Mechanochemically Synthesised Coal-Based Magnetic Carbon Composites for Removing As(V) and Cd(II) from Aqueous Solutions
Abstract
1. Introduction
- (i)
- pure synthetic inorganic compounds/oxides of metals such as Fe, Co, Ni, and Cu (e.g., Fe3O4, α-Fe2O3, γ-Fe2O3, β-FeOOH, and CuFe2O4),
- (ii)
- magnetic silica-based composites (Si-containing polymers such as zeolites and natural or synthetic aluminosilicate compounds used as matrices modified/treated by magnetic particles, mainly iron oxides), and
- (iii)
- magnetic carbon composites (carbon matrices modified with magnetic particles), e.g., activated carbon, biochars, fullerenes, nanotubes, and other carbon materials used as matrices.
2. Materials and Methods
2.1. Preparation of Magnetic Carbons
2.2. Volumetric Magnetic Susceptibility
2.3. X-ray Powder Diffraction (XRD)
2.4. Textural Analysis
2.5. Mössbauer Spectroscopy
2.6. Morphology
2.7. Elemental Analysis and Ash Content
2.8. XPS
2.9. Zeta Potential (ZP)
2.10. Sorption Experiments
3. Results and Discussion
3.1. Characterisation of Initial Materials (Coal and Char) and the Synthesised Magnetic Sorbents
3.2. Surface Analysis of the Magnetic Sorbents
3.3. Structural Differences/Similarities of the Prepared Magnetic Sorbents
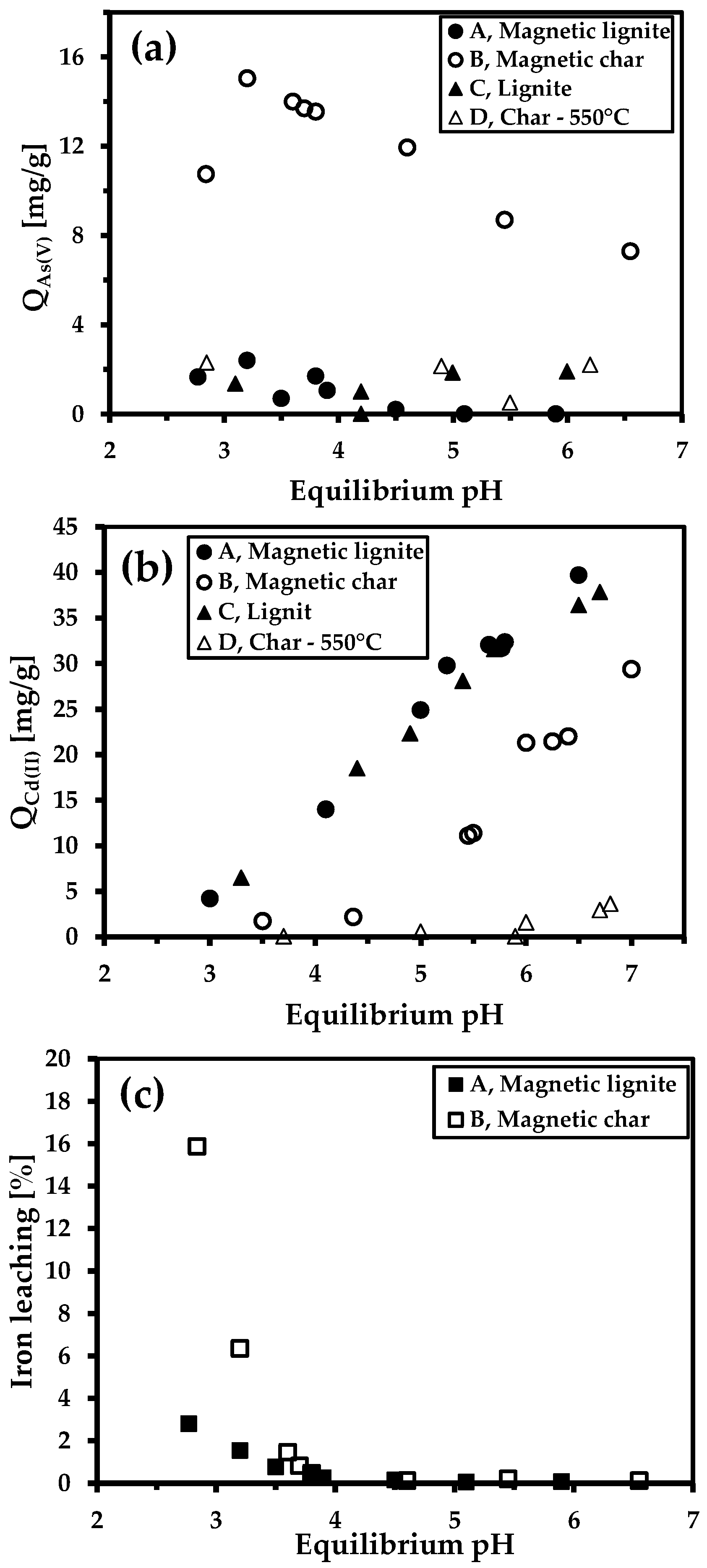
3.4. Effect of pH on Sorption Properties (Cd(II) and As(V))
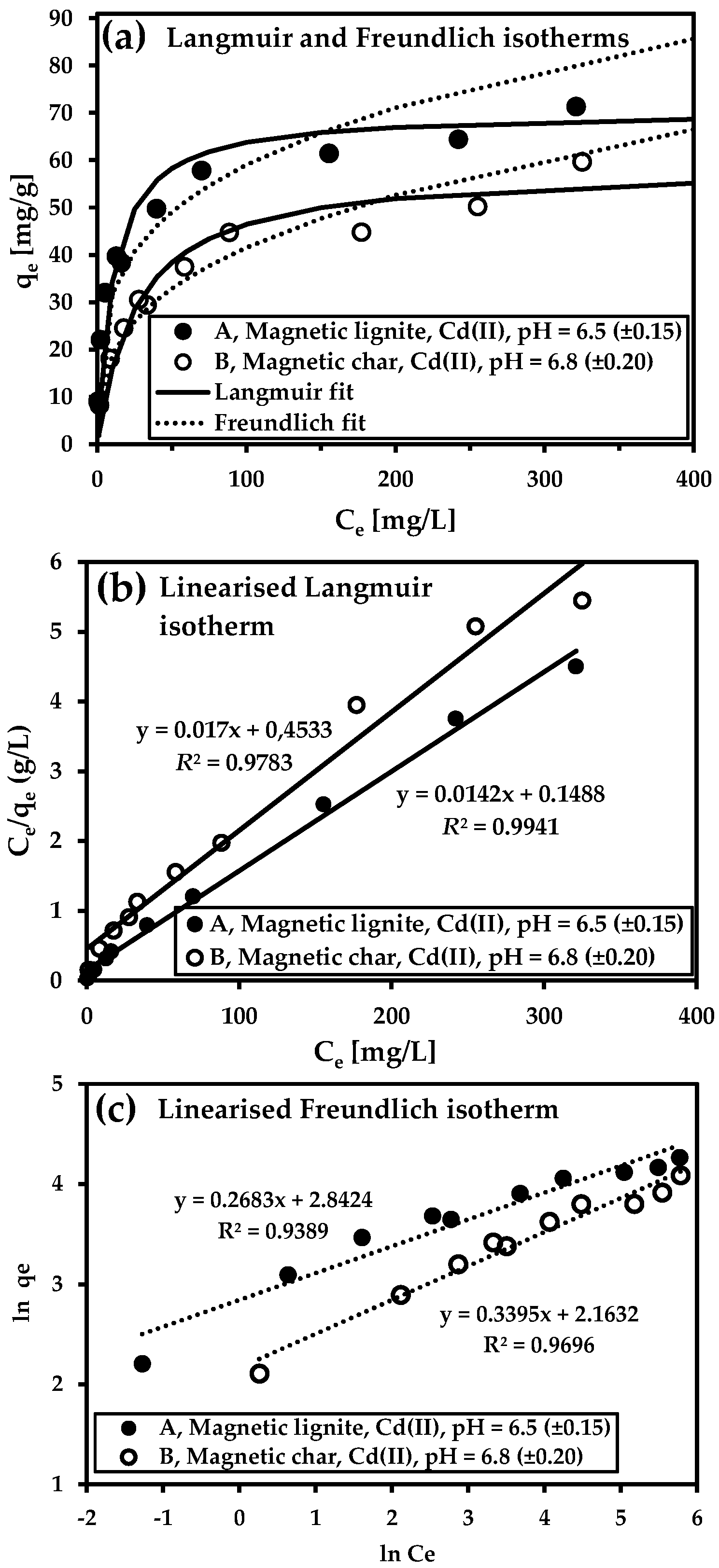
3.5. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karimifard, S.; Alavi Moghaddam, M.R. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total. Environ. 2018, 640–641, 772–797. [Google Scholar] [CrossRef]
- Zubrik, A.; Matik, M.; Hredzák, S.; Lovás, M.; Danková, Z.; Kováčová, M.; Briančin, J. Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J. Clean. Prod. 2017, 143, 643–653. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Yang, Q.; Chen, H.; Chen, X.; Jiao, T.; Peng, Q. Distinguished Cr(VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism. J. Hazard. Mater. 2018, 342, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.-F.; Lee, C.-L.; Hsieh, P.-Y.; Chen, C.-S.; Kang, Y.-Y.; Chin, W.-C.; Tai, N.-H. Superhydrophobic graphene-based sponge as a novel sorbent for crude oil removal under various environmental conditions. Chemosphere 2018, 207, 110–117. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Pashaei, S.; Hosseinzadeh, S.; Khodaparast, Z.; Ramin, S.; Saadat, Y. Preparation of novel multi-walled carbon nanotubes nanocomposite adsorbent via raft technique for the adsorption of toxic copper ions. Sci. Total. Environ. 2018, 640–641, 303–314. [Google Scholar] [CrossRef]
