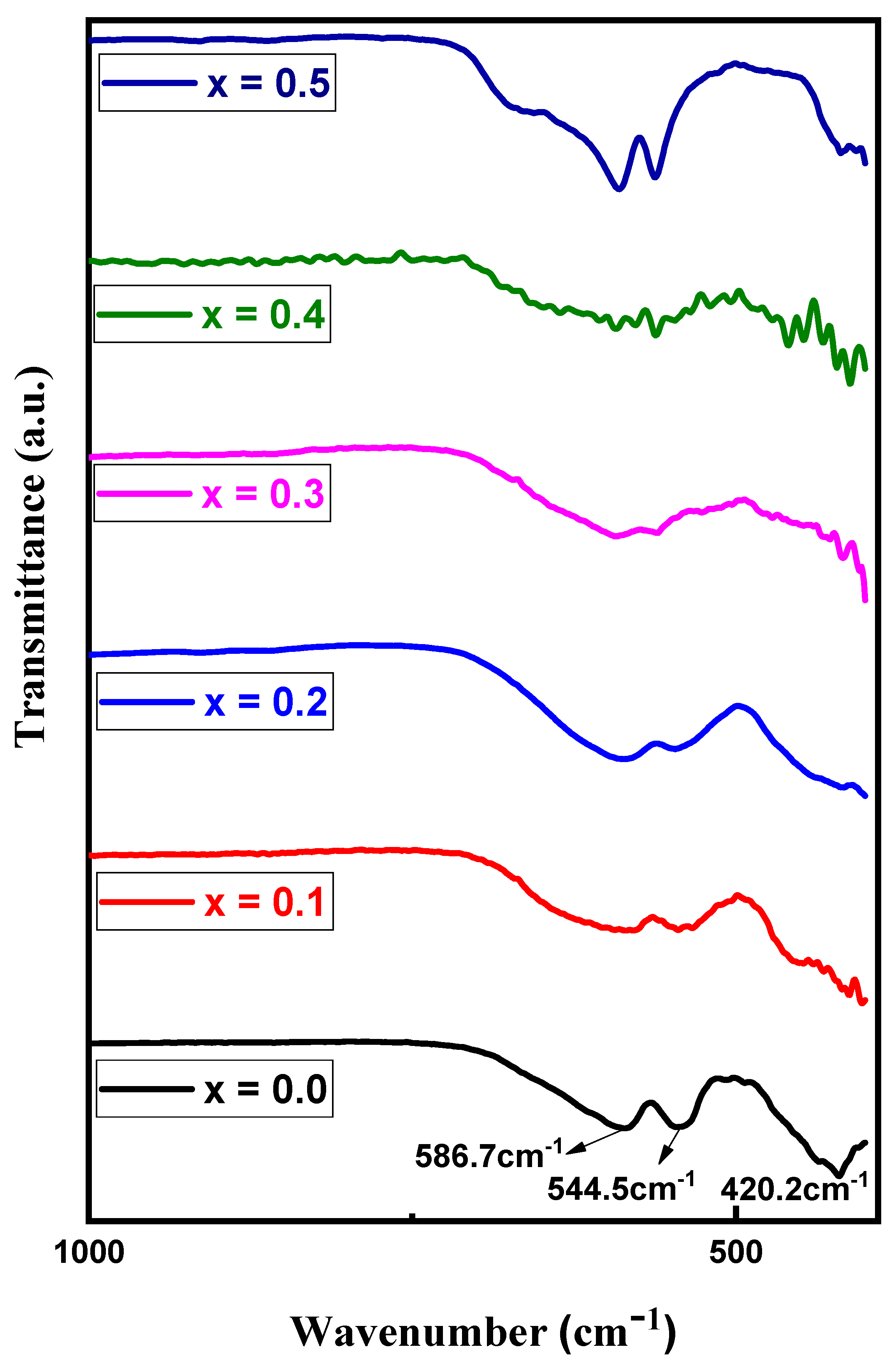
3.3. FTIR Spectra
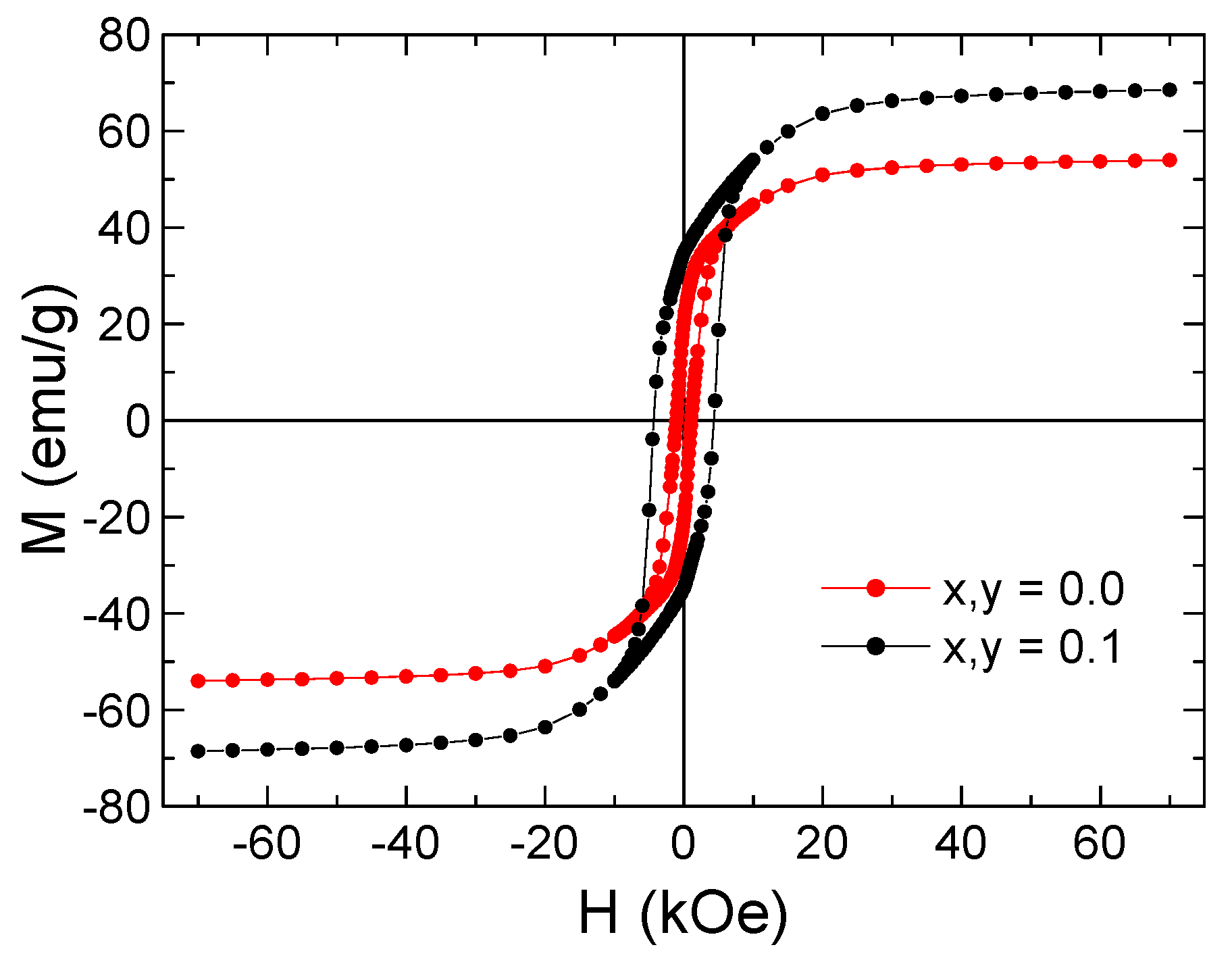
