Pd-Functionalized SnO2 Nanofibers Prepared by Shaddock Peels as Bio-Templates for High Gas Sensing Performance toward Butane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Pd-Functionalized SnO2 Nanofibers
2.2. Characterization of As-Prepared Products
2.3. Fabrication and Measurement of Gas Sensors
3. Results and Discussion
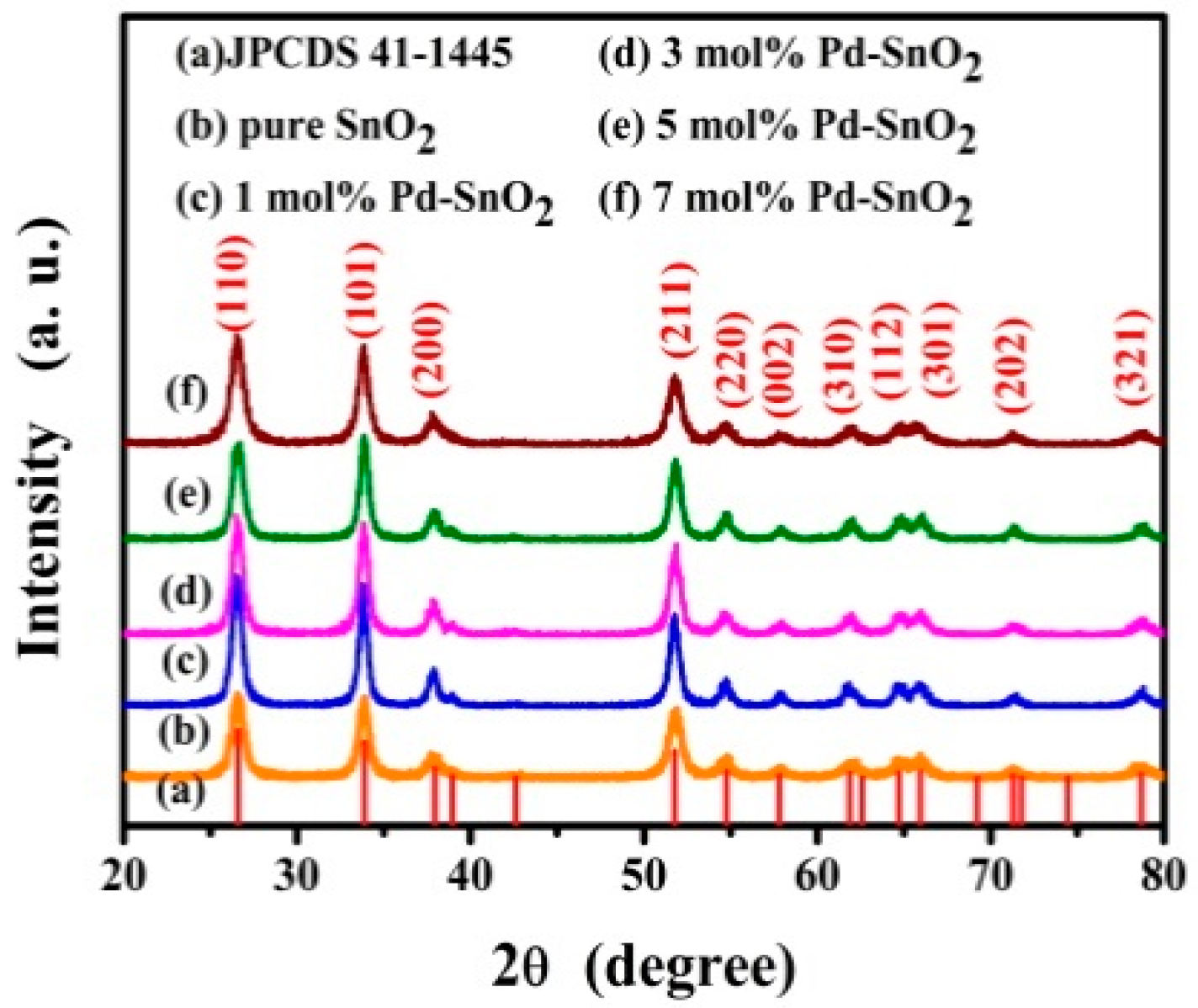
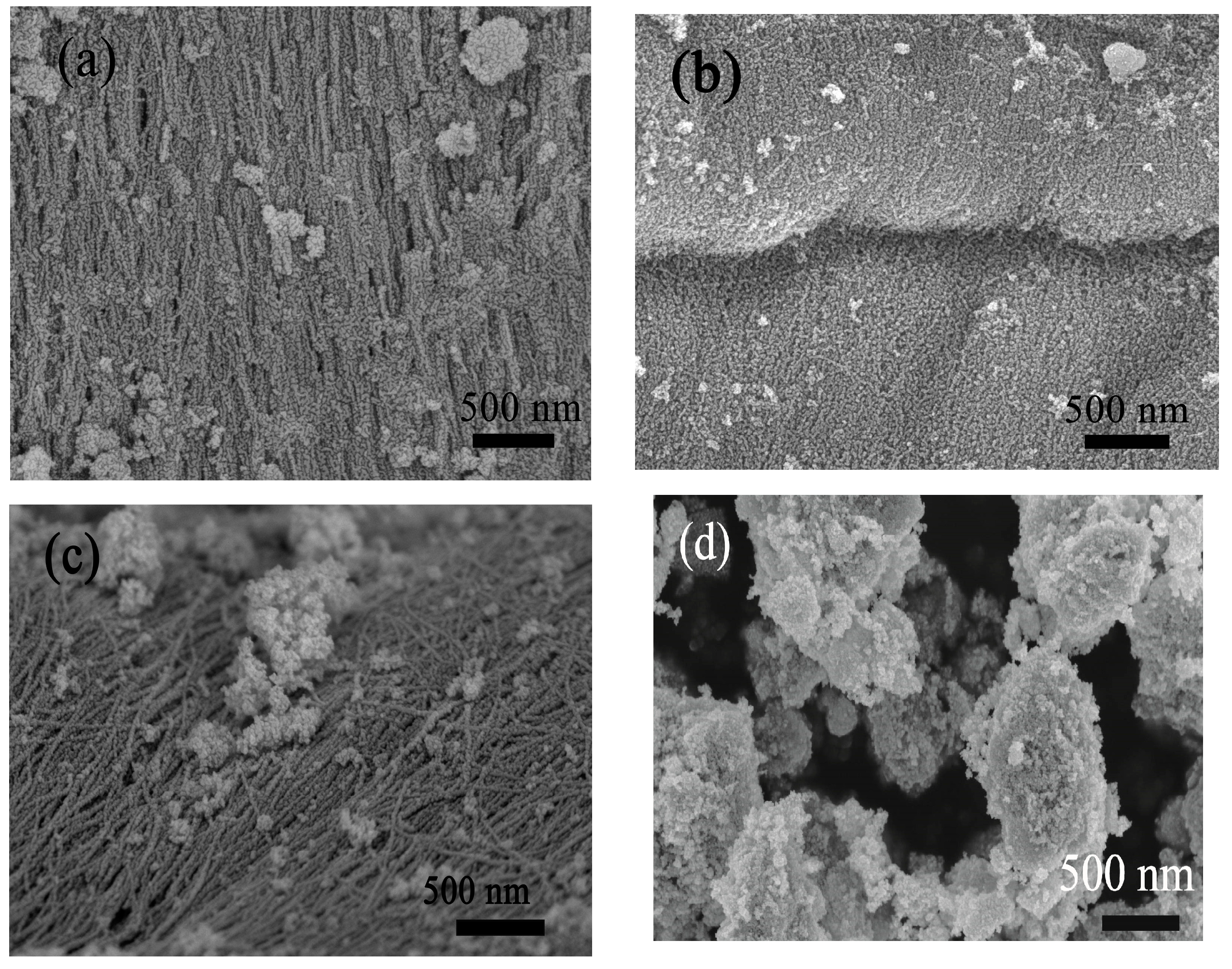
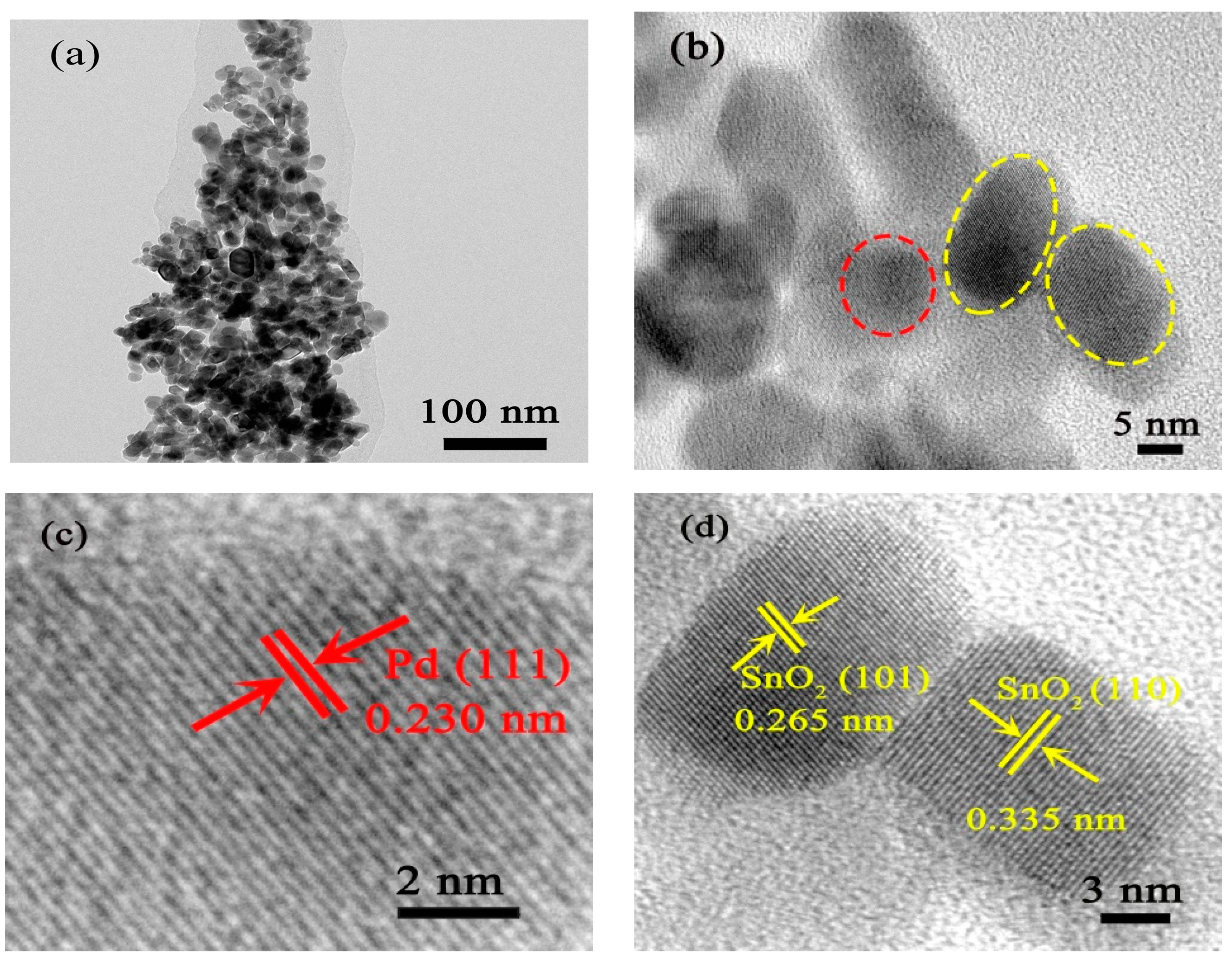
3.1. Structural and Morphological Characteristics
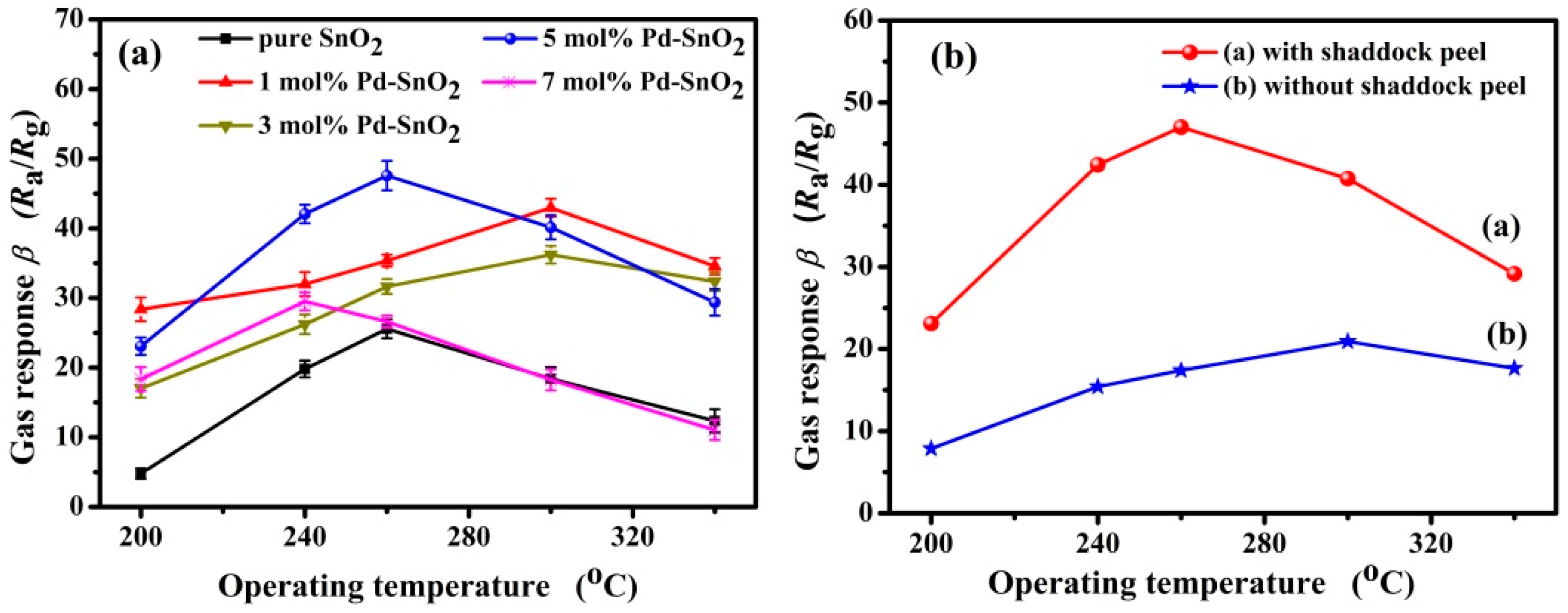
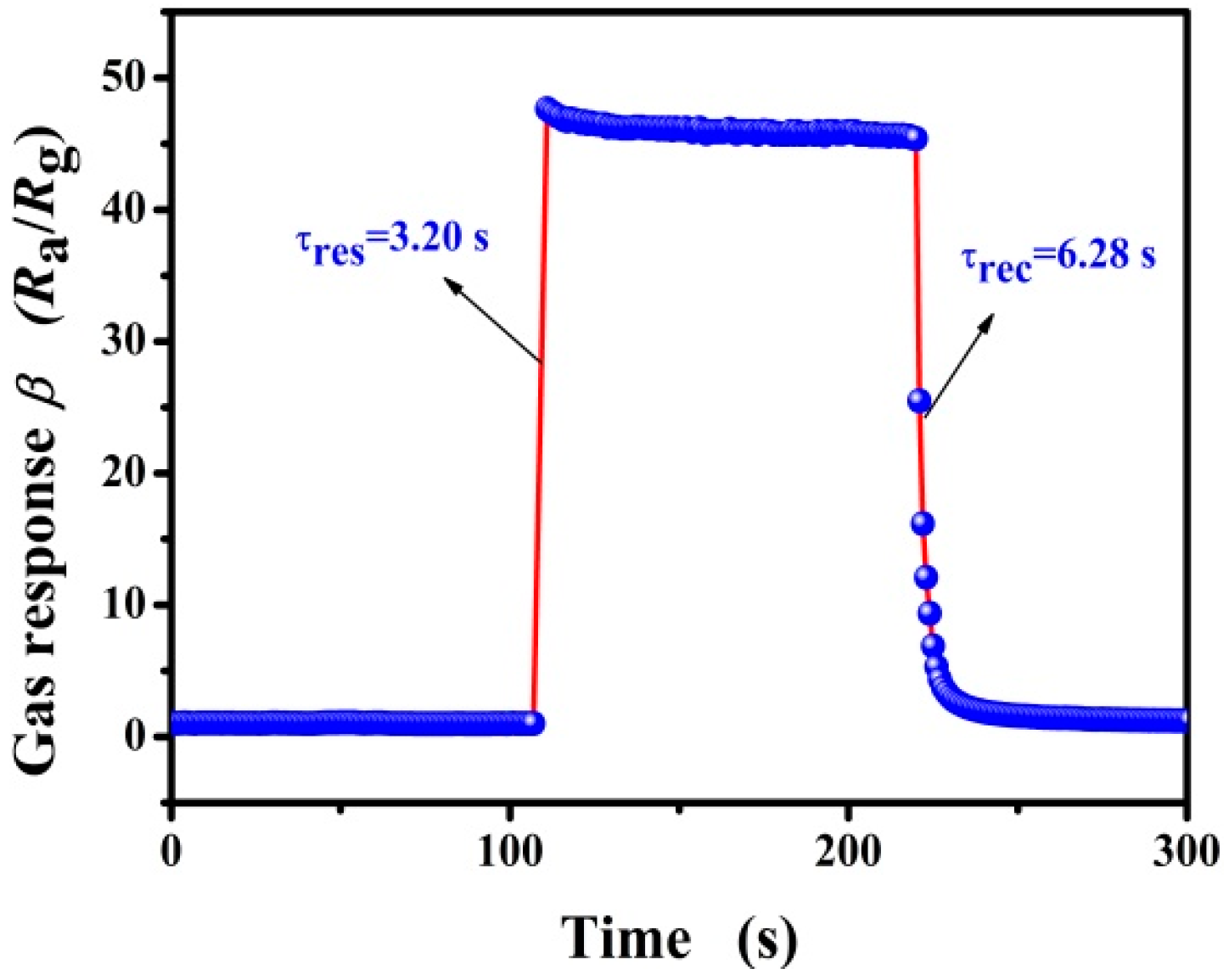
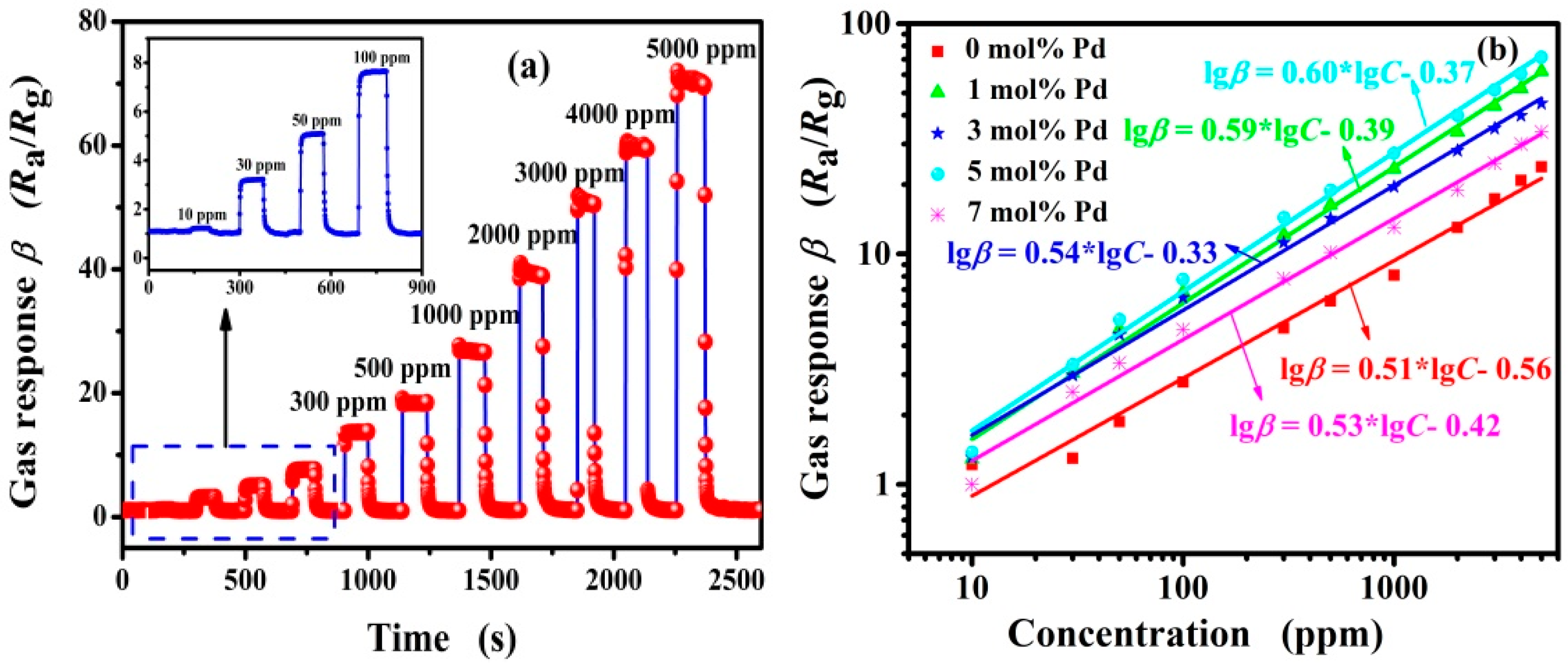
3.2. Gas-Sensing Properties of Pd-Functionalized SnO2
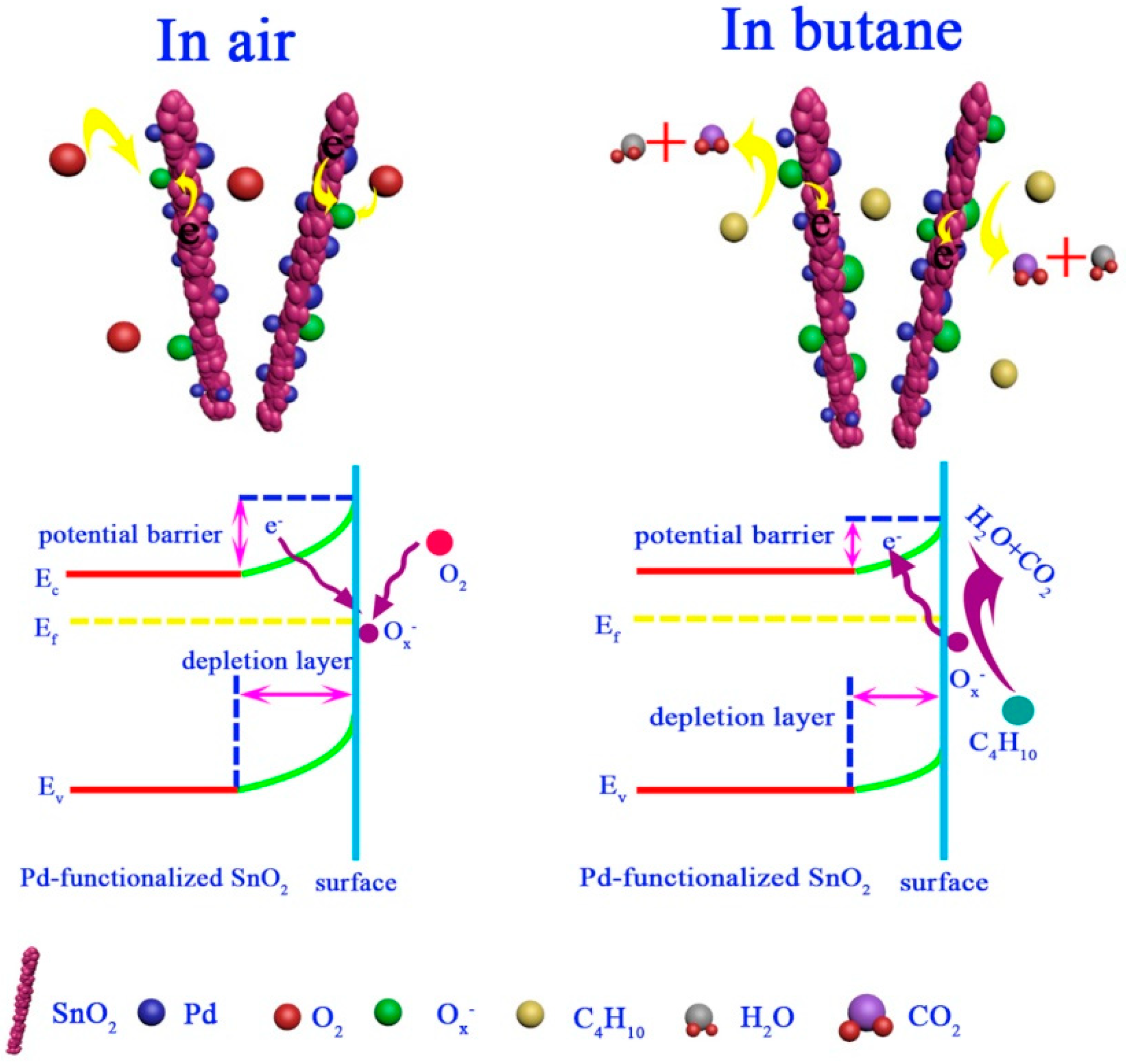
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhargav, K.K.; Maity, A.; Ram, S.; Majumder, S.B. Low temperature butane sensing catalytic nano-crystalline lanthanum ferrite sensing element. Sens. Actuators B 2014, 195, 303–312. [Google Scholar] [CrossRef]
- Liu, X.; Pan, K.M.; Wang, L.H.; Dong, C.J.; Xiao, X.C.; Wang, Y.D. Butane detection: W-doped TiO2 nanoparticles for a butane gas sensor with high sensitivity and fast response/recovery. RSC Adv. 2015, 5, 96539–96546. [Google Scholar] [CrossRef]
- Fan, H.T.; Zeng, Y.; Xu, X.J.; Lv, N.; Zhang, T. Hydrothermal synthesis of hollow ZnSnO3 microspheres and sensing properties toward butane. Sens. Actuators B 2011, 153, 170–175. [Google Scholar] [CrossRef]
- Wan, W.J.; Li, Y.H.; Zhao, Y.P.; Gao, F.; Zhao, H.Y. 2D SnO2 nanosheets: Synthesis, characterization, structures, and excellent sensing performance to ethylene glycol. Nanomaterials 2018, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.X.; Chen, T.; Zhao, R.J.; Wang, Z.Z.; Wang, Y.D. A low temperature butane gas sensor used Pt nanoparticles-modified AZO macro/mesoporous nanosheets as sensing material. Sens. Actuators B 2018, 254, 227–238. [Google Scholar] [CrossRef]
