The Recognition of Sweat Latent Fingerprints with Green-Emitting Carbon Dots
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Apparatus
2.2. Synthesis of Green-Emissioned Carbon Dots (G-CDs)
2.3. Application in Latent Fingerprint Development
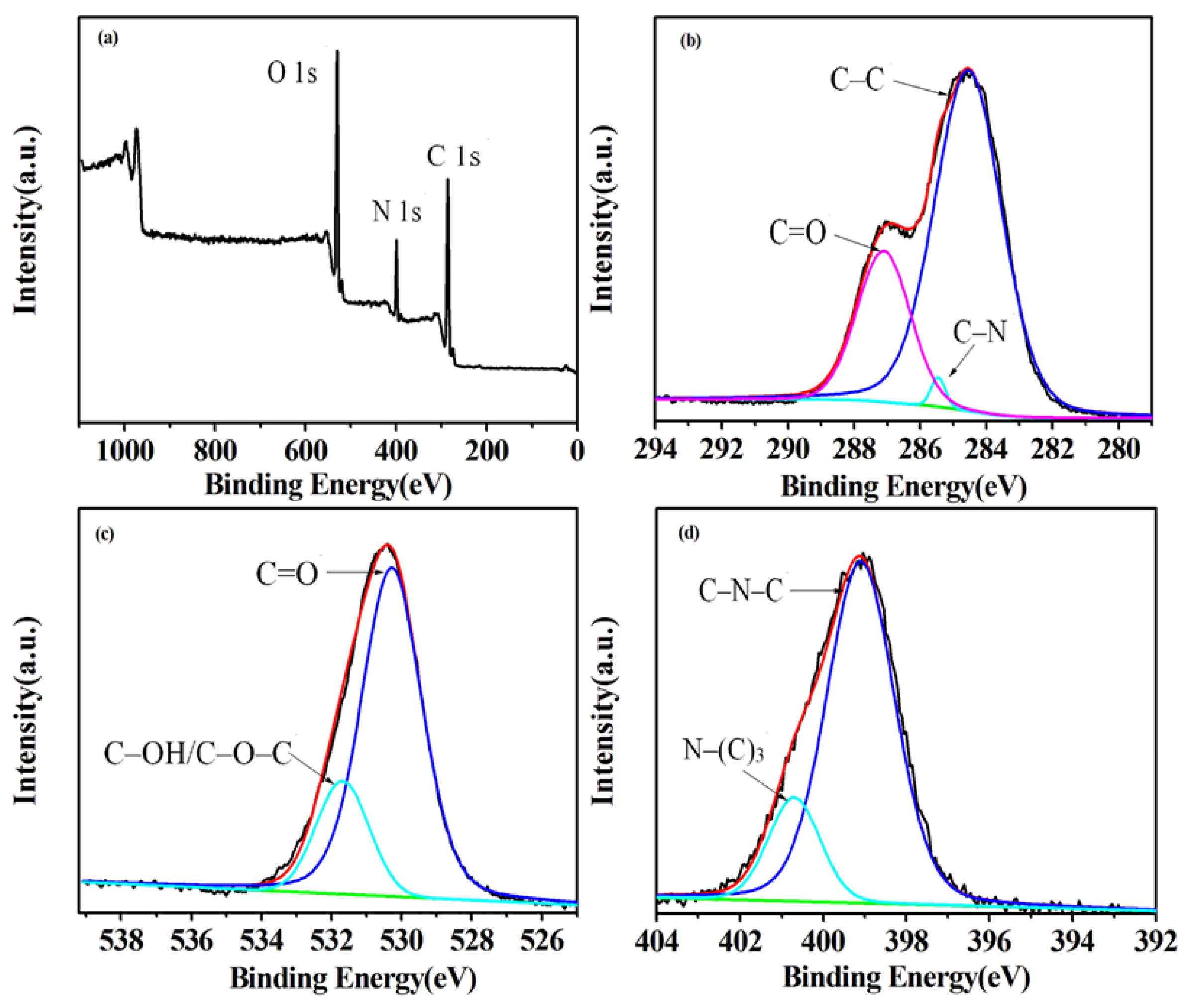
3. Results and Discussion
3.1. G-CDs Spectral Characterization and Application in Latent Fingerprint Development
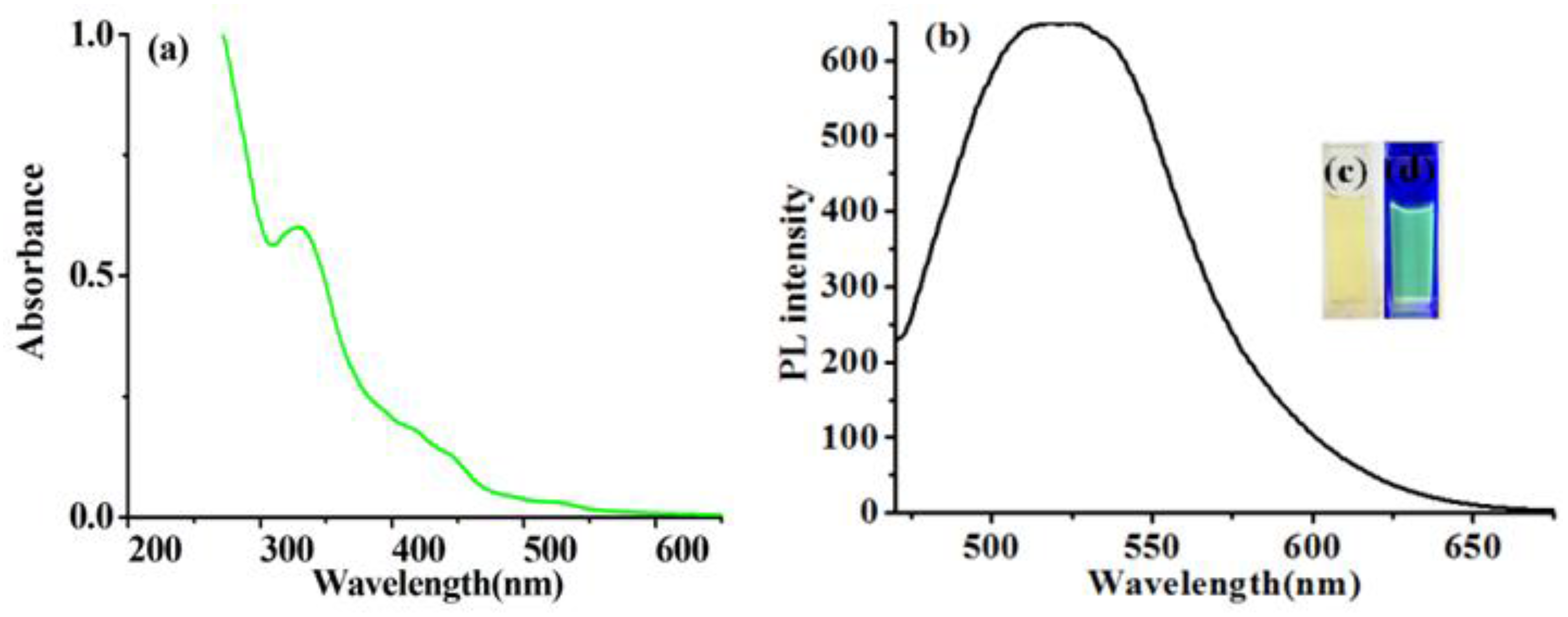
3.1.1. UV-Vis Spectrum and Fluorescence Spectrum
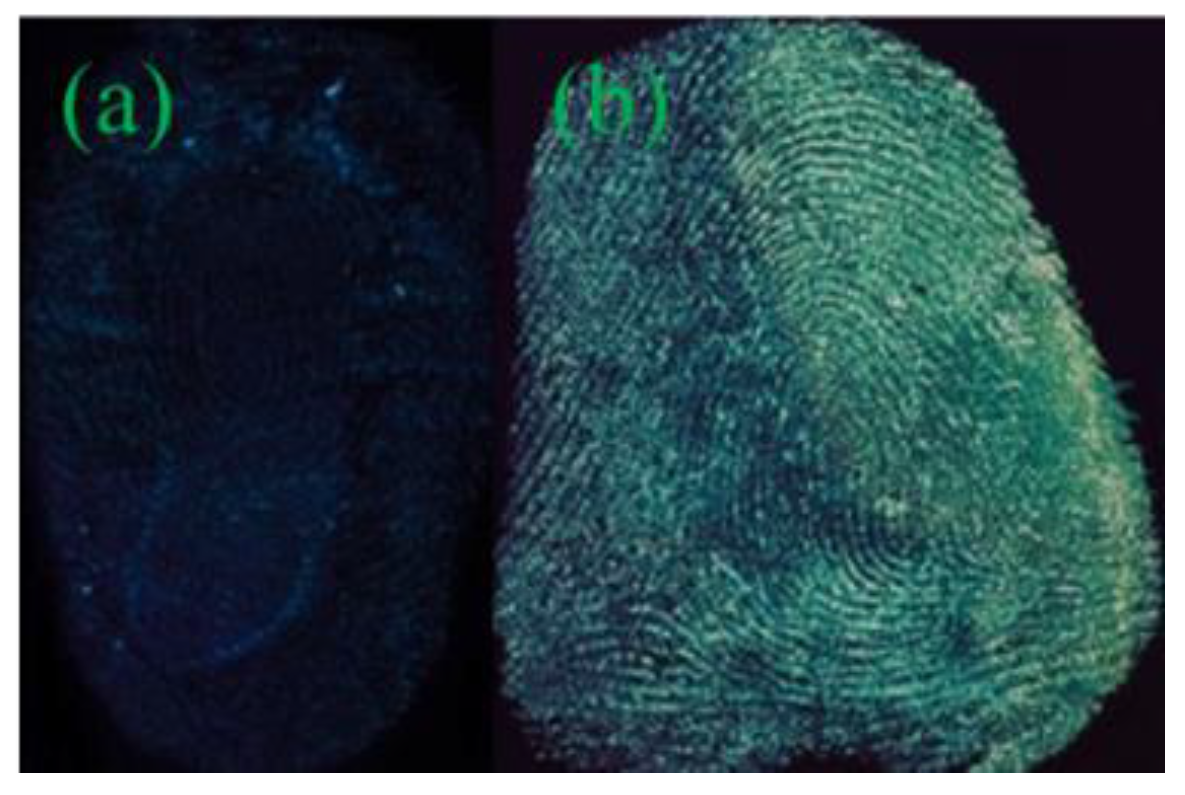
3.1.2. Fingerprints Developed by Various QDs
3.2. The Optimization of Fingerprint Development in G-CDs Solution
3.2.1. Fingerprints on Various Substrates
3.2.2. Effect of pH
3.2.3. The Impact of Developing Time
3.3. Application of Actual Samples
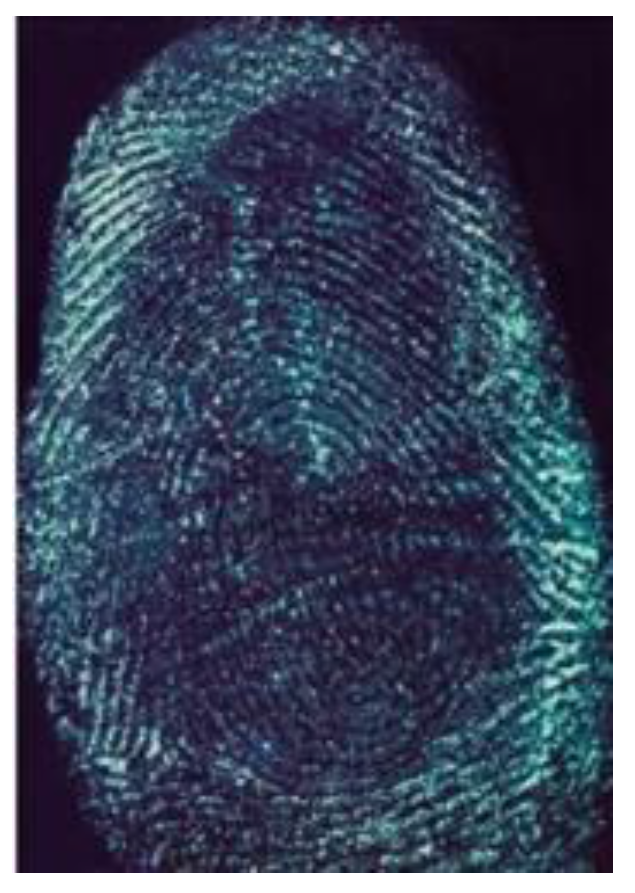
3.3.1. Fingermarks Developed by G-CDs of Preserving Time
3.3.2. The Development of Water-Soaked Latent Fingerprint
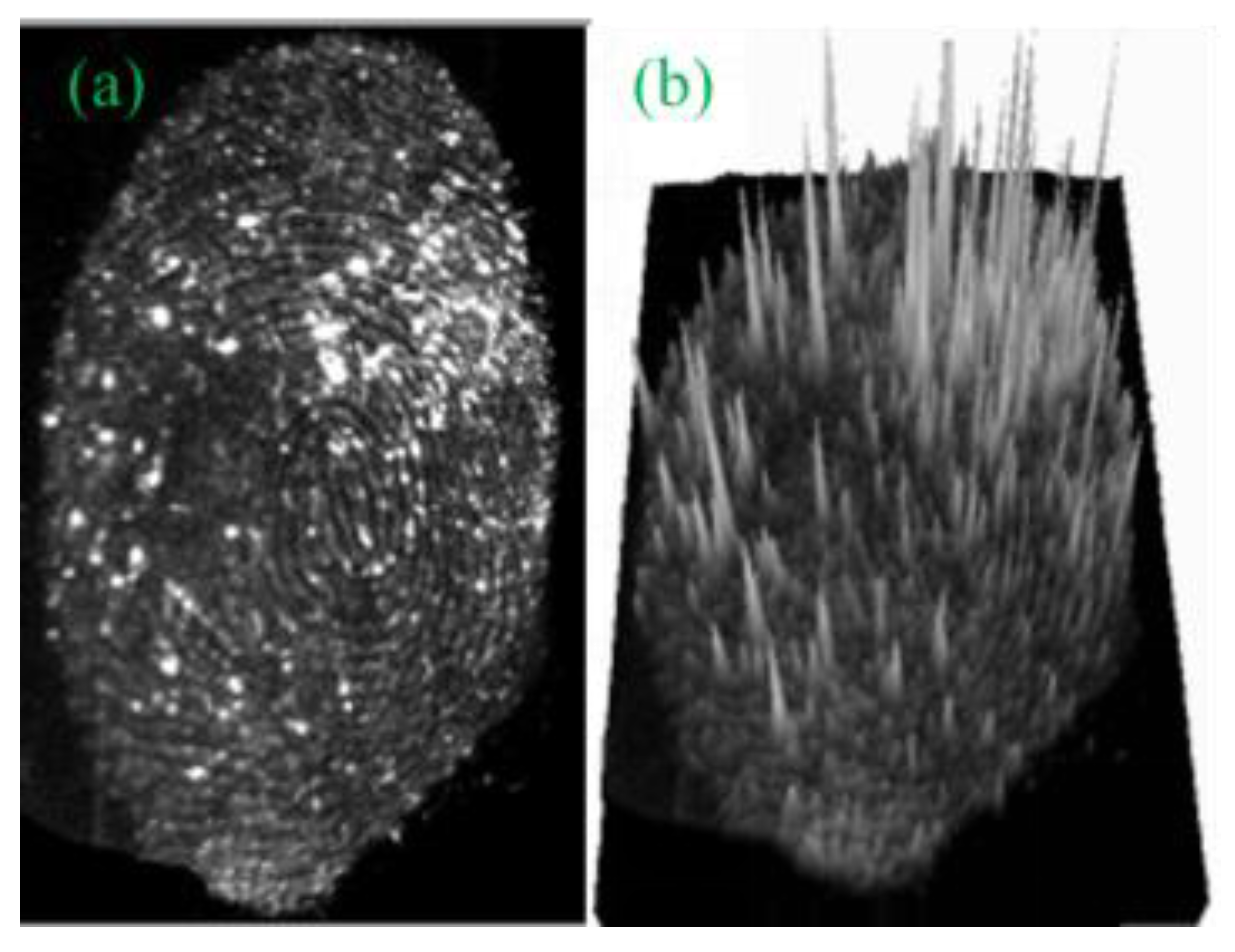
3.3.3. Three-Dimensional Image of Fingerprint developed in the G-CDs Solution
3.4. Mechanism of Latent Fingerprints Development by G-CDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, F.; Liu, S.Q.; Qi, R.Y.; Li, H.R.; Cui, T.F. Effective visualization of latent fingerprints with red fluorescent La2 (MoO4)3:Eu3+ microcrystals. J. Alloys Compd. 2017, 727, 919–924. [Google Scholar] [CrossRef]