- Zhao, H.-T.; Ma, S.; Zheng, S.-Y.; Han, S.-W.; Yao, F.-X.; Wang, X.-Z.; Wang, S.-S.; Feng, K. B–cyclodextrin functionalized biochars as novel sorbents for high-performance of Pb2+ removal. J. Hazard. Mater. 2019, 362, 206–213. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Huang, W.; Tan, X.; Dong, H.; Goodman, B.A.; Du, H.; Lei, F.; Diao, K. Adsorption of phenolic compounds from water by a novel ethylenediamine rosin-based resin: Interaction models and adsorption mechanisms. Chemosphere 2019, 214, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Li, X.; Wu, Z.; Alsaedi, A.; Hayat, T.; Chen, C.; Li, J. Adsorption of 17β-estradiol from aqueous solutions by a novel hierarchically nitrogen-doped porous carbon. J. Colloid Interface Sci. 2019, 533, 700–708. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. Novel and sustainable precursor for high-quality activated carbon preparation by conventional pyrolysis: Optimization of produce conditions and feasibility in adsorption studies. Adv. Powder Technol. 2018, 29, 726–736. [Google Scholar] [CrossRef]
- Mehta, D.; Mazumdar, S.; Singh, S.K. Magnetic adsorbents for the treatment of water/wastewater-a review. J. Water Process Eng. 2015, 7, 244–265. [Google Scholar] [CrossRef]
- Zubrik, A.; Matik, M.; Lovás, M.; Štefušová, K.; Danková, Z.; Hredzák, S.; Václavíková, M.; Bendek, F.; Briančin, J.; Machala, L. One-step microwave synthesis of magnetic biochars with sorption properties. Carbon Lett. 2018, 26, 31–42. [Google Scholar] [CrossRef]
- Jabasingh, A.; Ravi, T.; Yimam, A. Magnetic hetero-structures as prospective sorbents to aid arsenic elimination from life water streams. Water Sci. 2018, 32, 151–170. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Sasai, R. Arsenate removal from water using Fe3O4-loaded activated carbon prepared from waste biomass. Chem. Eng. J. 2010, 160, 57–62. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, J.; Liu, H.; Cooper, A.T.; Wu, R. CuFe2O4/activated carbon composite: A novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration. Chemosphere 2007, 68, 1058–1066. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Angelova, R.; Baldikova, E.; Pospiskova, K.; Maderova, Z.; Safarikova, M.; Safarik, I. Magnetically modified sargassum horneri biomass as an adsorbent for organic dye removal. J. Clean. Prod. 2016, 137, 189–194. [Google Scholar] [CrossRef]
