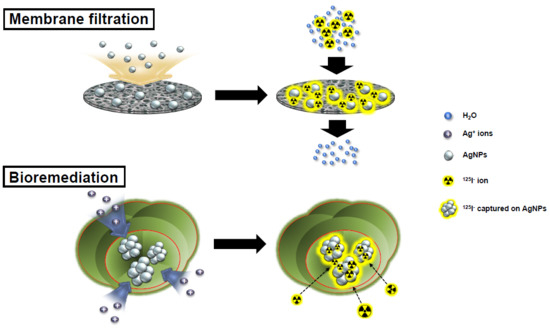
Silver Nanomaterial-Immobilized Desalination Systems for Efficient Removal of Radioactive Iodine Species in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis of Citrate-Stabilized AgNPs
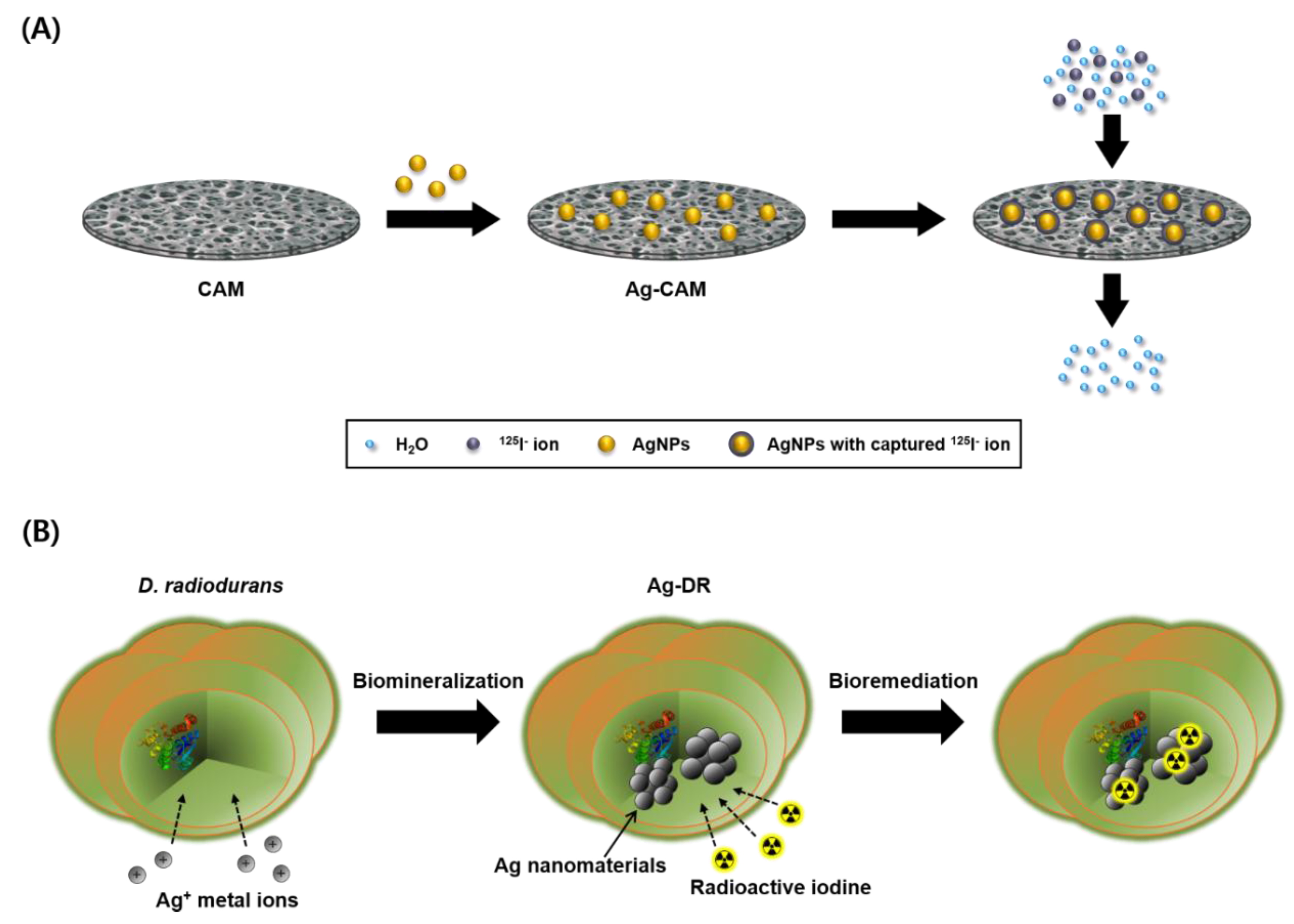
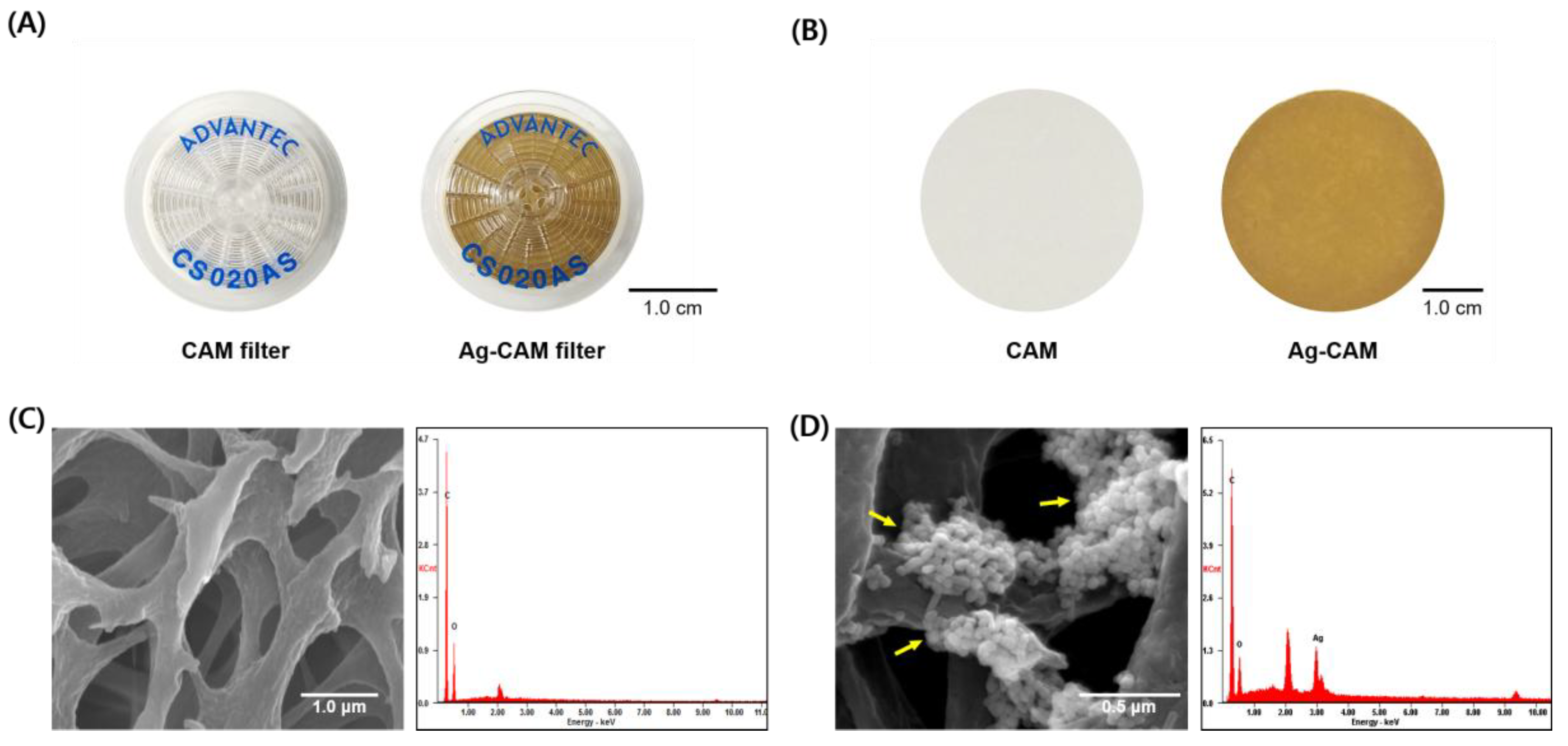
2.3. Preparation of the Ag-CAM
2.4. Desalination of Radioactive Iodine Using Ag-CAM Filter Unit under Continuous In-Flow Conditions
2.5. Preparation of Silver Nanomaterial-Containing Deinococcus radiodurans R1
2.6. Remediation Procedure of Radioactive Iodine Using Ag-DR
2.7. SPECT/CT Imaging of Radioactive Iodine Captured by Ag-DR
3. Results and Discussion
3.1. Preparation of Ag-CAM
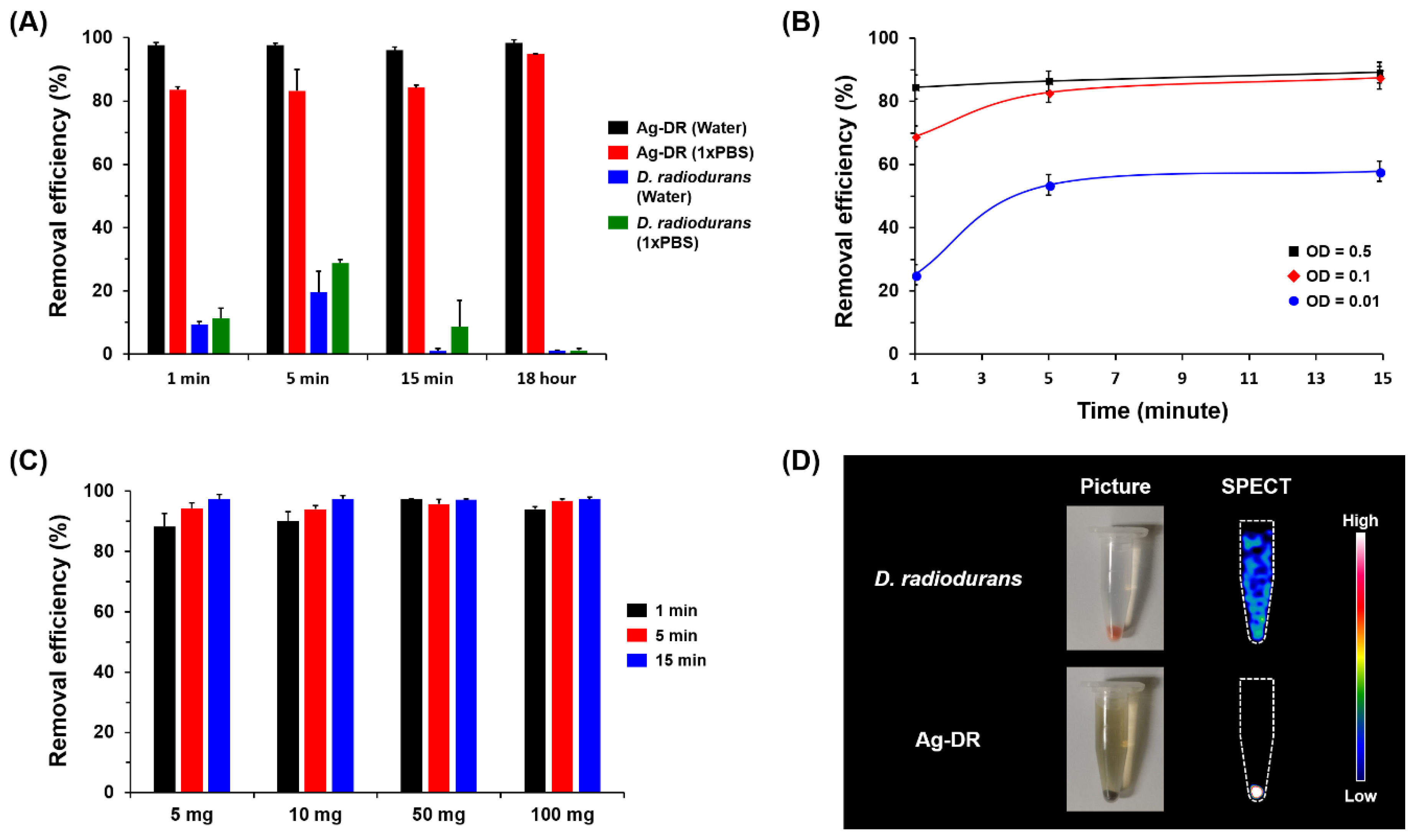
3.2. Desalination of Radioactive Iodine Using Ag-CAM
3.3. Remediation of Radioactive Iodine Using Ag-DR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khayet, M.; Matsuura, T. Radioactive decontamination of water. Desalination 2013, 321, 1–2. [Google Scholar] [CrossRef]
- Abdel Rahman, R.O.; Ibrahium, H.A.; Hung, Y.-T. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar] [CrossRef]
- Bonnema, S.J.; Hegedüs, L. Radioiodine therapy in benign thyroid diseases effects, side effects, and factors affecting therapeutic outcome. Endocr. Rev. 2012, 33, 920–980. [Google Scholar] [CrossRef] [PubMed]
- Prpic, M.; Dabelic, N.; Stanicic, J.; Jukic, T.; Milosevic, M.; Kusic, Z. Adjuvant thyroid remnant ablation in patients with differentiated thyroid carcinoma confined to the thyroid: A comparison of ablation success with different activities of radioiodine (I-131). Ann. Nucl. Med. 2012, 26, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sabra, M.M.; Grewal, R.K.; Ghossein, R.A.; Tuttle, R.M. Higher administered activities of radioactive iodine are