Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of the MBGs as Powders and Processing into Disks
2.2. Physicochemical Characterization of Samples
2.3. In Vitro Studies
2.3.1. Adsorption and Release of Osteostatin
2.3.2. Assays in SBF
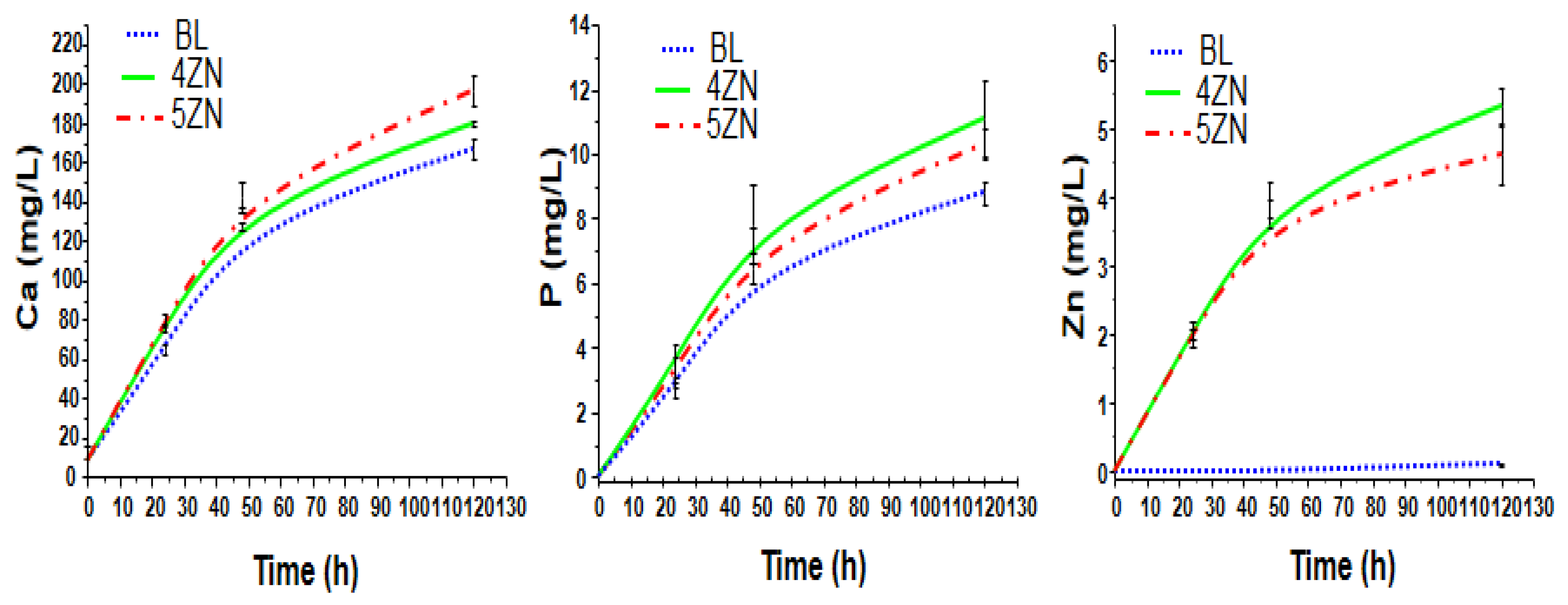
2.3.3. Ions Release from Disks
2.3.4. Culture Cell Studies
2.3.5. Statistical Analysis