- Šetka, M.; Drbohlavová, J.; Hubálek, J. Nanostructured polypyrrole-based ammonia and volatile organic compound sensor. Sensor 2017, 17, 562–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Deng, D.Y.; Li, Y.X.; Liu, X.; Xing, X.X.; Xiao, X.C.; Wang, Y.D. TiO2 nanoparticles functionalized by Pd nanoparticles for gas-sensing application with enhanced butane response performances. Sci. Rep. 2017, 7, 7692–7703. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Jiang, M.; Tao, Y.; Shen, Y.Y.; Lu, Y.X.; Yuan, Y. Nonaqueous synthesized of Pd-functionalized SnO2/In2O3 nanocomposites for excellent butane sensing properties. Sens. Actuators B 2018, 257, 419–426. [Google Scholar] [CrossRef]
- Bearzotti, A.; Macagnano, A.; Pantalei, S.; Zampetti, E.; Venditti, I.; Fratoddi, I.; Russo, M.V. Alcohol vapor sensory properties of nanostructured conjugated polymers. J. Phys. Condens. Matter 2008, 20, 474207. [Google Scholar] [CrossRef]
- Stephens, J.; Batra, A.K.; Currie, J.R. Characteristics of Nanoparticles-Based Chemical Sensors. Mater. Sci. Appl. 2012, 3, 448–452. [Google Scholar] [CrossRef]
- Venditti, I.; Fratoddi, I.; Russo, M.V.; Bearzotti, A. A nanostructured composite based on polyaniline and gold nanoparticles: Synthesis and gas sensing properties. Nanotechnology 2013, 24, 155503. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, N.; Cohen, A.B.; Vaknin, Y.; Henning, A.; Hayon, J.; Shimanovich, K.; Greenspan, H.; Rosenwaks, Y. Electrostatic selectivity of volatile organic compounds using electrostatically formed nanowire sensor. ACS Sens. 2018, 3, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Wang, J.; Hu, R.J.; Qiao, Q.; Li, X.G. Synthesis and gas sensing properties of porous hierarchical SnO2 by grapefruit exocarp biotemplate. Sens. Actuators B 2016, 222, 1134–1143. [Google Scholar] [CrossRef]