3.4. Mössbauer Spectral Analysis
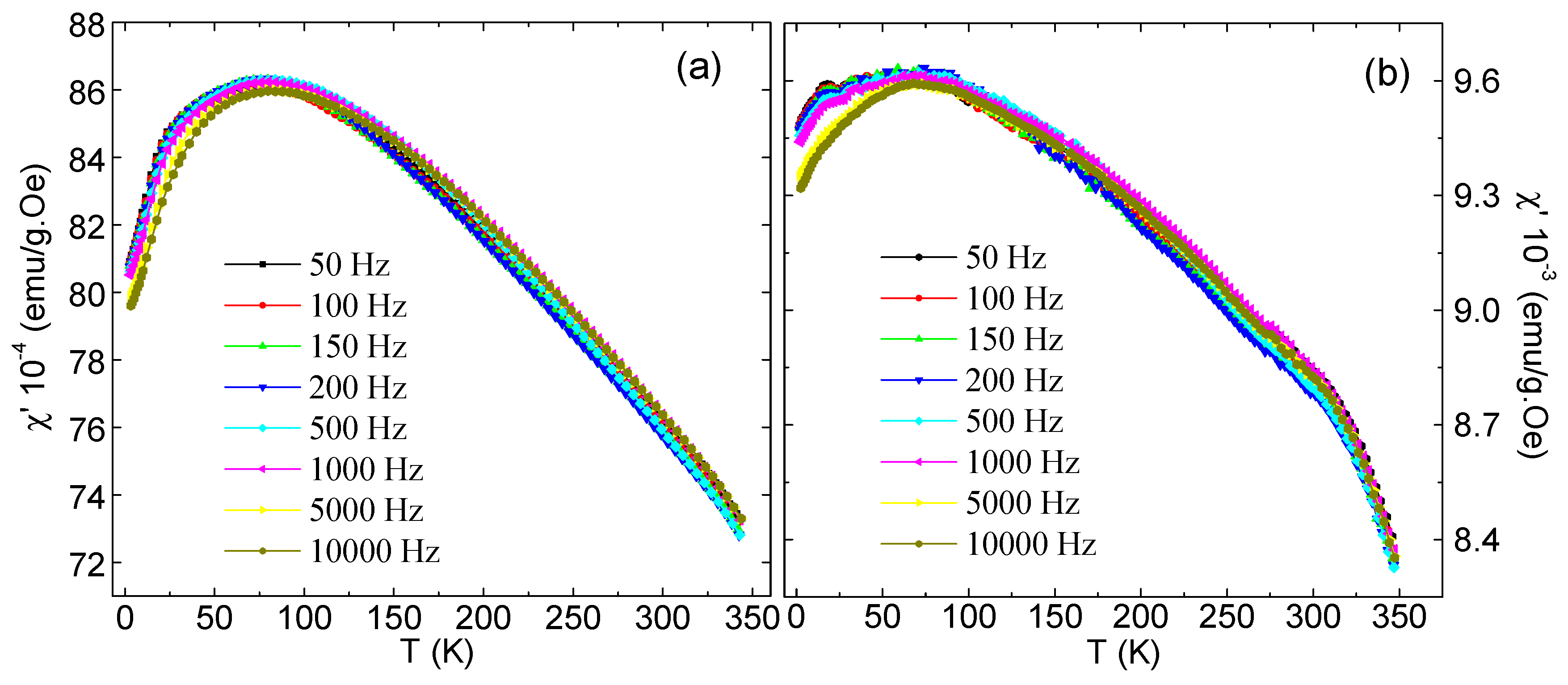