associated with less structural persistent response in older, but not younger, papillary thyroid cancer patients with lateral neck lymph node metastases. Thyroid 2014, 24, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R. Management of radioactive wastes in a hospital environment. In Modelling Trends in Solid and Hazardous Waste Management; Sengupta, D., Agrahari, S., Eds.; Springer: Singapore, 2017; pp. 1–14. [Google Scholar]
- Ravichandran, R.; Binukumar, J.P.; Sreeram, R.; Arunkumar, L.S. An overview of radioactive wastes disposal procedures of a nuclear medicine department. J. Med. Phys. 2011, 36, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Fenner, F.D. Radioactivity in municipal sewage and sludge. Public Health Rep. 1997, 112, 308–316. [Google Scholar] [PubMed]
- Grossman, C.M.; Nussbaum, R.H.; Nussbaum, F.D. Thyrotoxicosis among Hanford, Washington, downwinders: A community-based health survey. Arch. Environ. Health Int. J. 2002, 57, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Povinec, P.P.; Zhang, L.; Shi, K.; Biddulph, D.; Chang, C.-C.; Fan, Y.; Golser, R.; Hou, Y.; Ješkovský, M.; et al. Iodine-129 in seawater offshore Fukushima: Distribution, inorganic speciation, sources, and budget. Environ. Sci. Technol. 2013, 47, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.D.; Smith, S.M.; Turcotte, J.A. Using public relations strategies to prompt populations at risk to seek health information: The Hanford community health project. Health Promot. Pract. 2009, 10, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Grossman, C.M.; Morton, W.E.; Nussbaum, R.H. Hypothyroidism and spontaneous abortions among Hanford, Washington, downwinders. Arch. Environ. Health Int. J. 1996, 51, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Grossman, C.M.; Morton, W.E.; Nussbaum, R.H.; Kordysh, E.A.; Quastel, M.R.; Sobel, R.B.; Nussbaum, F.D. Juvenile hypothyroidism among two populations exposed to radioiodine. Environ. Health Perspect. 