- Ambadiyil, S.; Prakash, D.; Sheeja, M.K.; Mahadevan Pillai, V.P. Secure storage and analysis of fingerprints for criminal investigation using holographic techniques. Mater. Today Proc. 2017, 4, 4389–4395. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xu, C.Y.; Shu, C.; Hou, X.D.; Wu, P. Simultaneous extraction of level 2 and level 3 characteristics from latent fingerprints imaged with quantum dots for improved fingerprint analysis. Chin. Chem. Lett. 2017, 28, 1961–1964. [Google Scholar] [CrossRef]
- Milasinovic, N. Polymers in criminalistics: Latent fingerprint detection and enhancement: From idea to practical application. Nauka Bezb. Polic. 2016, 21, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Bailey, M.J.; Ismail, M.; Bleay, S.; Bright, N.; Levin Elad, M.; Cohen, Y.; Geller, B.; Everson, D.; Costa, C.; Webb, R.P.; et al. Enhanced imaging of developed fingerprints using mass spectrometry imaging. Analyst 2013, 138, 6246–6250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Li, M.; Yu, A.; Zhu, Y.; Yang, M.Y.; Mao, C.B. Fluorescent nanomaterials for the development of latent fingerprints in forensic sciences. Adv. Funct. Mater. 2017, 27, 1606243. [Google Scholar] [CrossRef] [PubMed]
- Petretei, D.; Angyal, M. Recovering bloody fingerprints from skin. J. Forensic Identif. 2015, 65, 813–827. [Google Scholar]