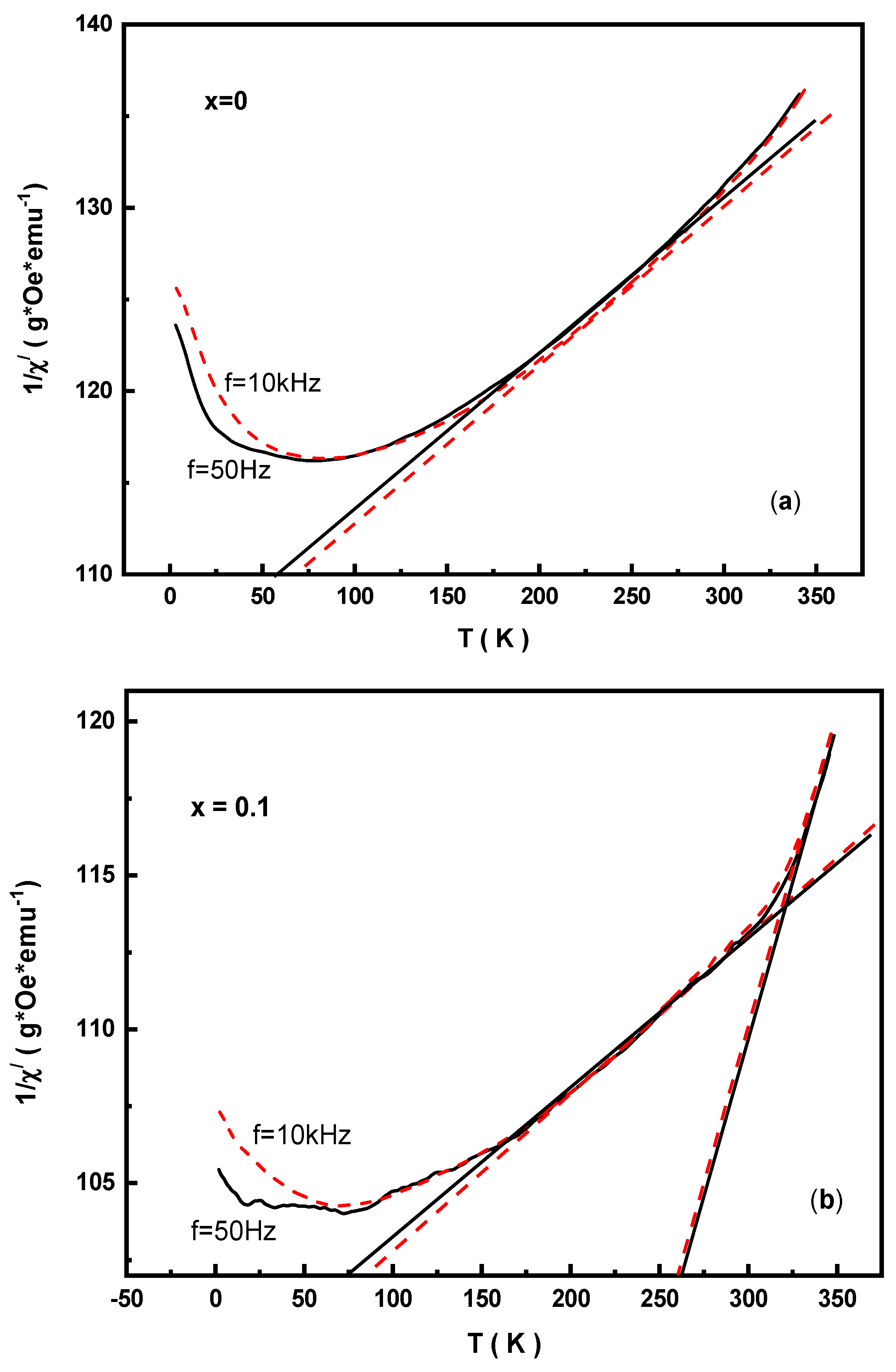
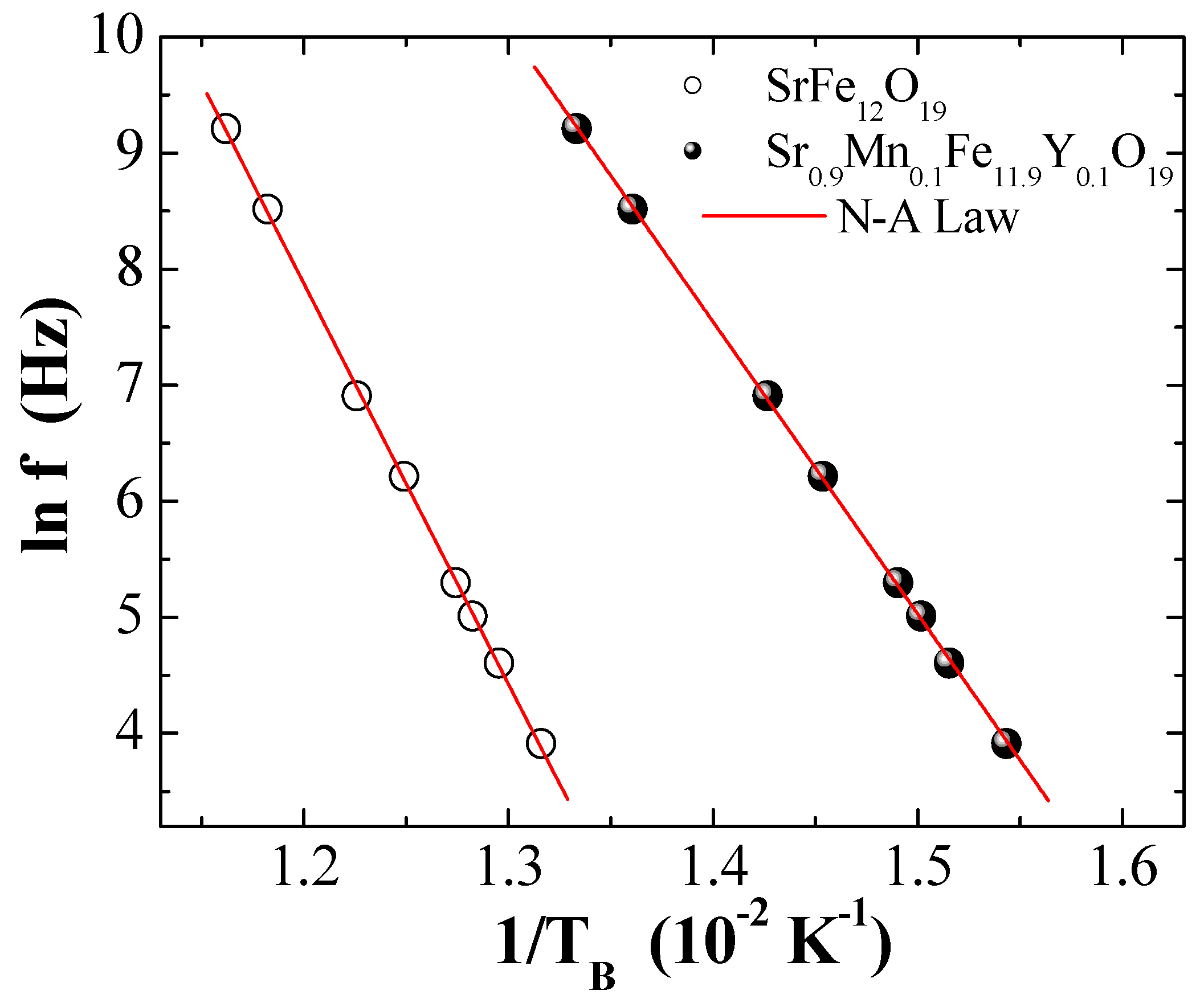
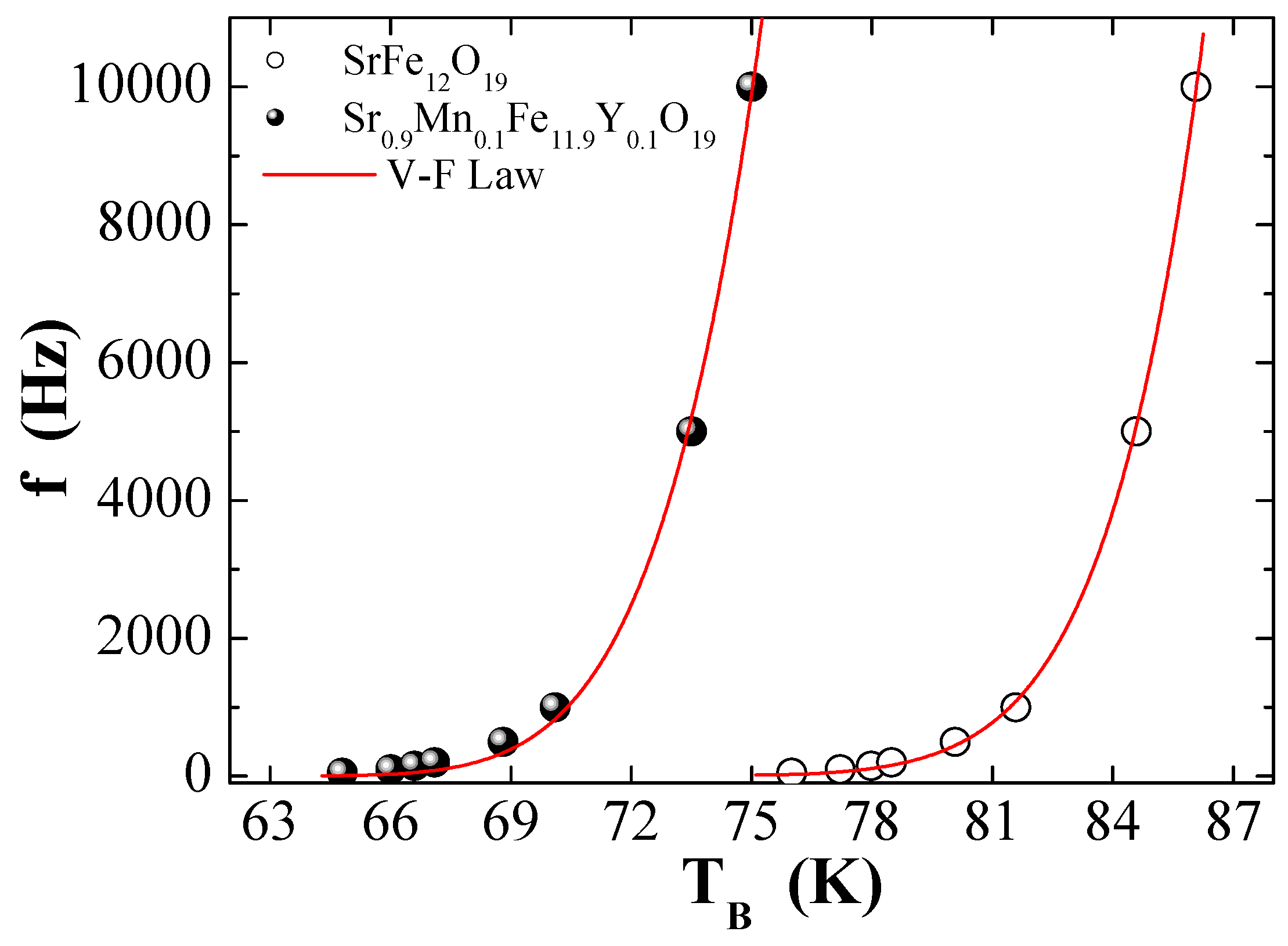
3.5. AC Magnetic Susceptibility
4. Conclusions
Author Contributions
- (1).