- Xi, L.J.; Qian, D.; Tang, X.C.; Chen, C.J. High surface area SnO2 nanoparticles: Synthesis and gas sensing properties. Mater. Chem. Phys. 2008, 108, 232–236. [Google Scholar] [CrossRef]
- Wang, Y.L.; Jiang, X.C.; Xia, Y.N. A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J. Am. Chem. Soc. 2003, 125, 16176–16177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Chen, N.; Deng, D.Y.; Xing, X.X.; Xiao, X.C.; Wang, Y.D. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Zhao, R.J.; Wang, Z.Z.; Yang, Y.; Xing, X.X.; Zou, T.; Wang, Z.D.; Wang, Y.D. Raspberry-like SnO2 hollow nanostructure as a high response sensing material of gas sensor toward n-butanol gas. J. Phys. Chem. Solids 2018, 120, 173–182. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.G.; Li, J.; Tang, C.; Zhang, H. Nanosheet-assembled flower-like SnO2 hierarchical structures with enhanced gas-sensing performance. Mater. Lett. 2015, 161, 499–502. [Google Scholar] [CrossRef]
- Li, Y.X.; Deng, D.Y.; Chen, N.; Xing, X.X.; Liu, X.; Xiao, X.C.; Wang, Y.D. Pd nanoparticles composited SnO2 microspheres as sensing materials for gas sensors with enhanced hydrogen response performances. J. Alloys Compd. 2017, 710, 216–224. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Han, B.Q.; Xiao, X.C.; Chen, G.; Djerdj, I.; Wang, Y.D. Nanoparticles cluster gas sensor: Pt activated SnO2 nanoparticles for NH3 detection with ultrahigh sensitivity. Nanoscale 2015, 7, 14872–14880. [Google Scholar] [CrossRef]
- Li, H.H.; He, Y.; Jin, P.P.; Cao, Y.; Fan, M.H.; Zou, X.X.; Li, G.D. Highly selective detection of trace hydrogen against CO and CH4 by Ag/Ag2O-SnO2 composite microstructures. Sens. Actuators B 2016, 228, 515–522. [Google Scholar] [CrossRef]