1999, 107, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Grossman, C.M.; Nussbaum, R.H.; Nussbaum, F.D. Cancers among residents downwind of Hanford, Washington, plutonium production site. Arch. Environ. Health Int. J. 2003, 58, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, H.; Hou, S.; Xia, D. Chapter 18. Applications of Nanomaterials in Nuclear Waste Management. In Multifunctional Nanocomposites for Energy and Environmental Applications; Wiley-VCH: Hoboken, NJ, USA, 2018; pp. 543–566. [Google Scholar]
- Mu, W.; Yu, Q.; Li, X.; Wei, H.; Jian, Y. Adsorption of radioactive iodine on surfactant-modified sodium niobate. RSC Adv. 2016, 6, 81719–81725. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Liu, L.; Sarina, S.; Zheng, Z.; Zhu, H. Silver oxide nanocrystals anchored on titanate nanotubes and nanofibers: Promising candidates for entrapment of radioactive iodine anions. Nanoscale 2013, 5, 11011–11018. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Sarina, S.; Zhu, H.; Liu, H.; Zheng, Z.; Xie, M.; Smith, S.V.; Komarneni, S. Capture of radioactive cesium and iodide ions from water by using titanate nanofibers and nanotubes. Angew. Chem. Int. Ed. 2011, 50, 10594–10598. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Wang, H.; Ma, D.; Tan, K.; Jensen, S.; Deibert, B.J.; Butler, J.; Cure, J.; Shi, Z.; et al. Capture of organic iodides from nuclear waste by metal-organic framework-based molecular traps. Nat. Commun. 2017, 8, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.W.; Chupas, P.J.; Nenoff, T.M. Radioactive iodine capture in silver-containing mordenites through nanoscale silver iodide formation. J. Am. Chem. Soc. 2010, 132, 8897–8899. [Google Scholar] [CrossRef] [PubMed]