- Synthesis of the sample, writing—original draft preparation, Munirah Abdullah Almessiere;
- (2).
- Investigation magnetic properties and writing, Yassine Slimani
- (3).
- Investigation and analysis the Mossbauer Spectra, Hakan Güngüneş
- (4).
- (Supervision, Abdulhadi Baykal and A.V. Trukhanov
- (5).
- Review and Editing, S.V. Trukhanov
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, C.N.R.; Cheetham, A.K. Science and technology of nanomaterials: Current status and future prospects. J. Mat. Chem. 2001, 12, 2887–2894. [Google Scholar] [CrossRef]
- Wang, X.X.; Ma, T.; Shu, J.C.; Cao, M.S. Confinedly tailoring Fe3O4 clusters-NG to tune electromagnetic parameters and Microwave absorption with broadened bandwidth. Chem. Eng. J. 2018, 332, 321–3300. [Google Scholar] [CrossRef]
- Gu, Z.; Yan, L.; Tian, G.; Li, S.; Chai, Z.; Zhao, Y. Recent Advances in Design and Fabrication of Upconversion Nanoparticles and Their Safe Theranostic Applications. Adv. Mater. 2013, 25, 3758–3779. [Google Scholar] [CrossRef] [PubMed]
- Katlakunta, S.; Meena, S.S.; Srinath, S.; Bououdina, M.; Sandhya, R.; Praveena, K. Improved magnetic properties of Cr3+ doped SrFe12O19 synthesized via microwave hydrothermal route. Mater. Res. Bull. 2015, 63, 58–66. [Google Scholar] [CrossRef]
- Jacobo, S.E.; Bercoff, P.G.; Herme, C.A.; Vives, L.A. Sr hexaferrite/Ni ferrite nanocomposites magnetic behavior and microwave absorbing properties in the X-band. Mater. Chem. Phys. 2015, 157, 124–129. [Google Scholar] [CrossRef]
- Auwal, I.A.; Baykal, A.; Güner, S.; Sertkol, M.; Sözeri, H. Magneto-optical properties BaBixLaxFe12−2xO19 (0.0 ≤ x ≤ 0.5) hexaferrites. J. Magn. Magn. Mater. 2016, 409, 92–98. [Google Scholar] [CrossRef]
- Harker, S.; Stewart, G.; Hutchison, W.; Amiet, A.; Tucker, D. Microwave absorption and 57Fe Mössbauer properties of Ni-Ti doped barium hexaferrite. Hyperfine Interact. 2015, 230, 205–211. [Google Scholar] [CrossRef]
- Dishovski, N.; Petkov, A.; Nedkov, I.; Razkazov, I. Hexaferrite contribution to microwave absorbers characteristics. IEEE Trans. Magn. 1994, 30, 969–971. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ashiq, M.N.; Gomez, P.H.; Munoz, J.M. Synthesis, physical, magnetic and electrical properties of Al–Ga substituted co-precipitated nanocrystalline strontium hexaferrite. J. Magn. Magn. Mater. 2008, 320, 881–886. [Google Scholar] [CrossRef]
- Obradors, X.; Collomb, A.; Pernet, M.; Joubert, J.C.; Isalgue, A. Structural and magnetic properties of BaFe12−xMnxO19 hexagonal ferrites. J. Magn. Magn. Mater. 1984, 44, 118–128. [Google Scholar] [CrossRef]
- Mocuta, H.; Lechevallier, L.; le Breton, J.M.; Wang, J.F.; Harris, I.R. Structural and magnetic properties of hydrothermally synthesized Sr1−xNdxFe12O19 hexagonal ferrites. J. Alloy. Compd. 2004, 364, 48–52. [Google Scholar] [CrossRef]
- Silva, W.M.S.; Ferreira, N.S.; Soares, J.M.; da Silva, R.B.; Macêdo, M.A. Investigation of structural and magnetic properties of nanocrystalline Mn-doped SrFe12O19 prepared by proteic sol–gel process. J. Magn. Magn. Mater. 2015, 395, 263–270. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Rehman, K.M.U.; Li, M.; Wu, Y. Synthesis and characterization of Sr1−xYxFe12O19 hexaferrites prepared by solid-state reaction method. J. Mater. Sci. Mater. Electron. 2016, 27, 12919–12924. [Google Scholar] [CrossRef]
- Niu, X.F.; Zhang, M.Y. Structure and magnetic properties of yttrium-doped M-type strontium ferrite. Asian J. Chem. 2014, 26, 6783–6786. [Google Scholar] [CrossRef]
- Shekhawat, D.; Roy, P.K. Impact of yttrium on the physical, electro-magnetic and dielectric properties of auto-combustion synthesized nanocrystalline strontium hexaferrite. J. Mater. Sci. Mater. Electron. 2018. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; el Sayed, H.S.; Baykal, A. Structural and magnetic properties of Ce-Y substituted strontium nanohexaferrites. Ceram. Int. 2018, 44, 12511–12519. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Baykal, A. Impact of Nd-Zn co-substitution on microstructure and magnetic properties of SrFe12O19 nanohexaferrite. Ceram. Int. 2019, 45, 963–969. [Google Scholar] [CrossRef]