- Maya, F.; Palomino Cabello, C.; Frizzarin, R.M.; Estela, J.M.; Turnes Palomino, G.; Cerdà, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TrAC-Trend. Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Safarik, I.; Horska, K.; Pospiskova, K.; Filip, J.; Safarikova, M. Mechanochemical synthesis of magnetically responsive materials from non-magnetic precursors. Mater. Lett. 2014, 126, 202–206. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Šepelák, V.; Düvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef]
- Baláž, P.; Dutková, E. Fine milling in applied mechanochemistry. Miner. Eng. 2009, 22, 681–694. [Google Scholar] [CrossRef]
- Zubrik, A.; Turčániová, Ľ.; Ježová, V.; Čuvanová, S.; Skybová, M. Effect of the mechanochemical activation for the extraction of diterpenes from the brown coal. J. Alloy. Compd. 2007, 434–435, 837–841. [Google Scholar] [CrossRef]
- Turčániová, L.; Kádárová, J.; Imrich, P.; Liptaj, T.; Vidlář, J.; Vašek, J.; Foldyna, F.; Sitek, J.; Baláž, P. Reactivity of mechanical activated coals for special utilization. J. Mater. Sci. 2004, 39, 5467–5470. [Google Scholar] [CrossRef]
- Jakabsky, S.; Zatko, S.; Bakos, J.; Zalesakova, E. The Method of Magnetic Fluid Production. Czechoslovak Patent No. 223697, 15 March 1986. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.; Everett, D.; Haynes, J.; Pernicone, N.; Ramsay, J.; Sing, K.; Unger, K. Recommendations for the characterization of porous solids (technical report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part i. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Haasch, R.T. X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy (AES). In Practical Materials Characterization; Springer: New York, NY, USA, 2014; pp. 93–132. [Google Scholar]
- Wilson, D.; Langell, M.A. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Harinath, Y.; Seshaiah, K.; Reddy, A.V.R. Biosorption of Pb(II) from aqueous solutions using chemically modified moringa oleifera tree leaves. Chem. Eng. J. 2010, 162, 626–634. [Google Scholar] [CrossRef]
- Shen, G.; Xu, Y.; Liu, B. Preparation and adsorption properties of magnetic mesoporous Fe3C/carbon aerogel for arsenic removal from water. Desalin. Water Treat. 2016, 57, 24467–24475. [Google Scholar] [CrossRef]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- National Research Council (US). Chemistry and Analysis of Arsenic Species in Water, Food, Urine, Blood, Hair, and Nails. In Subcommittee on Arsenic in Drinking Water. Arsenic in Drinking Water; National Academies Press (US): Washington, DC, USA, 1999; Volume 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK230885/ (accessed on 21 December 2018).