- Yin, X.T.; Tao, L. Fabrication and gas sensing properties of Au-loaded SnO2 composite nanoparticles for low concentration hydrogen. J. Alloys Compd. 2017, 727, 254–259. [Google Scholar] [CrossRef]
- Du, H.Y.; Li, X.G.; Yao, P.J.; Wang, J.; Sun, Y.H.; Dong, L. Zinc oxide coated tin oxide nanofibers for improved selective acetone sensing. Nanomaterials 2018, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Li, Y.H.; Ren, X.P.; Gao, F.; Zhao, H.Y. The effect of Eu doping on microstructure, morphology and methanol-sensing performance of highly ordered SnO2 nanorods array. Nanomaterials 2017, 7, 410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, J.L.; Wang, Y.A.; Fang, X.T.; Huang, Y.Q.; Chen, W.X.; Liu, Q.L.; Zhang, D. Single porous SnO2 microtubes templated from Papiliomaacki bristles: New structure towards superior gas sensing. J. Mater. Chem. A 2014, 2, 4543–4550. [Google Scholar] [CrossRef]
- Zhu, S.M.; Zhang, D.; Gu, J.J.; Xu, J.Q.; Dong, J.P.; Li, J.L. Biotemplate fabrication of SnO2 nanotubular materials by a sonochemical method for gas sensors. J. Nanopart. Res. 2010, 12, 1389–1400. [Google Scholar] [CrossRef]
- Song, F.; Su, H.L.; Han, J.; Lau, W.M.; Moon, W.J.; Zhang, D. Bioinspried hierarchical tin oxide scaffolds for enhanced gas sensing properties. J. Phys. Chem. C 2012, 116, 10274–10281. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Wang, X.F.; Yu, J.Y.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 11, 16–27. [Google Scholar] [CrossRef]
- Wang, Y.D.; Djerdj, I.; Antonietti, M.; Smarsly, B. Polymer-assisted generation of antimony-doped SnO2 nanoparticles with high crystalline for application in gas sensors. Small 2008, 4, 1656–1660. [Google Scholar] [CrossRef]