- Peng, L.; Li, L.Z.; Wang, R.; Hu, Y.; Tu, X.Q.; Zhong, X.X. Effect of La–Co substitution on the crystal structure and magnetic properties of low temperature sintered Sr1−xLaxFe12−xCoxO19 (x=0–0.5) ferrites. J. Magn. Magn. Mater. 2015, 393, 399–403. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, X.S. Microstructure and magnetic properties of La– Cu doped M-type strontium ferrites prepared by ceramic process. Mater. Technol. Adv. Perform. Mater. 2014, 29, 232–236. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Shao, J.; Batoo, K.M. Microstructure and magnetic properties of Zr–Mn substituted M-type SrLa hexaferrites. Appl. Phys. A 2017, 123, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Liu, X.X.; Wang, X.J.; Peng, Y.; Li, R. Effect of Nd–Co substitution on magnetic and microwave absorption properties of SrFe12O19 hexaferrites. J. Alloy. Compd. 2012, 525, 114–119. [Google Scholar] [CrossRef]
- Lee, S.W.; An, S.Y. In-Bo Shim, Chul Sung Kim, Mossbauer studies of La–Zn substitution effect in strontium ferrite nanoparticles. J. Magn. Magn. Mater. 2005, 290–291, 231–233. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Shao, J.; Huang, D.; He, H.; Trukhanov, A.V.; Trukhanov, S.V. Influence of Nd-NbZn co-substitution on structural, spectral and magnetic properties of M-type calcium-strontium hexaferrites Ca0.4Sr0.6-xNdxFe12.0-x(Nb0.5Zn0.5)xO19. J. Alloy. Compd. 2018, 765, 616–623. [Google Scholar] [CrossRef]
- Ashiq, M.N.; Shakoor, S.; Najam-ul-Haq, M.; Warsi, M.F.; Ali, I.; Shakir, I. Structural, electrical, dielectric and magnetic properties of Gd-Sn substituted Sr-hexaferrite synthesized by sol–gel combustion method. J. Magn. Magn. Mater. 2015, 374, 173–178. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Farooq, S. Impact of Pr–Ni substitution on the electrical and magnetic properties of chemically derived nanosized strontium–barium hexaferrites. J. Alloy. Compd. 2010, 505, 560–567. [Google Scholar] [CrossRef]
- Shakoor, S.; Ashiq, M.N.; Marlana, M.A.; Mahmood, A.; Warsi, M.F.; Najam-ul-Haq, M.; Karamat, N. Electrical, dielectric and magnetic characterization of Bi–Cr substituted M-type strontium hexaferrite nanomaterials. J. Magn. Magn. Mater. 2014, 362, 110–114. [Google Scholar] [CrossRef]
- Kaur, P.; Chawla, S.K.; Meena, S.S.; Yusuf, S.M.; Pubby, K.; Narang, S.B. Modulation of physico-chemical, magnetic, microwave and electromagnetic properties of nanocrystalline strontium hexaferrite by Co-Zr doping synthesized using citrate precursor sol-gel method. Ceram. Int. 2017, 43, 590–598. [Google Scholar] [CrossRef]
- Joshi, R.; Singh, C.; Kaur, D.; Zaki, H.; Narang, S.B.; Jotania, R.; Mishra, S.; Singh, J.; Dhruv, P.; Ghimiree, M. Structural and magnetic properties of Co2+-W4+ ions doped M-type Ba-Sr hexaferrites synthesized by a ceramic method. J. Alloy. Compd. 2017, 695, 909. [Google Scholar] [CrossRef]
- Yang, Y.J.; Shao, J.X.; Wang, F.H.; Liu, X.S.; Huang, D.H. Impacts of MnZn doping on the structural and magnetic properties of M-type SrCaLa hexaferrites. Appl. Phys. A 2017, 123, 309. [Google Scholar] [CrossRef]
- Baniasadi, A.; Ghasemi, A.; Nemati, A.; Ghadikolaei, M.A.; Paimozd, E. Effect of Ti–Zn substitution on structural, magnetic and microwave absorption characteristics of strontium hexaferrite. J. Alloy. Compd. 2014, 583, 325–328. [Google Scholar] [CrossRef]
- Rostami, M.; Vahdani, M.R.K.; Moradi, M.; Mardani, R. Structural, magnetic, and microwave absorption properties of Mg–Ti–Zr–Co-substituted barium hexaferrites nanoparticles synthesized via sol–gel auto-combustion method. J. Sol-Gel Sci. Technol. 2017, 82, 783–794. [Google Scholar] [CrossRef]
- Zhang, T.; Peng, X.; Li, J.; Yang, Y.; Xu, J.; Wang, P.; Jin, D.; Jin, H.; Hong, B.; Wang, X.; et al. Platelet-like hexagonal SrFe12O19 particles: Hydrothermal synthesis and their orientation in a magnetic field. J. Magn. Magn. Mater. 2016, 412, 102–106. [Google Scholar] [CrossRef]
- Rezaie, E.; Rezanezhad, A.; Ghadimi, L.S.; Hajalilou, A.; Arsalani, N. Effect of calcination on structural and supercapacitance properties of hydrothermally synthesized plate-like SrFe12O19 hexaferrite nanoparticles. Ceram. Int. 2018, 44, 20285–20290. [Google Scholar] [CrossRef]