- Vaclavikova, M.; Gallios, G.P.; Hredzak, S.; Jakabsky, S. Removal of arsenic from water streams: An overview of available techniques. Clean Technol. Environ. 2008, 10, 89–95. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Kilianová, M.; Prucek, R.; Filip, J.; Kolařík, J.; Kvítek, L.; Panáček, A.; Tuček, J.; Zbořil, R. Remarkable efficiency of ultrafine superparamagnetic iron(III) oxide nanoparticles toward arsenate removal from aqueous environment. Chemosphere 2013, 93, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Carabante, I.; Grahn, M.; Holmgren, A.; Kumpiene, J.; Hedlund, J. Adsorption of As (V) on iron oxide nanoparticle films studied by in situ ATR-FTIR spectroscopy. Colloid. Surface. A 2009, 346, 106–113. [Google Scholar] [CrossRef]
- Sherman, D.M.; Randall, S.R. Surface complexation of arsenic(V) to iron(III) (hydr)oxides: Structural mechanism from ab initio molecular geometries and EXAFS spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 4223–4230. [Google Scholar] [CrossRef]
- Manceau, A. The mechanism of anion adsorption on iron oxides: Evidence for the bonding of arsenate tetrahedra on free Fe(O, OH)6 edges. Geochim. Cosmochim. Acta 1995, 59, 3647–3653. [Google Scholar] [CrossRef]
- Fendorf, S.; Eick, M.J.; Grossl, P.; Sparks, D.L. Arsenate and chromate retention mechanisms on goethite. 1. Surface structure. Environ. Sci. Technol. 1997, 31, 315–320. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Rea, B.A.; Fuller, C.C.; Davis, J.A. Surface chemistry of ferrihydrite: Part 1. EXAFS studies of the geometry of coprecipitated and adsorbed arsenate. Geochim. Cosmochim. Acta 1993, 57, 2251–2269. [Google Scholar] [CrossRef]