- Dong, C.J.; Liu, X.; Han, B.Q.; Deng, S.J.; Xiao, X.C.; Wang, Y.D. Nonaqueous synthesis of Ag-functionalized In2O3/ZnO nanocomposites for highly sensitive formaldehyde sensor. Sens. Actuators B 2016, 224, 193–200. [Google Scholar] [CrossRef]
- Aziz, M.; Abbas, S.S.; Baharom, W.R.W.; Mahmud, W.Z.W. Structure of SnO2 nanoparticles by sol–gel method. Mter. Lett. 2012, 74, 62–64. [Google Scholar] [CrossRef]
- Shirotori, M.; Nishimura, S.; Ebitani, K. Fine-crystallized LDHs prepared with SiO2 spheresas highly active solid base catalysts. J. Mater. Chem. A 2017, 5, 6947–6957. [Google Scholar] [CrossRef]
- Hu, D.; Han, B.Q.; Han, R.; Deng, S.J.; Wang, Y.; Li, Q.; Wang, Y.D. SnO2 nanorods based sensing material as an isopropanol vapor sensor. New J. Chem. 2014, 38, 2443–2450. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, W.; Li, Y.J.; Li, F.; Zhou, J.R.; Sun, D.M.; Chen, Y.; Ruan, S.P. Preparation of Pd nanoparticle-decorated hollow SnO2 nanofibers and their enhanced formaldehyde sensing properties. J. Alloys Compd. 2015, 65, 690–698. [Google Scholar] [CrossRef]
- Becker, T.; Ahlers, S.; Braunmuhl, C.B.; Müller, G.; Kiesewetter, O. Gas sensing properties of thin- and thick-film tin-oxide materials. Sens. Actuators B 2001, 77, 55–61. [Google Scholar] [CrossRef]
- Ahlers, S.; Müller, G.; Becker, T.; Doll, T. Factors Influencing the Gas Sensitivity of Metal Oxide Materials. Encycl. Sens. 2006, 3, 413–447. [Google Scholar]
- Banerjee, S.; Nag, P.; Majumdar, S.; Devi, P.S. High butane sensitivity and selectivity exhibited by combustion synthesized SnO2 nanoparticles. Mater. Res. Bull. 2015, 65, 216–223. [Google Scholar] [CrossRef]
- Cai, P.; Dong, C.J.; Jiang, M.; Shen, Y.Y.; Tao, Y.; Wang, Y.D. SnO2 quantum dots with rapid butane detection at lower ppm-level. Appl. Phys. A 2018, 124, 293–299. [Google Scholar] [CrossRef]