- Annapureddya, V.; Kang, J.H.; Palneedi, H.; Kima, J.W.; Ahna, C.W.; Choi, S.Y.; Johnson, S.D.; Ryu, J. Growth of self-textured barium hexaferrite ceramics by normal sintering process and their anisotropic magnetic properties. J. Eur. Ceram. Soc. 2017, 37, 4701–4706. [Google Scholar] [CrossRef]
- Pradeep, A.; Chandrasekaran, G. FTIR study of Ni, Cu and Zn substituted nano-particles of MgFe2O4. Mater. Lett. 2006, 60, 371–374. [Google Scholar] [CrossRef]
- Pereira, F.M.M.; Junior, C.A.R.; Santos, M.R.P.; Sohn, R.S.T.M.; Freire, F.N.A.; Sasaki, J.M.; De-Paiva, J.A.C.; Sombra, A.S.B. Structural and dielectric spectroscopy studies of the M-type barium strontium hexaferrite alloys (BaxSr1−xFe12O19). J. Mater. Sci. Mater. Electron. 2008, 19, 627–638. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, R.R.; Barman, P.B. Synthesis and characterizations of Nd3+ doped SrFe12O19 nanoparticles. Mater. Chem. Phys. 2013, 141, 562–569. [Google Scholar] [CrossRef]
- Tenorio-Gonzalez, F.N.; Bolarín-Miro, A.M.; Jesús, F.Sa.; Vera-Serna, P.; Menendez-Gonzalez, N.; Sanchez-Marcos, J. Crystal structure and magnetic properties of high Mn-doped strontium hexaferrite. J. Alloy. Compd. 2017, 695, 2083–2090. [Google Scholar] [CrossRef]
- Solovyova, E.D.; Pashkova, E.V.; Ivanitski, V.P.; Vyunov, O.I.; Belous, A.G. Mössbauer and X-ray diffraction study of Co2+–Si4+ substituted M-type barium hexaferrite BaFe12−2xCoxSixO19±γ. J. Magn. Magn. Mater. 2013, 330, 72–75. [Google Scholar] [CrossRef]
- Belous, A.G.; Vyunov, O.I.; Pashkova, E.V.; Ivanitski, V.P.; Gavrilenko, O.N. Mössbauer Study and Magnetic Properties of M-Type Barium Hexaferrite Doped with Co + Ti and Bi + Ti Ions. Phys. Chem. B 2006, 110, 26477–26481. [Google Scholar] [CrossRef]
- Chawla, S.K.; Mudsainiyan, R.K.; Meena, S.S.; Yusuf, S.M. Sol–gel synthesis, structural and magnetic properties of nanoscale M-type barium hexaferrites BaCoxZrxFe(12−2x)O19. J. Magn. Magn. Mater. 2014, 350, 23–29. [Google Scholar] [CrossRef]
- Auwal, I.A.; Güngüneş, H.; Güner, S.; Shirsath, S.E.; Sertkol, M.; Structural, A.B. magneto-optical properties and cation distribution of SrBixLaxYxFe12−3xO19 (0.0 ≤ x ≤ 0.33) hexaferrites. Mater. Res. Bull. 2016, 80, 263–272. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; el Sayed, H.S.; Baykal, A. AC susceptibility and Mossbauer study of Ce3+ ion substituted SrFe12O19 nanohexaferrites. Ceram. Int. 2018. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; el Sayed, H.S.; Baykal, A. Ce-Y co-substituted Strontium nanohexaferrites: AC susceptibility and Mossbauer studies. Ceram. Int. 2018. [Google Scholar] [CrossRef]
- Slimani, Y.; Baykal, A.; Manikandan, A. Effect of Cr3+ substitution on AC susceptibility of Ba hexaferrite nanoparticles. J. Magn. Magn. Mater. 2018, 458, 204–212. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Bahadur, D.; Aslam, M. Superspin glass behavior of self-interacting CoFe2O4 nanoparticles. J. Alloy. Compd. 2015, 628, 416–423. [Google Scholar] [CrossRef]
- Kaul, S.N.; Methfessel, S. Effect of field and frequency on the temperature dependence of a.c. susceptibility of the (La, Gd) Ag spin-glass. Solid State Comm. 1983, 47, 147–151. [Google Scholar] [CrossRef]
- Fiorani, D.; Viticoli, S.; Dormann, J.L.; Tholence, J.L.; Murani, A.P. Spin-glass behavior in an antiferromagnetic frustrated spinel: ZnCr1.6Ga0.4O4. Phys. Rev. B 1984, 30, 2776–2786. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Troyanchuk, I.O.; Fita, I.M.; Szymczak, H.; Bärner, K. Comparative study of the magnetic and electrical properties of Pr1−xBaxMnO3-δ manganites depending on the preparation conditions. J. Magn. Magn. Mater. 2001, 237, 276–282. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Lobanovski, L.S.; Bushinsky, M.V.; Khomchenko, V.A.; Pushkarev, N.V.; Tyoyanchuk, I.O.; Maignan, A.; Flahaut, D.; Szymczak, H.; Szymczak, R. Influence of oxygen vacancies on the magnetic and electrical properties of La1−xSrxMnO3−x/2 manganites. Eur. Phys. J. B 2004, 42, 51–61. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Vasiliev, A.N.; Szymczak, H. Frustrated exchange interactions formation at low temperatures and high hydrostatic pressures in La0.70Sr0.30MnO2.85. J. Exp. Theor. Phys. 2010, 111, 209–214. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Kostishyn, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Structure and magnetic properties of BaFe11.9In0.1O19 hexaferrite in a wide temperature range. J. Alloy. Compd. 2016, 689, 383–393. [Google Scholar] [CrossRef]