- D’Arcy, M.; Weiss, D.; Bluck, M.; Vilar, R. Adsorption kinetics, capacity and mechanism of arsenate and phosphate on a bifunctional TiO2–Fe2O3 bi-composite. J. Colloid Interface Sci. 2011, 364, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wainipee, W.; Weiss, D.J.; Sephton, M.A.; Coles, B.J.; Unsworth, C.; Court, R. The effect of crude oil on arsenate adsorption on goethite. Water Res. 2010, 44, 5673–5683. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Cope, C.O.; Webster, D.S.; Sabatini, D.A. Arsenate adsorption onto iron oxide amended rice husk char. Scie. Total Environ. 2014, 488–489, 554–561. [Google Scholar] [CrossRef]
- Park, H.; Myung, N.V.; Jung, H.; Choi, H. As(V) remediation using electrochemically synthesized maghemite nanoparticles. J. Nanopart. Res. 2008, 11, 1981. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Gu, Z.; Fang, J.; Deng, B. Preparation and evaluation of GAC-based iron-containing adsorbents for arsenic removal. Environ. Sci. Technol. 2005, 39, 3833–3843. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Chai, L.; Liu, Y.; Zeng, G.; Wang, X.; Zeng, W.; Shang, M.; Deng, J.; Zhou, Z. Enhancement of As(V) adsorption from aqueous solution by a magnetic chitosan/biochar composite. RSC Adv. 2017, 7, 10891–10900. [Google Scholar] [CrossRef]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. J. Environ. Health Sci. 2014, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.H.K.; Lee, S.M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloid. Surface. A 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Ruthiraan, M.; Mubarak, N.M.; Thines, R.K.; Abdullah, E.C.; Sahu, J.N.; Jayakumar, N.S.; Ganesan, P. Comparative kinetic study of functionalized carbon nanotubes and magnetic biochar for removal of Cd2+ ions from wastewater. Korean J. Chem. Eng. 2015, 32, 446–457. [Google Scholar] [CrossRef]
