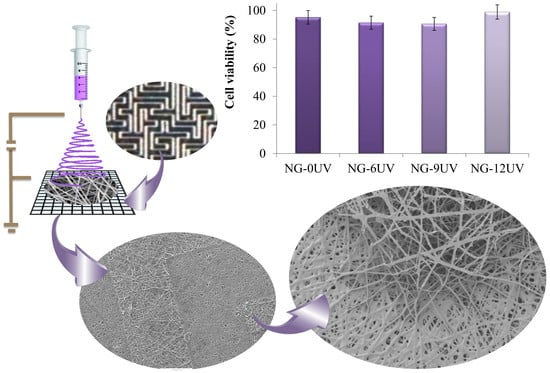
Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GelMA
2.3. Fabrication of Electrospun GelMA Nanofibers
2.4. Photocrosslinking of GelMA Nanofibers
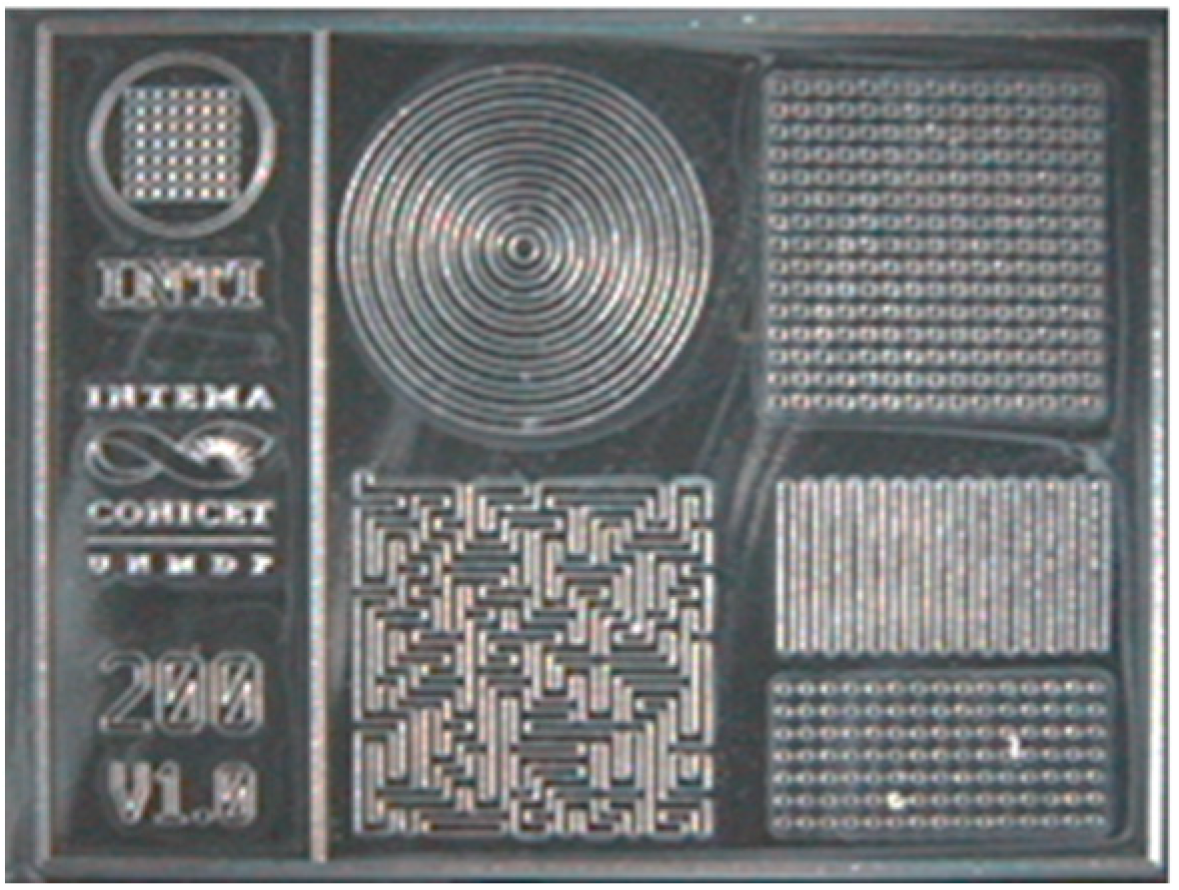
2.5. Fabrication of Micropatterned Molds
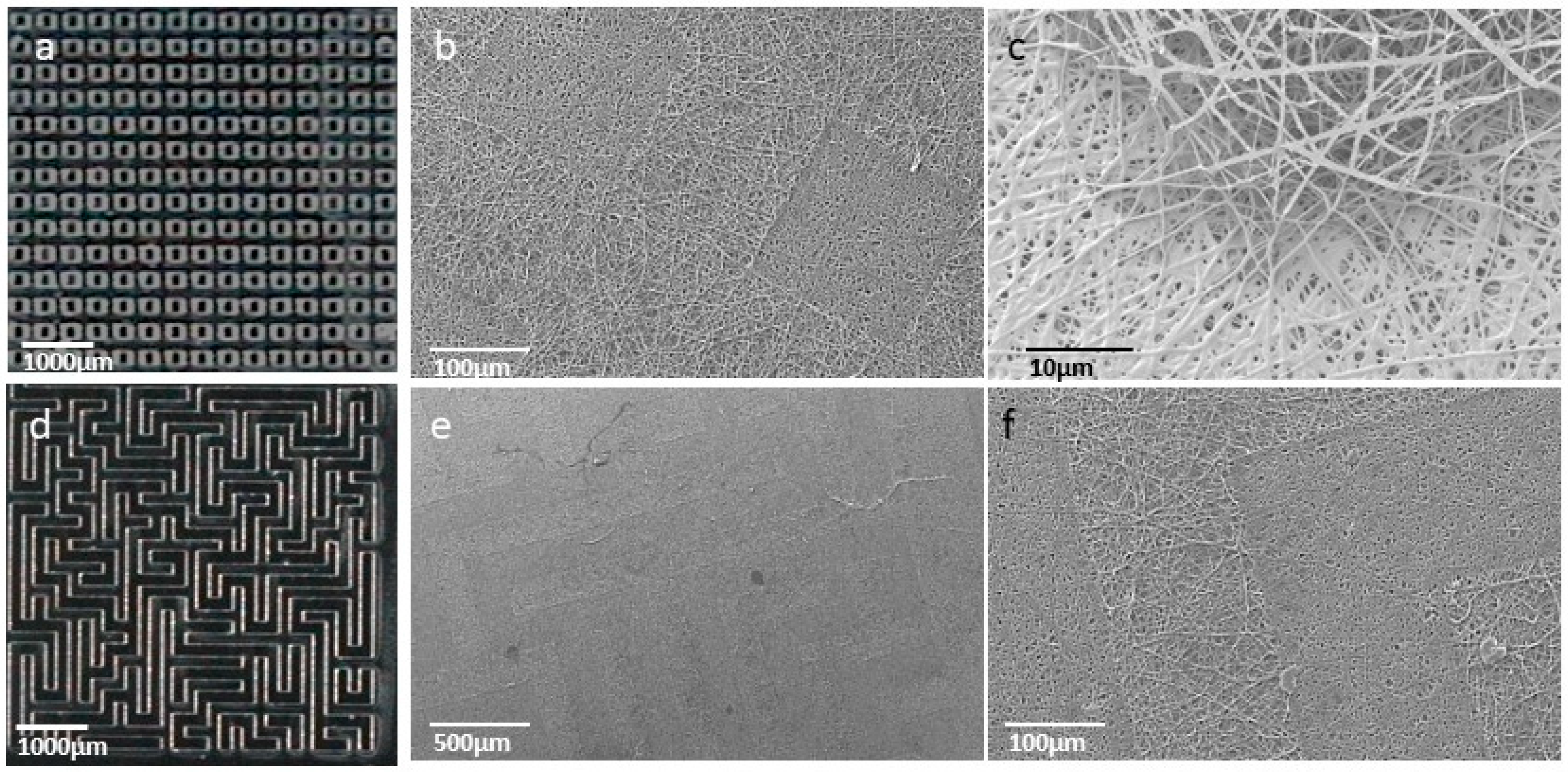
2.6. Fabrication of Micropatterned Nanofibrous GelMA Scaffolds
2.7. Morphology Characterization
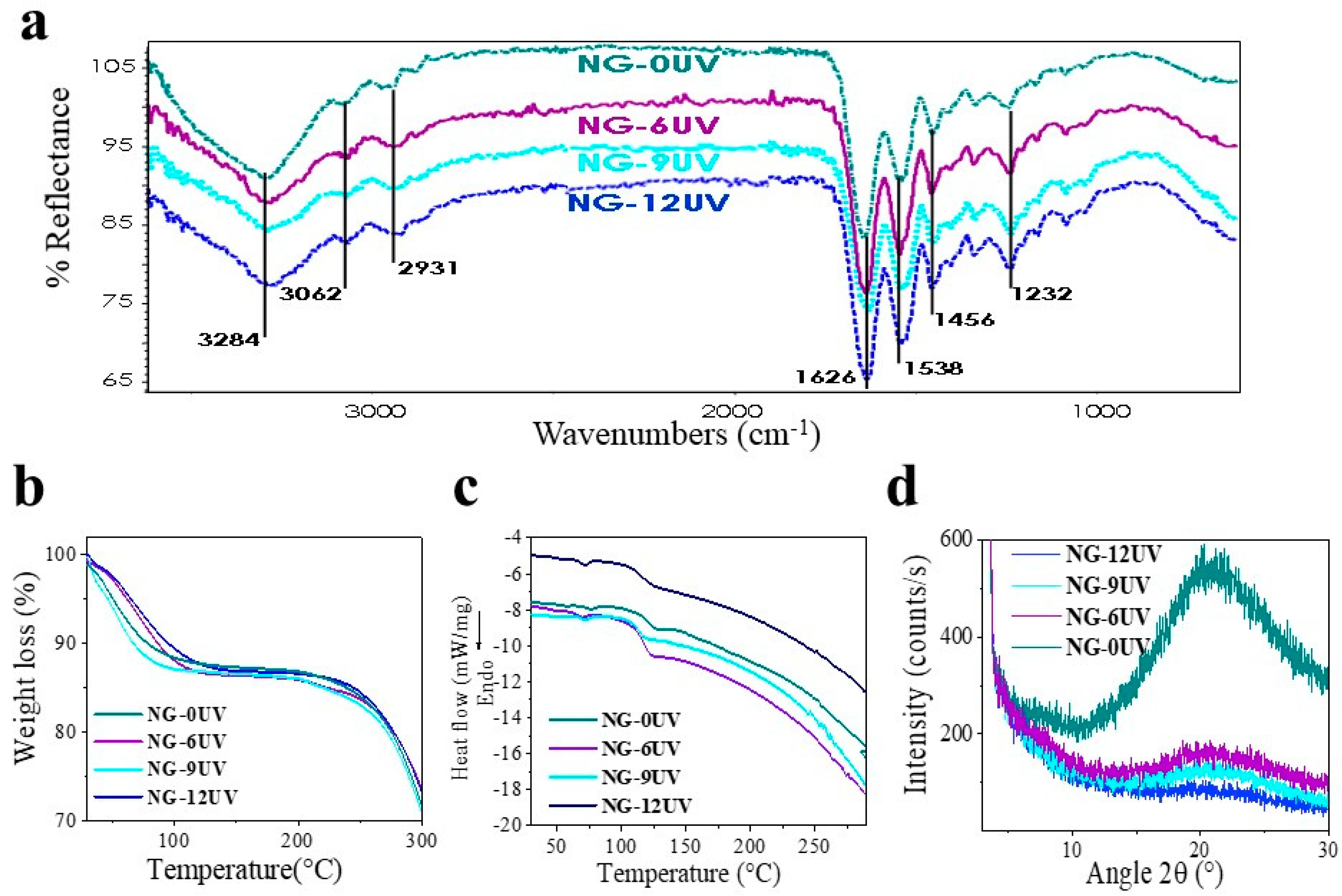
2.8. Infrared Analysis
2.9. Contact Angle Measurements
2.10. Water Uptake Measurements
2.11. Tensile Testing
2.12. Thermal Analysis
2.13. X-ray Diffraction
2.14. Roughness Measurements
2.15. Cell Viability
3. Results
3.1. GelMA Nanofibrous Mats
3.1.1. Synthesis of GelMA
3.1.2. Fabrication of Electrospun GelMA Matrices
3.2. Micropatterned Nanofibrous GelMA Scaffolds
3.2.1. Fabrication of Micropatterned Molds
3.2.2. Fabrication of Micropatterned Nanofibrous GelMA Scaffolds