| x | a = b (Å) | c (Å) | DXRD (nm) | χ2 |
|---|---|---|---|---|
| 0.0 | 5.881 | 23.048 | 55.1 | 1.8 |
| 0.1 | 5.881 | 23.021 | 69.1 | 2.1 |
| 0.2 | 5.882 | 23.023 | 55.9 | 2.4 |
| 0.3 | 5.883 | 23.039 | 59.8 | 2.6 |
| 0.4 | 5.883 | 23.029 | 49.5 | 2.6 |
| 0.5 | 5.884 | 23.050 | 37.4 | 3.2 |
| x | Site | Bhf (T) | I.S (mm/s) | Q.S (mm/s) | W (mm/s) | RA (%) |
|---|---|---|---|---|---|---|
| (±0.01) | (±0.002) | (±0.001) | (±0.006) | |||
| 0 | 12k | 41.183 | 0.353 | 0.396 | 0.277 | 48.381 |
| 4f1 | 49.157 | 0.259 | 0.176 | 0.238 | 17.771 | |
| 4f2 | 51.835 | 0.379 | 0.292 | 0.244 | 13.924 | |
| 2a | 50.885 | 0.323 | 0.016 | 0.363 | 11.679 | |
| 2b | 40.937 | 0.279 | 2.279 | 0.988 | 8.2449 | |
| 0.1 | 12k | 41.13 | 0.353 | 0.401 | 0.249 | 43.331 |
| 12k1 | 38.761 | 0.305 | 0.223 | 0.348 | 4.1509 | |
| 4f1 | 49.106 | 0.261 | 0.179 | 0.248 | 16.983 | |
| 4f2 | 51.896 | 0.375 | 0.285 | 0.254 | 12.616 | |
| 2a | 50.558 | 0.342 | 0.069 | 0.311 | 13.347 | |
| 2b | 40.952 | 0.292 | 2.247 | 0.251 | 5.3067 | |
| Db | - | 0.232 | 0.703 | 680 | 4.2661 | |
| 0.2 | 12k | 41.089 | 0.351 | 0.399 | 0.251 | 39.926 |
| 12k1 | 38.557 | 0.305 | 0.271 | 0.266 | 7.995 | |
| 4f1 | 48.994 | 0.261 | 0.171 | 0.267 | 18.46 | |
| 4f2 | 51.814 | 0.362 | 0.317 | 0.146 | 10.953 | |
| 2a | 50.464 | 0.356 | 0.058 | 0.313 | 15.042 | |
| 2b | 40.879 | 0.305 | 2.216 | 0.264 | 4.3767 | |
| Db | - | 0.293 | 0.871 | 0.814 | 3.2476 | |
| 0.3 | 12k | 41.154 | 0.354 | 0.4 | 0.294 | 33.051 |
| 12k1 | 38.652 | 0.284 | 0.222 | 0.234 | 9.0202 | |
| 4f1 | 49.08 | 0.279 | 0.127 | 0.343 | 19.982 | |
| 4f2 | 52.551 | 0.422 | −0.041 | 0.2 | 8.7147 | |
| 2a | 50.885 | 0.384 | 0.015 | 0.449 | 23.917 | |
| 2b | 40.983 | 0.297 | 2.244 | 0.268 | 3.5987 | |
| Db | - | 0.438 | 0.835 | 0.812 | 1.7174 | |
| 0.4 | 12k | 41.113 | 0.346 | 0.389 | 0.302 | 28.237 |
| 12k1 | 38.762 | 0.27 | 0.217 | 0.304 | 16.523 | |
| 4f1 | 48.732 | 0.292 | 0.111 | 0.402 | 21.383 | |
| 4f2 | 52.555 | 0.423 | −0.078 | 0.18 | 5.1586 | |
| 2a | 50.816 | 0.373 | 0.089 | 0.471 | 24.509 | |
| 2b | 40.906 | 0.282 | 2.187 | 0.249 | 3.2939 | |
| Db | - | 0.249 | 0.39 | 0.92 | 0.89625 | |
| 0.5 | 12k | 41.103 | 0.35 | 0.385 | 0.463 | 25.222 |
| 12k1 | 38.589 | 0.258 | 0.225 | 0.461 | 20.37 | |
| 4f1 | 48.464 | 0.315 | 0.063 | 0.511 | 22.301 | |
| 4f2 | 52.221 | 0.403 | −0.07 | 0.467 | 10.131 | |
| 2a | 50.509 | 0.376 | 0.04 | 0.547 | 19.531 | |
| 2b | 40.914 | 0.284 | 2.14 | 0.21 | 2.4454 |
| Models | Parameters | Values | |
|---|---|---|---|
| SrFe12O19 | Sr0.9Mn0.1Fe11.9Y0.1O19 | ||
| Neel–Arrhenius | (s) | 3.85 × 10−22 | 2.58 × 10−19 |
| (K) | 3452.59 | 2518.3 | |
| (erg/cm3) | 1.25 × 103 | 2.02 × 103 | |
| Vogel–Fulcher | (s) | 1.18 × 10−11 | 7.39 × 10−11 |
| (K) | 587.83 | 226.66 | |
| (K) | 49.22 | 51.26 | |
| (erg/cm3) | 213.33 | 181.94 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almessiere, M.A.; Slimani, Y.; Güngüneş, H.; Baykal, A.; Trukhanov, S.V.; Trukhanov, A.V. Manganese/Yttrium Codoped Strontium Nanohexaferrites: Evaluation of Magnetic Susceptibility and Mossbauer Spectra. Nanomaterials 2019, 9, 24. https://doi.org/10.3390/nano9010024
Almessiere MA, Slimani Y, Güngüneş H, Baykal A, Trukhanov SV, Trukhanov AV. Manganese/Yttrium Codoped Strontium Nanohexaferrites: Evaluation of Magnetic Susceptibility and Mossbauer Spectra. Nanomaterials. 2019; 9(1):24. https://doi.org/10.3390/nano9010024
Chicago/Turabian StyleAlmessiere, Munirah Abdullah, Yassine Slimani, Hakan Güngüneş, Abdulhadi Baykal, S.V. Trukhanov, and A.V. Trukhanov. 2019. "Manganese/Yttrium Codoped Strontium Nanohexaferrites: Evaluation of Magnetic Susceptibility and Mossbauer Spectra" Nanomaterials 9, no. 1: 24. https://doi.org/10.3390/nano9010024
APA StyleAlmessiere, M. A., Slimani, Y., Güngüneş, H., Baykal, A., Trukhanov, S. V., & Trukhanov, A. V. (2019). Manganese/Yttrium Codoped Strontium Nanohexaferrites: Evaluation of Magnetic Susceptibility and Mossbauer Spectra. Nanomaterials, 9(1), 24. https://doi.org/10.3390/nano9010024