- Song, Q.; Yang, B.; Wang, H.; Xu, S.; Cao, Y. Effective removal of copper (II) and cadmium (II) by adsorbent prepared from chitosan-modified magnetic biochar. J. Residuals Sci. Technol. 2016, 13, 197–205. [Google Scholar] [CrossRef]
- Jafari Kang, A.; Baghdadi, M.; Pardakhti, A. Removal of cadmium and lead from aqueous solutions by magnetic acid-treated activated carbon nanocomposite. Desalin. Water Treat. 2016, 57, 18782–18798. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Yap, M.W.; Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from douglas fir. Chem. Eng. J. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Ruthiraan, M.; Abdullah, E.C.; Mubarak, N.M.; Noraini, M.N. A promising route of magnetic based materials for removal of cadmium and methylene blue from waste water. J. Environ. Chem. Eng. 2017, 5, 1447–1455. [Google Scholar] [CrossRef]
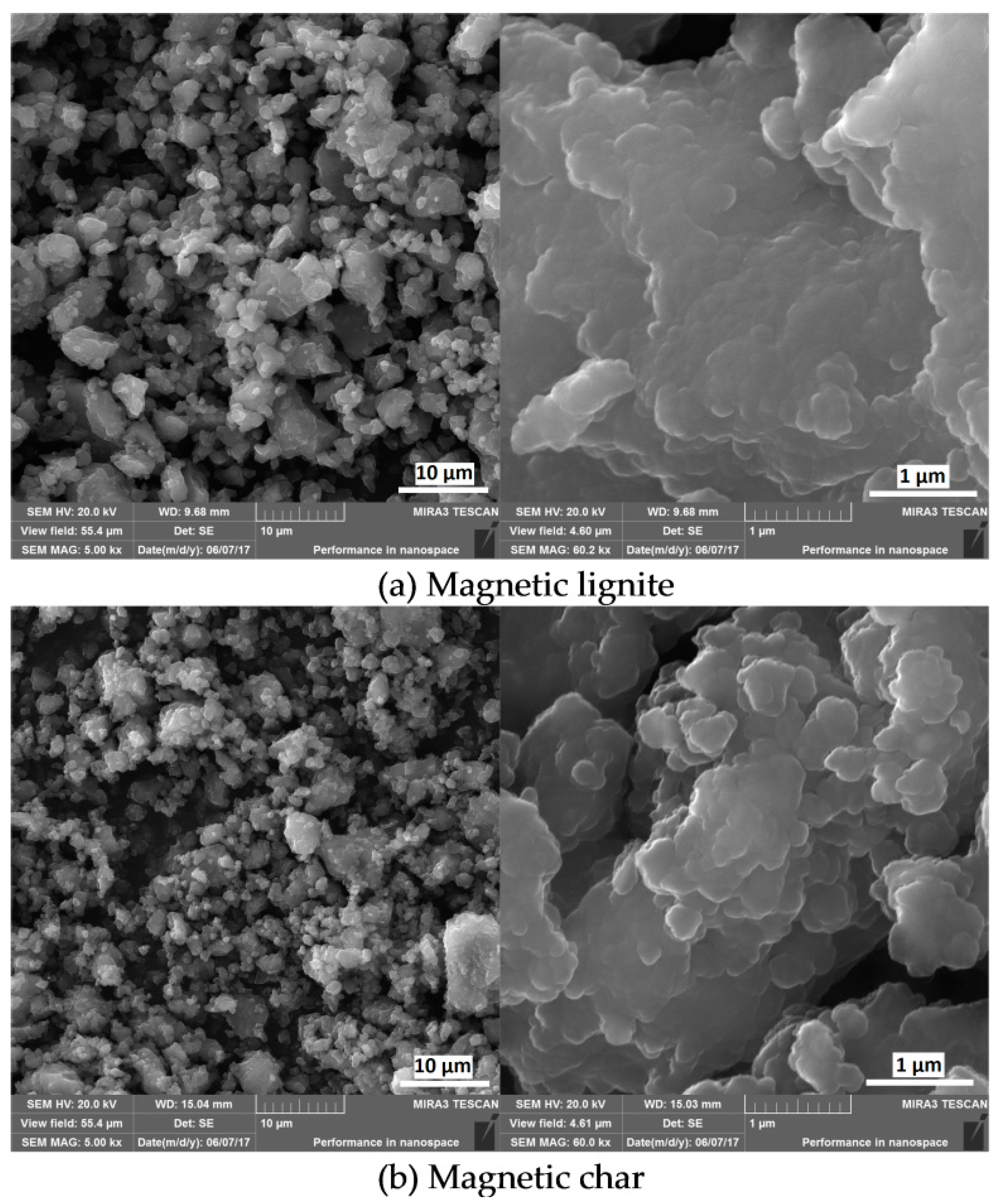
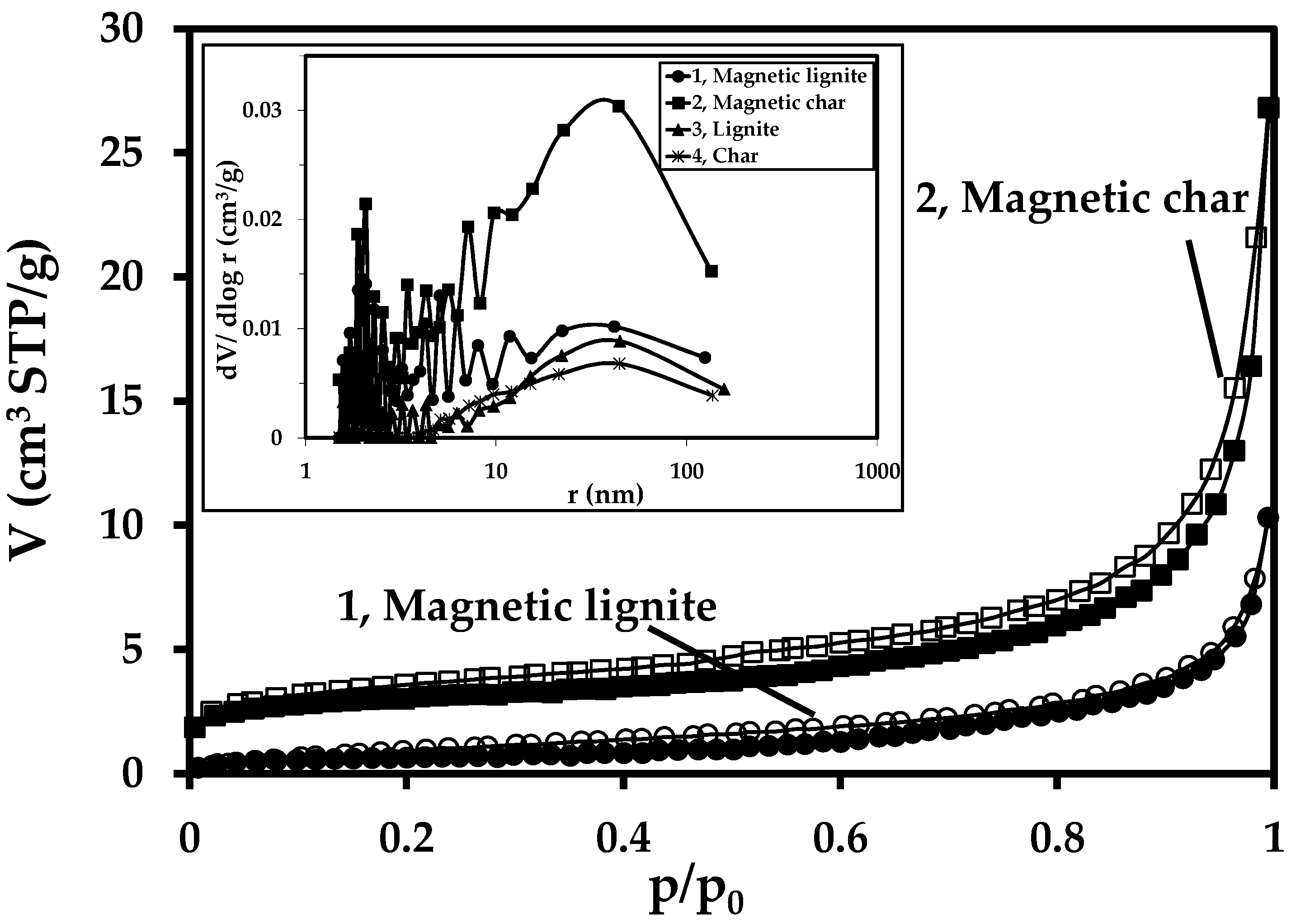
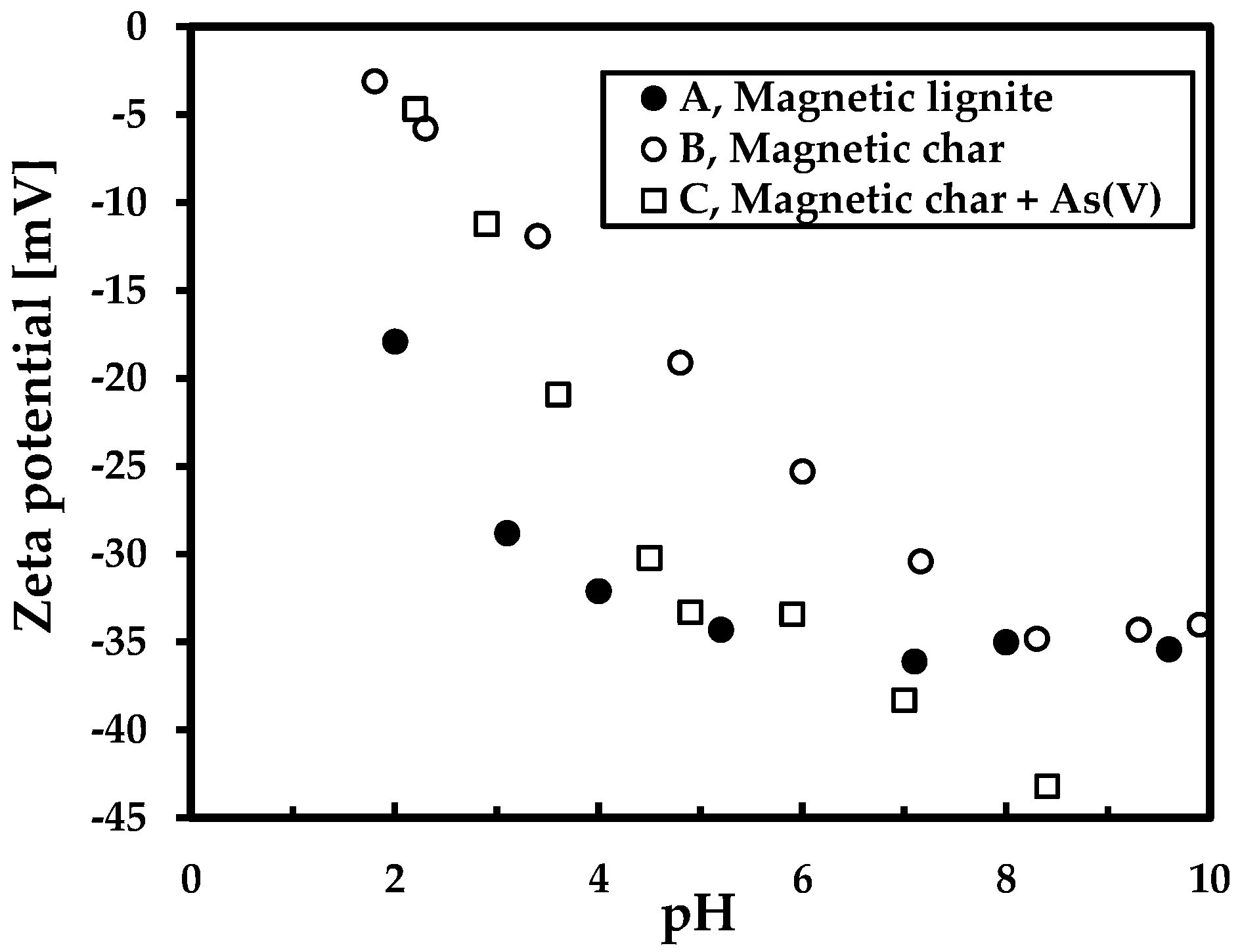


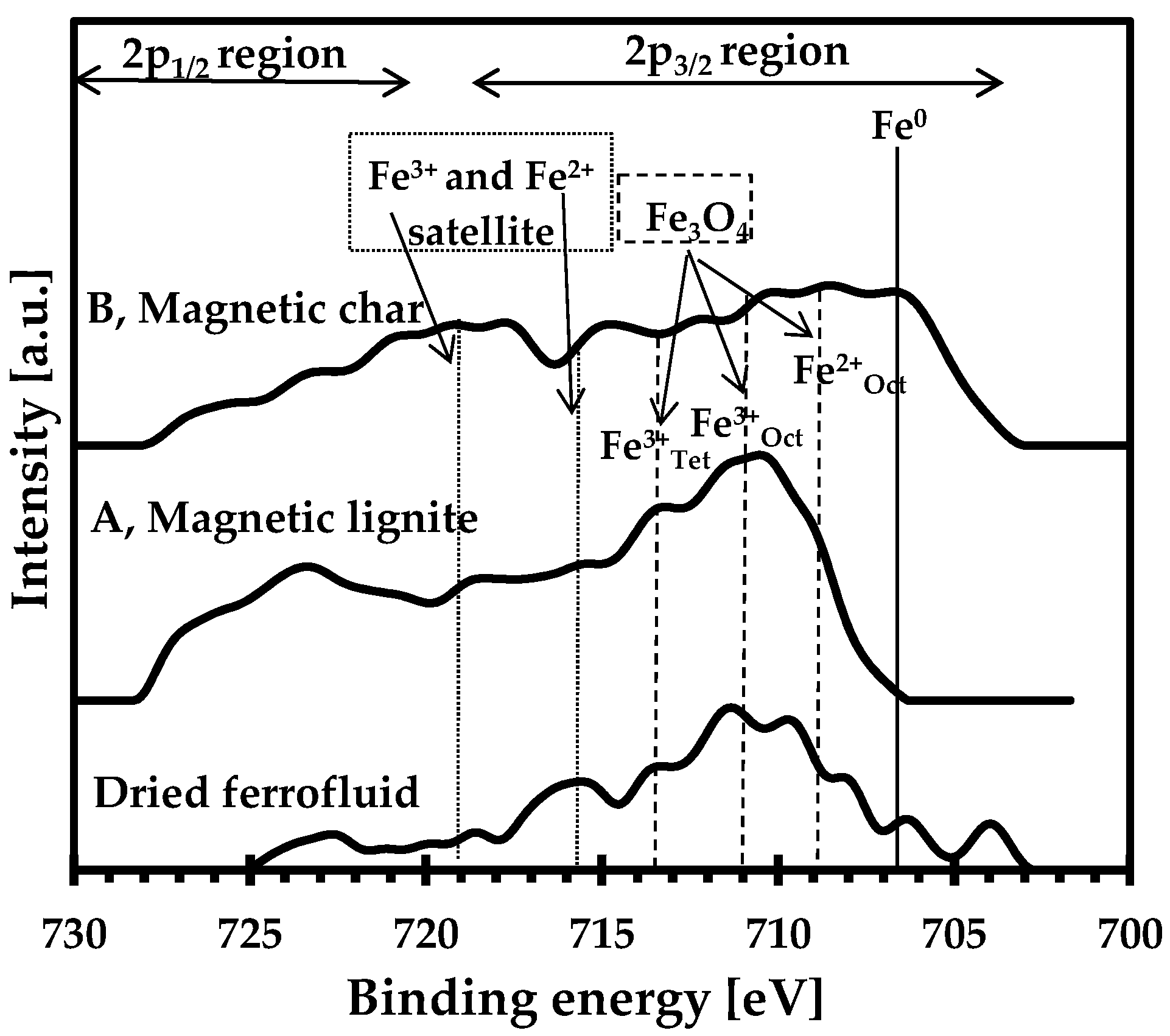

| Sample | Ad (%) | Cd (%) | Hd (%) | Nd (%) | Sd (%) | Od (%) | FeTotal (%) | κ (SI Units) |
|---|---|---|---|---|---|---|---|---|
| Lignite | 13.8 | 51.9 | 5.4 | 0.8 | 2.3 | 25.8 | - | 89 × 10−6 |
| Char | 21.6 | 62.9 | 3.1 | 1.0 | 2.4 | 9.0 | - | 223 × 10−6 |
| Magnetic lignite | - | 46.7 | 5.4 | 0.7 | 2.0 | - | 7.7 | 343,333 × 10−6 |
| Magnetic char | - | 55.5 | 3.5 | 0.9 | 2.2 | - | 8.5 | 316,897 × 10−6 |
| Sample | SBET (m2/g) | CBET | Vtot (cm3/g) | Vmicro (cm3/g) | Sext (m2/g) |
|---|---|---|---|---|---|
| Lignite | 1.0 | 41.2 | 0.0099 | 0 | 1.0 |