- Song, Z.P.; Li, Z.H.; Lin, L.H.; Zhang, Y.F.; Lin, T.R.; Chen, L.; Cai, Z.; Lin, S.; Guo, L.Q.; Fu, F.F.; et al. Phenyl-doped graphitic carbon nitride: Photoluminescence mechanism and latent fingerprint imaging. Nanoscale 2017, 9, 17737–17742. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, H.R.; Cui, T.F. One-step synthesis of solid state luminescent carbon-based silica nanohybrids for imaging of latent fingerprints. Optmat 2017, 73, 459–465. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yang, R.Q.; Shi, Z.X.; Liu, J.J.; Zhao, K.; Wang, Y.J. The effectiveness of CdSe nanoparticle suspension for developing latent fingermarks. J. Saudi Chem. Soc. 2014, 18, 13–18. [Google Scholar] [CrossRef]
- Menzel, E.R.; Savoy, S.M.; Ulvick, S.J.; Cheng, K.H.; Murdock, R.H.; Sudduth, M.R. Photoluminescent semiconductor nanocrystals for fingerprint detection. J. Forensic Sci. 2000, 45, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Luo, Y.J.; Li, G.P.; Li, J.; Wang, Y.F.; Yang, R.Q.; Lu, W.T. Application of photoluminescent CdS/PAMAM nanocomposites in fingerprint detection. Forensic Sci. Int. 2008, 179, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Yang, R.Q.; Wang, Y.J.; Shi, Z.X.; Liu, J.J. Application of CdSe nanoparticle suspension for developing latent fingermarks on the sticky side of adhesives. Forensic Sci. Int. 2009, 185, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Shi, Z.X.; Yu, Y.C.; Yang, R.Q.; Zuo, S.L. Water-soluble multicolored fluorescent CdTe quantum