3.3. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kang, D.-H.; Kim, M.S.; Jiao, A.; Kim, D.-H.; Suh, K.-Y. Patterning methods for polymers in cell and tissue engineering. Ann. Biomed. Eng. 2012, 40, 1339–1355. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Iijima, K.; Otsuka, H. Nanofabrication technologies to control cell and tissue function for biomedical applications. In Nanobiomaterials; Narayan, R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 385–409. ISBN 9780081007167. [Google Scholar]
- Petrie, R.J.; Doyle, A.D.; Yamada, K.M. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical attributes of nanofibers: Preparation, drug loading, and tissue regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Ngadiman, N.; Noordin, M.; Idris, A.; Kurniawan, D.; Fallahiarezoudar, E.; Sudin, I. Developments in tissue engineering scaffolding using an electrospinning process. In Electrospinning and Electroplating: Fundamentals, Methods and Applications; Jacobs, T., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 87–125. ISBN 9781536123890. [Google Scholar]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Electrospinning: A versatile processing technology for producing nanofibrous materials for biomedical and tissue-engineering applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Uyar, T., Kny, E., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 3–41. ISBN 9780081010228. [Google Scholar]
- Gao, Y.; Yan, J.; Cui, X.-J.; Wang, H.-Y.; Wang, Q. Aligned fibrous scaffold induced aligned growth of corneal stroma cells in vitro culture. Chem. Res. Chin. Univ. 2012, 28, 1022–1025. [Google Scholar]
- Shalumon, K.T.; Deepthi, S.; Anupama, M.S.; Nair, S.V.; Jayakumar, R.; Chennazhi, K.P. Fabrication of poly (I-lactic acid)/gelatin composite tubular scaffolds for vascular tissue engineering. Int. J. Biol. Macromol. 2015, 72, 1048–1055. [Google Scholar] [CrossRef]
- Martínez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behavior. Ann. Anat. 2009, 191, 126–135. [Google Scholar] [CrossRef]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- Zheng, L.; Jiang, J.; Gui, J.; Zhang, L.; Liu, X.; Sun, Y.; Fan, Y. Influence of Micropatterning on Human Periodontal Ligament Cells’ Behavior. Biophys. J. 2018, 114, 1988–2000. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, D.; Levenberg, S. Effect of Matrix Mechanical Forces and Geometry on Stem Cell Behavior. In Biology and Engineering of Stem Cell Niches; Vishwakarma, A., Karp, J.M., Eds.; Academic Press: London, UK, 2017; pp. 233–243. ISBN 9780128027349. [Google Scholar]
- ICH. Q3C Guideline for Residual Solvents (R5). In International Conference Harmon Tech Requir Regist Pharm Hum Use 29; ICH: Geneva, Switzerland, 2011. [Google Scholar]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef]
- Chou, S.-F.; Luo, L.-J.; Lai, J.-Y.; Ma, D.H.-K. Role of solvent-mediated carbodiimide cross-linking in fabrication of electrospun gelatin nanofibrous membranes as ophthalmic biomaterials. Mater. Sci. Eng. C 2017, 71, 1145–1155. [Google Scholar] [CrossRef]
- Van den Bulcke, A.I.; Bogdanov, B.; Cornelissen, M.; Schacht, E.H.; de Rooze, N.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly(ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Aldana, A.A.; Rial-Hermida, M.I.; Abraham, G.A.; Concheiro, A.; Alvarez-Lorenzo, C. Temperature-sensitive biocompatible IPN hydrogels based on poly(NIPA-PEGdma) and photocrosslinkable gelatin methacrylate. Soft Mater. 