| Char | 1.8 | −350.5 | 0.0084 | 0.0019 | 0 |
| Magnetic lignite | 2.3 | 69.1 | 0.0159 | 0 | 2.2 |
| Magnetic char | 10.6 | −343.5 | 0.0415 | 0.0024 | 5.3 |
| Sample | IS (mm/s) | QS (mm/s) | H (T) | I (%) | Component Description |
|---|---|---|---|---|---|
| Magnetic lignite | 0.31 | 0.67 | - | 10.6 | Doublet |
| 0.45 | 0.001 | 45.0 | 33.6 | Sextet | |
| 0.31 | −0.09 | 22.1 | 55.8 | Relaxation component | |
| Magnetic char | 0.32 | 0.72 | - | 9.5 | Doublet |
| 0.47 | 0.005 | 43.4 | 23.6 | Sextet | |
| 0.31 | 0.009 | 0 | 66.9 | Relaxation component |
| Sample Description | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | b (L/mg) | R2 | KF (L/g) | n | R2 | |
| Magnetic char—As(V), pH 3.9 | 19.9 | 0.02715 | 0.9917 | 4.13 | 4.10 | 0.9549 |
| Magnetic lignite—Cd(II), pH 6.5 | 70.4 | 0.09543 | 0.9941 | 17.16 | 2.95 | 0.9389 |
| Magnetic char—Cd(II), pH 6.8 | 58.8 | 0.03750 | 0.9873 | 8.70 | 3.73 | 0.9696 |
| Sorption of Arsenic (V) | Qm (mg/g) | Reference |
| Adsorbsia, Dow Water Solutions (Launch: 2005, USA) | 12–15 | [34] |
| ArsenX, SolrneteX, Inc. (Launch: 2004, USA) | 38 | [34] |
| Magnetic biochar (wheat straw) | 25.6 | [11] |
| Iron-impregnated biochar | 2.2 | [47] |
| Biochar/γ-Fe2O3 composite | 3.2 | [48] |
| Iron-oxide amended rice-husk char | 1.5 | [49] |
| γ-Fe2O3 | 4.6 | [50] |
| Fe3O4 | 4.7 | [51] |
| Flowerlike γ-Fe2O3 | 4.8 | [51] |
| α-Fe2O3 | 5.3 | [51] |
| Iron-modified activated carbon | 1.9–6.6 | [52] |
| Magnetic mesoporous carbon aerogel | 56.2 | [33] |
| Magnetic chitosan biochar | 17.9 | [53] |
| Iron oxide/activated carbon | 20.2 | [54] |
| Hematite-modified biochar | 0.43 | [15] |
| Magnetic char | 19.9 | Present study |
| Sorption of Cadmium (II) | ||