dots: Synthesis and application for fingerprint developing. J. Colloid Interface Sci. 2010, 342, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Han, J.X.; Zhang, J.; Li, Q.; Sun, X.F.; Zheng, J.C.; Bao, L.R.; Li, X.; Liu, Z.L. The synthesis of newly modified CdTe quantum dots and their application for improvement of latent fingerprint detection. Nanotechnology 2011, 22, 075705. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Zhou, R.H.; He, W.W.; Wu, L.; Wu, P.; Hou, X.D. Fast imaging of eccrine latent fingerprints with nontoxic Mn-doped ZnS QDs. Anal. Chem. 2014, 86, 3279–3283. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Wei, S.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2015, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Lan, M.H.; Zhu, X.Y.; Xue, H.T.; Ng, T.W.; Meng, X.M.; Lee, C.S.; Wang, P.F.; Zhang, W.J. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.P.; Yu, Y.X.; Guo, X.L.; Ding, Z.Y.; Zhou, B.; Liang, H.; Shen, X.C. White-emitting carbon dots with long alkyl-chain structure: Effective inhibition of aggregation caused quenching effect for label-free imaging of latent fingerprint. Carbon 2018, 128, 12–20. [Google Scholar] [CrossRef]
- Khan, W.U.; Wang, D.Y.; Zhang, W.; Tang, Z.B.; Ma, X.L.; Ding, X.; Du, S.S.; Wang, Y.H. High quantum yield green-emitting carbon dots for Fe (ІІІ) detection, biocompatible fluorescent ink and cellular imaging. Sci. Rep. 2017, 7, 14866. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, J.S.; Zhang, P.; Niu, X.Q.; Zhao, W.; Zhu, Z.Y.; Ding, H.; Xiong, H.M. Red-emissive carbon dots for fingerprints detection by spray method: Coffee ring effect and unquenched fluorescence in drying process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, J.D.; Lu, L.L.; Song, Q.J. Rapid visualization of latent fingerprints with color-tunable solid fluorescent carbon dots. Part. Part. Syst. Charact. 2018, 35, 1700387. [Google Scholar] [CrossRef]
- Gao, F.; Ma, S.Y.; Li, J.; Dai, K.; Xiao, X.C.; Zhao, D.; Gong, W.F. Rational design of high quality citric acid-derived carbon dots by selecting efficient chemical structure motifs. Carbon 2017, 112, 131–141. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, Y.; Shu, Q.W.; Liang, J.M.; Zhang, F.; Chen, X.P.; Deng, X.Y.; Swihart, M.T.; Tan, K.J. One-pot hydrothermal synthesis of carbon dots with efficient up-and down-converted photoluminescence for the sensitive detection of morin in a dual-readout assay. Langmuir 2017, 33, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cheng, Y.Z.; Lin, C.; Fang, J.L.; Xiang, W.D.; Liang, X.J. Carbon nanodots with intense emission from green to red and their multifunctional applications. J. Alloys Compd. 2018, 742, 212–219. [Google Scholar] [CrossRef]