- Sarina, S.; Bo, A.; Liu, D.; Liu, H.; Yang, D.; Zhuo, C.; Maes, N.; Komarneni, S.; Zhu, H. Separate or simultaneous removal of radioactive cations and anions from water by layered sodium vanadate-based sorbents. Chem. Mater. 2014, 26, 4788–4795. [Google Scholar] [CrossRef]
- Mu, W.; Li, X.; Liu, G.; Yu, Q.; Xie, X.; Wei, H.; Jian, Y. Safe disposal of radioactive iodide ions from solutions by Ag2O grafted sodium niobate nanofibers. Dalton Trams. 2016, 45, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Bo, A.; Sarina, S.; Liu, H.; Zheng, Z.; Xiao, Q.; Gu, Y.; Ayoko, G.A.; Zhu, H. Efficient removal of cationic and anionic radioactive pollutants from water using hydrotalcite-based getters. ACS Appl. Mater. Interfaces 2016, 8, 16503–16510. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Park, H.-S.; Cho, Y.-Z. Al2O3-containing silver phosphate glasses as hosting matrices for radioactive iodine. J. Nucl. Sci. Technol. 2017, 54, 1330–1337. [Google Scholar] [CrossRef]
- Liu, S.; Wang, N.; Zhang, Y.; Li, Y.; Han, Z.; Na, P. Efficient removal of radioactive iodide ions from water by three-dimensional Ag2O–Ag/TiO2 composites under visible light irradiation. J. Hazard. Mater. 2015, 284, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bo, A.; Sarina, S.; Zheng, Z.; Yang, D.; Liu, H.; Zhu, H. Removal of radioactive iodine from water using Ag2O grafted titanate nanolamina as efficient adsorbent. J. Hazard. Mater. 2013, 246–247, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Yu, S.-H.; Yao, Q.-Z.; Fu, S.-Q.; Zhuo, G.