2017, 15, 341–349. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, M.J.; Ki, C.S.; Kim, H.J.; Park, Y.H. Fabrication of bi-layer scaffold of keratin nanofiber and gelatin-methacrylate hydrogel: Implications for skin graft. Int. J. Biol. Macromol. 2017, 105, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Habeeb, A.F.S.A. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14. [Google Scholar] [CrossRef]
- Schumacher, M.; Uhl, F.; Detsch, R.; Deisinger, U.; Ziegler, G. Indirect rapid prototyping of biphasic calcium phosphate scaffolds as bone substitutes: Influence of phase composition, macroporosity and pore geometry on mechanical properties. J. Mater. Sci. Mater. Med. 2010, 21, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I.; Rosolen, M.A. Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J. Therm. Anal. Calorim. 2013, 114, 1161–1166. [Google Scholar] [CrossRef]
- Ferreira, P.; Santos, P.; Alves, P.; Carvalho, M.P.; de Sá, K.D.; Miguel, S.P.; Correia, I.J.; Coimbra, P. Photocrosslinkable electrospun fiber meshes for tissue engineering applications. Eur. Polym. J. 2017, 97, 210–219. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, P.; Santos, P.; Alves, P.; Miguel, S.P.; Carvalho, M.P.; de Sá, K.D.; Correia, I.J.; Ferreira, P. Coaxial electrospun PCL/Gelatin-MA fibers as scaffolds for vascular tissue engineering. Colloids Surf. B Biointerfaces 2017, 159, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y.; Luo, L.-J. Effect of riboflavin concentration on the development of photo-cross-linked amniotic membranes for cultivation of limbal epithelial cells. RSC Adv. 2015, 5, 3425–3434. [Google Scholar] [CrossRef]
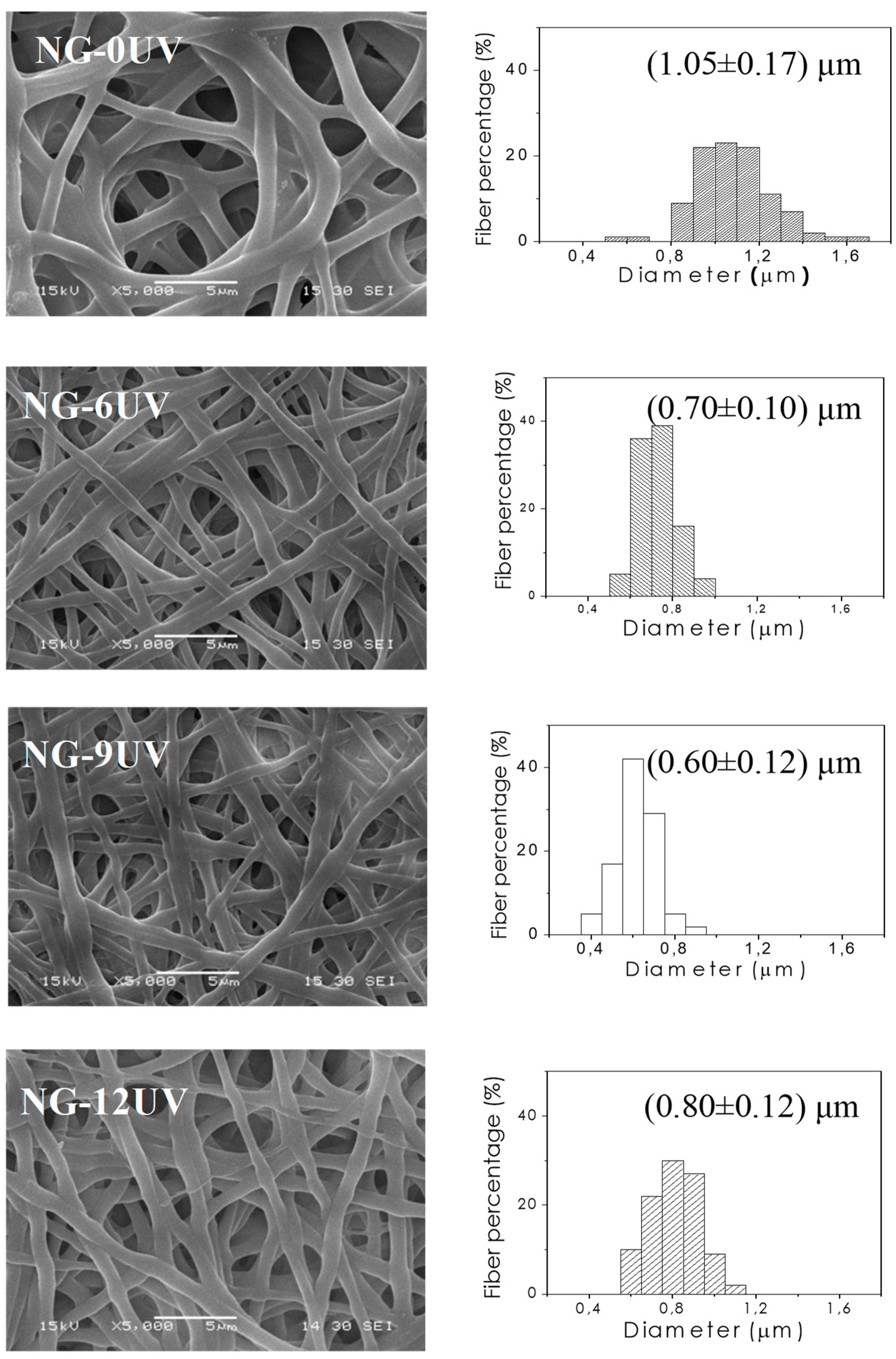
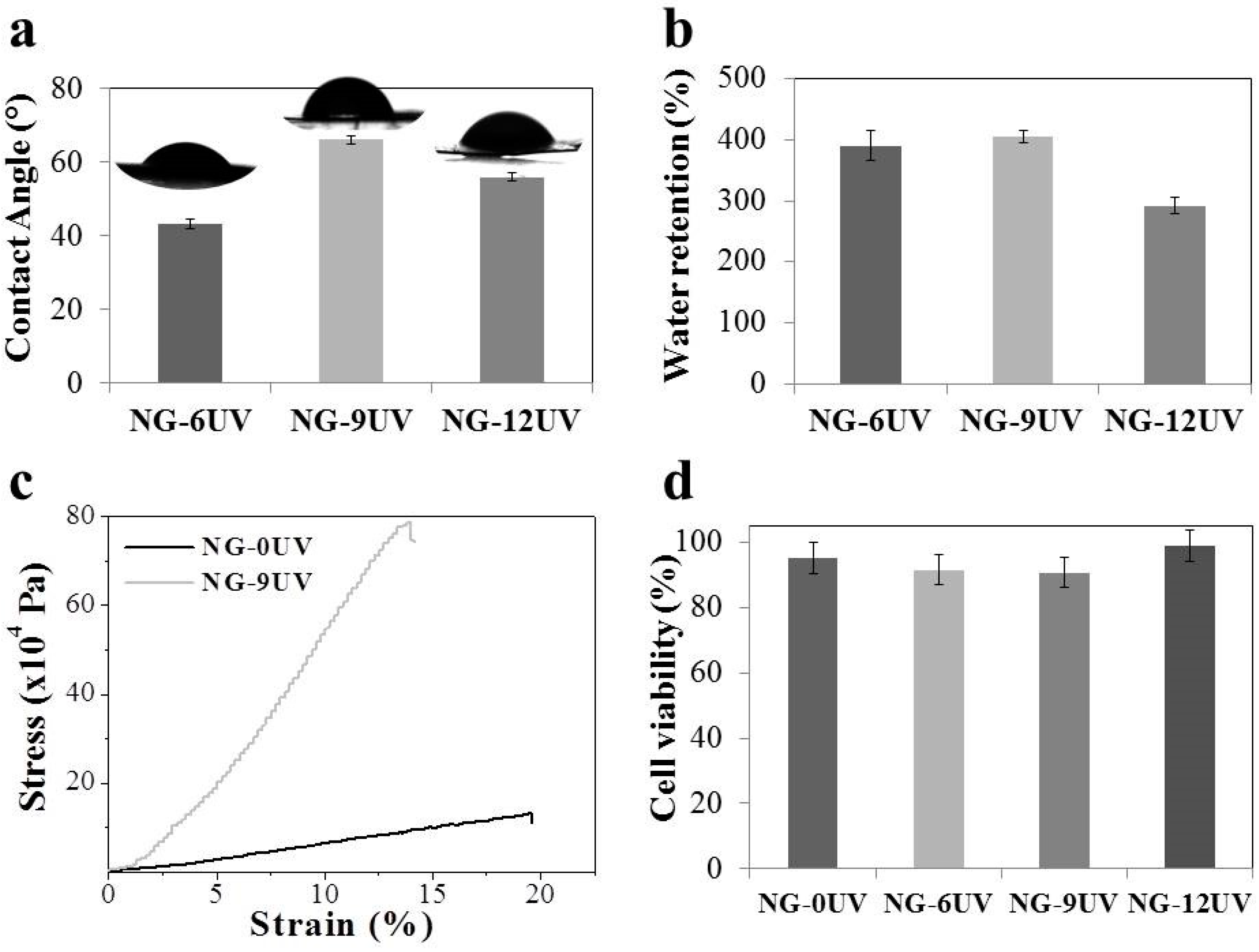
| Matrix. | Mean Diameter (µm) | Contact Angle (°) | WR (%) | Tgonset (°C) | Tmonset (°C) |
|---|---|---|---|---|---|
| NG-0UV | 1.05 ± 0.17 | 0 | n/d | 57.6 | 74.1 |
| NG-6UV | 0.70 ± 0.10 | 43.2 | 390 ± 21 | 52.3 | 66.5 |
| NG-9UV | 0.60 ± 0.12 | 66.1 | 405 ± 10 | 52.9 | 71.2 |