| Magnetic biochar | 15.0 | [55] |
| Magnetic biochar | 62.5 | [56] |
| Chitosan-modified magnetic biochar | 105.3 | [57] |
| Magnetic activated carbon | 49.8 | [58] |
| Magnetic oak-bark biochar | 8.3 | [59] |
| Magnetic biochar (coconut shell) | 3.9 | [60] |
| Magnetic biochar (Douglas fir biochar) | 11.3 | [61] |
| Magnetic biochar (mangosteen peels) | 45.7 | [62] |
| Magnetic char | 58.8 | Present study |
| Magnetic lignite | 70.4 | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubrik, A.; Matik, M.; Lovás, M.; Danková, Z.; Kaňuchová, M.; Hredzák, S.; Briančin, J.; Šepelák, V. Mechanochemically Synthesised Coal-Based Magnetic Carbon Composites for Removing As(V) and Cd(II) from Aqueous Solutions. Nanomaterials 2019, 9, 100. https://doi.org/10.3390/nano9010100
Zubrik A, Matik M, Lovás M, Danková Z, Kaňuchová M, Hredzák S, Briančin J, Šepelák V. Mechanochemically Synthesised Coal-Based Magnetic Carbon Composites for Removing As(V) and Cd(II) from Aqueous Solutions. Nanomaterials. 2019; 9(1):100. https://doi.org/10.3390/nano9010100
Chicago/Turabian StyleZubrik, Anton, Marek Matik, Michal Lovás, Zuzana Danková, Mária Kaňuchová, Slavomír Hredzák, Jaroslav Briančin, and Vladimír Šepelák. 2019. "Mechanochemically Synthesised Coal-Based Magnetic Carbon Composites for Removing As(V) and Cd(II) from Aqueous Solutions" Nanomaterials 9, no. 1: 100. https://doi.org/10.3390/nano9010100
APA StyleZubrik, A., Matik, M., Lovás, M., Danková, Z., Kaňuchová, M., Hredzák, S., Briančin, J., & Šepelák, V. (2019). Mechanochemically Synthesised Coal-Based Magnetic Carbon Composites for Removing As(V) and Cd(II) from Aqueous Solutions. Nanomaterials, 9(1), 100. https://doi.org/10.3390/nano9010100