- Ray, I.; Chakraborty, S.; Chowdhury, A.; Majumdar, S.; Prakash, A.; Pyare, R.; Sen, A. Room-temperature synthesis of γ-Fe2O3 by sonochemical route and its response towards butane. Sens. Actuators B 2008, 130, 882–888. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sen, A.; Maiti, H.S. Selective detection of methane and butane by temperature modulation in iron doped tin oxide sensors. Sens. Actuators B 2006, 115, 610–613. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Feng, W.; Sun, P.; Li, X.W.; Lu, G.Y. Hollow α-Fe2O3 quasi-cubic structures: Hydrothermal synthesis and gas sensing properties. Mater. Lett. 2014, 120, 5–8. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.D.; Mi, R.N.; Liu, M.; Chen, Y.; Chen, C.; Ruan, S.P. On the high response towards TEA of gas sensors based on Ag-loaded 3D porous ZnO microspheres. Sens. Actuators B 2018, 270, 492–499. [Google Scholar] [CrossRef]
- Chen, N.; Deng, D.Y.; Li, Y.X.; Xing, X.X.; Liu, X.; Xiao, X.C.; Wang, Y.D. The xylene sensing performance of WO3 decorated anatase TiO2 nanoparticles as a sensing material for a gas sensor at a low operating temperature. RSC Adv. 2016, 6, 49692–49701. [Google Scholar] [CrossRef]
- Jing, Z.H.; Zhan, J.H. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008, 20, 4547–4551. [Google Scholar] [CrossRef]
- Rai, P.; Kwak, W.K.; Yu, Y.T. Solvothermal synthesis of ZnO nanostructures and their morphology-dependent gas-sensing properties. ACS Appl. Mater. Interfaces 2013, 5, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.D.; Shi, B.; Dai, R.R.; Jia, X.H.; Wu, X.Y. Synthesis and enhanced acetone gas-sensing performance of ZnSnO3/SnO2 hollow urchin nanostructures. J. Nanopart. Res. 2017, 19, 401–419. [Google Scholar] [CrossRef]