3. Results and Discussion
3.1. Glass Powders Characterization
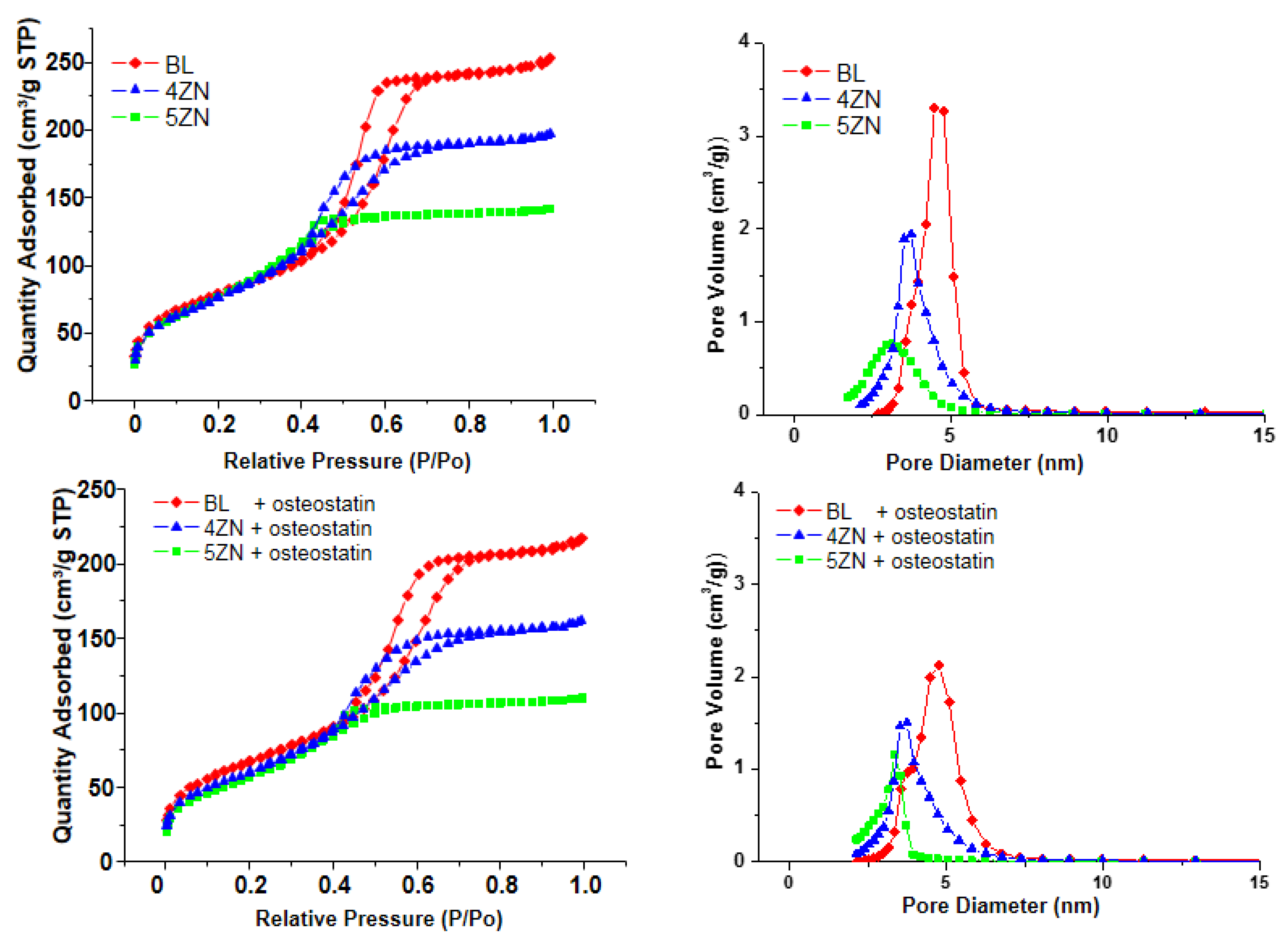
3.2. Textural Properties of MBG Disks
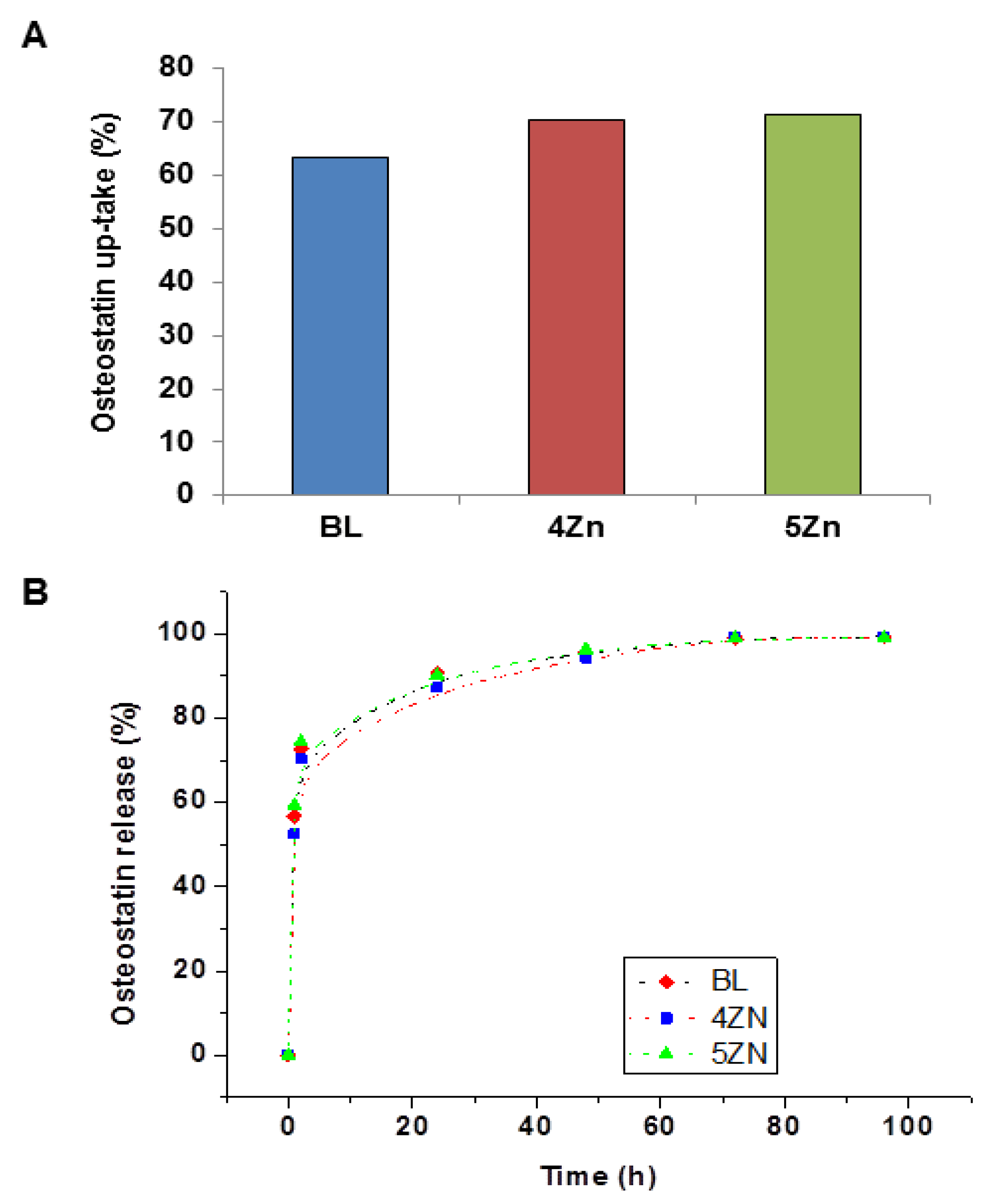