| NG-12UV | 0.80 ± 0.12 | 56.0 | 290 ± 15 | 53.9 | 68.6 |
| Sample | Ra (µm) | Rz (µm) | Rmax (µm) |
|---|---|---|---|
| NG-P400 | 1.5 ± 0.5 | 4.0 ± 0.2 | 14 ± 1 |
| NG-P200 | 0.7 ± 0.05 | 2.5 ± 0.4 | 6 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldana, A.A.; Malatto, L.; Rehman, M.A.U.; Boccaccini, A.R.; Abraham, G.A. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials 2019, 9, 120. https://doi.org/10.3390/nano9010120
Aldana AA, Malatto L, Rehman MAU, Boccaccini AR, Abraham GA. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials. 2019; 9(1):120. https://doi.org/10.3390/nano9010120
Chicago/Turabian StyleAldana, Ana Agustina, Laura Malatto, Muhammad Atiq Ur Rehman, Aldo Roberto Boccaccini, and Gustavo Abel Abraham. 2019. "Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features" Nanomaterials 9, no. 1: 120. https://doi.org/10.3390/nano9010120
APA StyleAldana, A. A., Malatto, L., Rehman, M. A. U., Boccaccini, A. R., & Abraham, G. A. (2019). Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials, 9(1), 120. https://doi.org/10.3390/nano9010120