-T. One-step synthesis of Ag2O@Mg(OH)2 nanocomposite as an efficient scavenger for iodine and uranium. J. Colloid. Interface Sci. 2018, 510, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L., Jr. Materials and processes for the effective capture and immobilization of radioiodine: A review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.-K.; Lee, S.; Lee, J.S.; Kim, S.W. Development of silver nanoparticle-doped adsorbents for the separation and recovery of radioactive iodine from alkaline solutions. Appl. Radiat. Isot. 2017, 129, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Conde-González, J.E.; Peña-Méndez, E.M.; Rybáková, S.; Pasán, J.; Ruiz-Pérez, C.; Havel, J. Adsorption of silver nanoparticles from aqueous solution on copper-based metal organic frameworks (HKUST-1). Chemosphere 2016, 150, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Sohsah, M.A.; Ghoneim, M.M.; Sokkar, H.H.; Badawy, S.M.; El-Anadouli, B.E. Adsorption of hazardous ions from radioactive waste on chelating cloth filter. Radiat. Phys. Chem. 2006, 75, 278–285. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 11, 1–16. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Shaiwale, N.S.; Deobagkar, D.N.; Deobagkar, D.D. Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int. J. Nanomed. 2015, 10, 963–974. [Google Scholar]
- Amini, E.; Azadfallah, M.; Layeghi, M.; Talaei-Hassanloui, R. Silver-nanoparticle-impregnated cellulose nanofiber coating for packaging paper. Cellolose 2016, 23, 557–570. [Google Scholar] [CrossRef]
- Kaushil, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef]
- Choi, M.H.; Shim, H.E.; Yun, S.-J.; Park, S.H.; Choi, D.S.; Jang, B.-S.; Choi, Y.J.; Jeon, J. Gold-nanoparticle-immobilized desalting columns for highly efficient and specific removal of radioactive iodine in aqueous media. ACS Appl. Mater. Interfaces 2016, 8, 29227–29231. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Yun, S.-J.; Yang, J.E.; Jeong, S.-W.; Shim, H.E.; Choi, M.H.; Park, S.H.; Choi, Y.J.; Jeon, J. Efficient and selective removal of radioactive iodine anions using engineered nanocomposite membranes. Environ. Sci. Nano 2017, 4, 2157–2163. [Google Scholar] [CrossRef]