- Xiao, L.; Shu, S.M.; Liu, S.T. A facile synthesis of Pd-doped SnO2 hollow microcubes with enhanced sensing performance. Sens. Actuators B 2015, 221, 120–126. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chen, T.; Mu, Q.Y.; Wang, G.F. A nonaqueous sol-gel route to synthesize CdIn2O4 nanoparticles for the improvement of formaldehyde-sensing performance. Scr. Mater. 2009, 61, 935–938. [Google Scholar] [CrossRef]
- Ruiz, A.M.; Cornet, A.; Shimanoe, K.; Morante, J.R.; Yamazoe, N. Effects of various metal additives on the gas sensing performances of TiO2 nanocrystals obtained from hydrothermal treatments. Sens. Actuators B 2005, 108, 34–40. [Google Scholar] [CrossRef]
- Schierbaum, K.D.; Kirner, U.K.; Geiger, J.F.; Gopel, W. Schottky-barrier and conductivity gas sensors based upon Pd/SnO2 and Pt/TiO2. Sens. Actuators B 1991, 4, 87–97. [Google Scholar] [CrossRef]
- Fu, J.C.; Zhao, C.H.; Zhang, J.L.; Peng, Y.; Xie, E.Q. Enhanced gas sensing performance of electrospun Pt-functionalized NiO nanotubes with chemical and electronic sensitization. ACS Appl. Mater. Interfaces 2013, 5, 7410–7416. [Google Scholar] [CrossRef] [PubMed]
- Sennik, E.; Alve, O.; Öztürk, Z. The effect of Pd on the H2 and VOC sensing properties of TiO2 nanorods. Sens. Actuators B 2016, 229, 692–700. [Google Scholar] [CrossRef]
- Dong, C.J.; Li, Q.; Chen, G.; Xiao, X.C.; Wang, Y.D. Enhanced formaldehyde sensing performance of 3D hierarchical porous structure Pt-functionalized NiO via a facile solution combustion synthesis. Sens. Actuators B 2015, 220, 171–179. [Google Scholar] [CrossRef]

| Materials | C (ppm) | OT (°C) | τres/τrec (s) | β | Ref. |
|---|---|---|---|---|---|
| W-doped TiO2 nanoparticle | 3000 | 420 | 2./12 | 17.80 | [2] |
| Hollow ZnSnO3 | 500 | 380 | 0.3/0.65 | 5.79 | [3] |