- Pang, S.B.; Zhang, Y.; Wu, C.K.; Feng, S.L. Fluorescent carbon dots sensor for highly sensitive detection of guanine. Sens. Actuators B Chem. 2016, 222, 857–863. [Google Scholar] [CrossRef]
- Xiao, D.L.; Yuan, D.H.; He, H.; Gao, M.M. Microwave assisted one-step green synthesis of fluorescent carbon nanoparticles from ionic liquids and their application as novel fluorescence probe for quercetin determination. J. Lumin. 2013, 140, 120–125. [Google Scholar] [CrossRef]
- Cai, K.Y.; Yang, R.Q.; Wang, Y.J.; Yu, X.J.; Liu, J.J. Super fast detection of latent fingerprints with water soluble CdTe quantum dots. Forensic Sci. Int. 2013, 226, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, R.; Sodhi, G.S.; Kapoor, A.K. Small particle reagent based on crystal violet dye for developing latent fingerprints on non-porous wet surfaces. Egypt. J. Forensic Sci. 2015, 5, 162–165. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Ma, W.; Xiao, X. The Recognition of Sweat Latent Fingerprints with Green-Emitting Carbon Dots. Nanomaterials 2018, 8, 612. https://doi.org/10.3390/nano8080612
Zhao D, Ma W, Xiao X. The Recognition of Sweat Latent Fingerprints with Green-Emitting Carbon Dots. Nanomaterials. 2018; 8(8):612. https://doi.org/10.3390/nano8080612
Chicago/Turabian StyleZhao, Dan, Wenting Ma, and Xincai Xiao. 2018. "The Recognition of Sweat Latent Fingerprints with Green-Emitting Carbon Dots" Nanomaterials 8, no. 8: 612. https://doi.org/10.3390/nano8080612