- Choi, M.H.; Jeong, S.-W.; Shim, H.E.; Yun, S.-J.; Mushtaq, S.; Choi, D.S.; Jang, B.-S.; Yang, J.E.; Choi, Y.J.; Jeon, J. Efficient bioremediation of radioactive iodine using biogenic gold nanomaterial-containing radiation-resistant bacterium, Deinococcus radiodurans R1. Chem. Commun. 2017, 53, 3937–3940. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, H.E.; Yang, J.E.; Jeong, S.-W.; Lee, C.H.; Song, L.; Mushtaq, S.; Choi, D.S.; Choi, Y.J.; Jeon, J. Silver Nanomaterial-Immobilized Desalination Systems for Efficient Removal of Radioactive Iodine Species in Water. Nanomaterials 2018, 8, 660. https://doi.org/10.3390/nano8090660
Shim HE, Yang JE, Jeong S-W, Lee CH, Song L, Mushtaq S, Choi DS, Choi YJ, Jeon J. Silver Nanomaterial-Immobilized Desalination Systems for Efficient Removal of Radioactive Iodine Species in Water. Nanomaterials. 2018; 8(9):660. https://doi.org/10.3390/nano8090660
Chicago/Turabian StyleShim, Ha Eun, Jung Eun Yang, Sun-Wook Jeong, Chang Heon Lee, Lee Song, Sajid Mushtaq, Dae Seong Choi, Yong Jun Choi, and Jongho Jeon. 2018. "Silver Nanomaterial-Immobilized Desalination Systems for Efficient Removal of Radioactive Iodine Species in Water" Nanomaterials 8, no. 9: 660. https://doi.org/10.3390/nano8090660
APA StyleShim, H. E., Yang, J. E., Jeong, S.-W., Lee, C. H., Song, L., Mushtaq, S., Choi, D. S., Choi, Y. J., & Jeon, J. (2018). Silver Nanomaterial-Immobilized Desalination Systems for Efficient Removal of Radioactive Iodine Species in Water. Nanomaterials, 8(9), 660. https://doi.org/10.3390/nano8090660