| Pt-AZO nanosheets | 3000 | 200 | 31/28 | 56.52 | [5] |
| 7.5 mol% Pd-TiO2 | 3000 | 340 | 13/8 | 33.93 | [7] |
| SnO2/In2O3 | 3000 | 320 | 3.40/5.82 | 29.27 | [8] |
| 7 at% Pd-SnO2/In2O3 | 3000 | 320 | 3.51/7.86 | 71.28 | [8] |
| SnO2 quantum dots | 100 | 400 | 3/8 | 5.10 | [38] |
| γ-Fe2O3 nanocrystalline | 1000 | 300 | 12/120 | 14.29 | [39] |
| Fe-doped SnO2 powder | 1000 | 325 | -/- | 5.88 | [40] |
| 5 mol% Pd-SnO2 | 100 | 260 | 2.53/9.21 | 7.78 | This work |
| 500 | 0.83/9.24 | 18.95 | |||
| 1000 | 0.86/8.63 | 27.38 | |||
| 3000 | 3.20/6.28 | 47.58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Wang, Z.; Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Hong, P.; Peng, S.; Wang, Y. Pd-Functionalized SnO2 Nanofibers Prepared by Shaddock Peels as Bio-Templates for High Gas Sensing Performance toward Butane. Nanomaterials 2019, 9, 13. https://doi.org/10.3390/nano9010013
Zhao R, Wang Z, Yang Y, Xing X, Zou T, Wang Z, Hong P, Peng S, Wang Y. Pd-Functionalized SnO2 Nanofibers Prepared by Shaddock Peels as Bio-Templates for High Gas Sensing Performance toward Butane. Nanomaterials. 2019; 9(1):13. https://doi.org/10.3390/nano9010013
Chicago/Turabian StyleZhao, Rongjun, Zhezhe Wang, Yue Yang, Xinxin Xing, Tong Zou, Zidong Wang, Ping Hong, Sijia Peng, and Yude Wang. 2019. "Pd-Functionalized SnO2 Nanofibers Prepared by Shaddock Peels as Bio-Templates for High Gas Sensing Performance toward Butane" Nanomaterials 9, no. 1: 13. https://doi.org/10.3390/nano9010013
APA StyleZhao, R., Wang, Z., Yang, Y., Xing, X., Zou, T., Wang, Z., Hong, P., Peng, S., & Wang, Y. (2019). Pd-Functionalized SnO2 Nanofibers Prepared by Shaddock Peels as Bio-Templates for High Gas Sensing Performance toward Butane. Nanomaterials, 9(1), 13. https://doi.org/10.3390/nano9010013