3.3. Uptake and Release of Osteostatin
3.4. In Vitro Bioactivity Assay
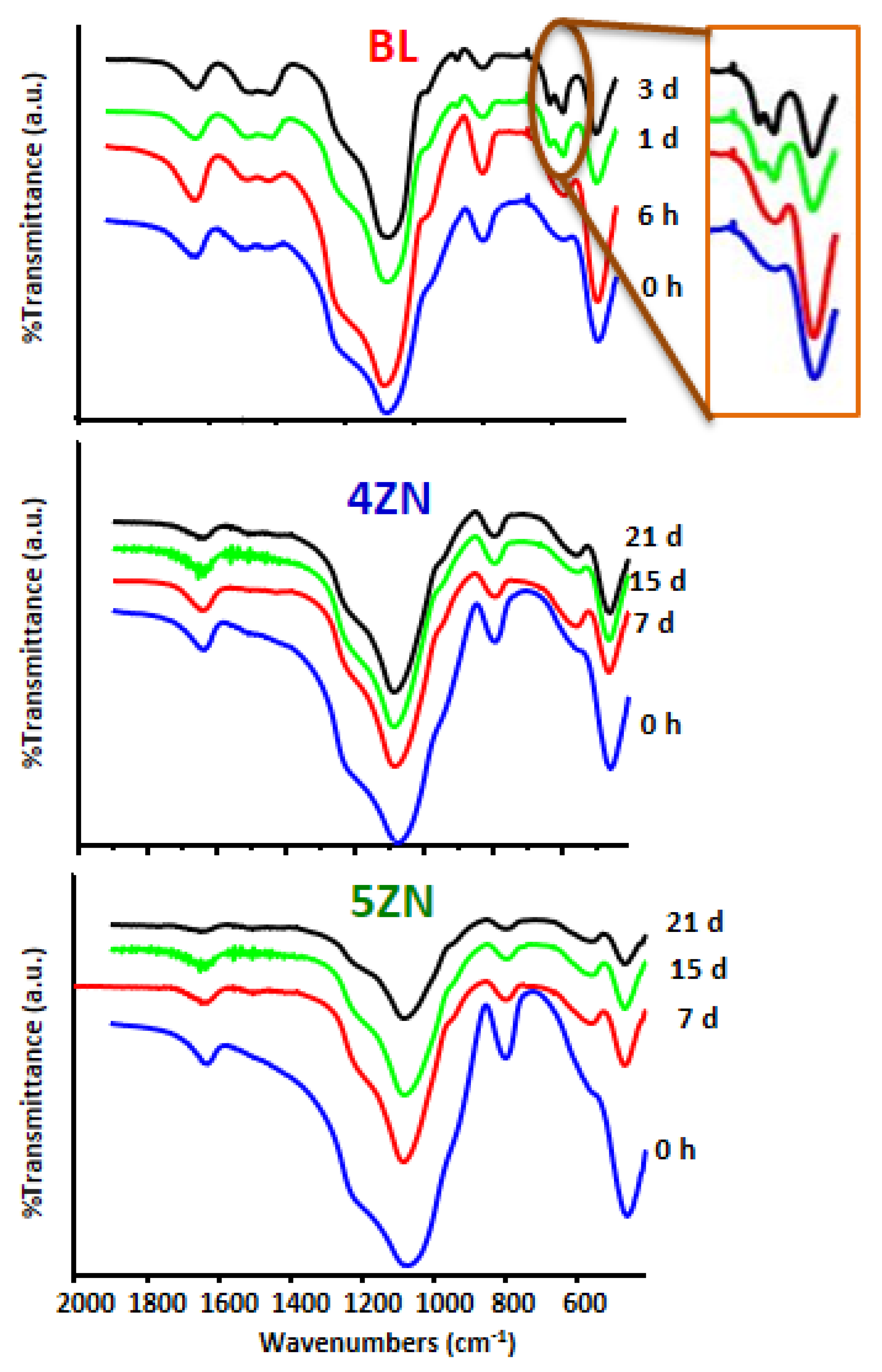
3.5. Degradability of Disks in Complete Medium
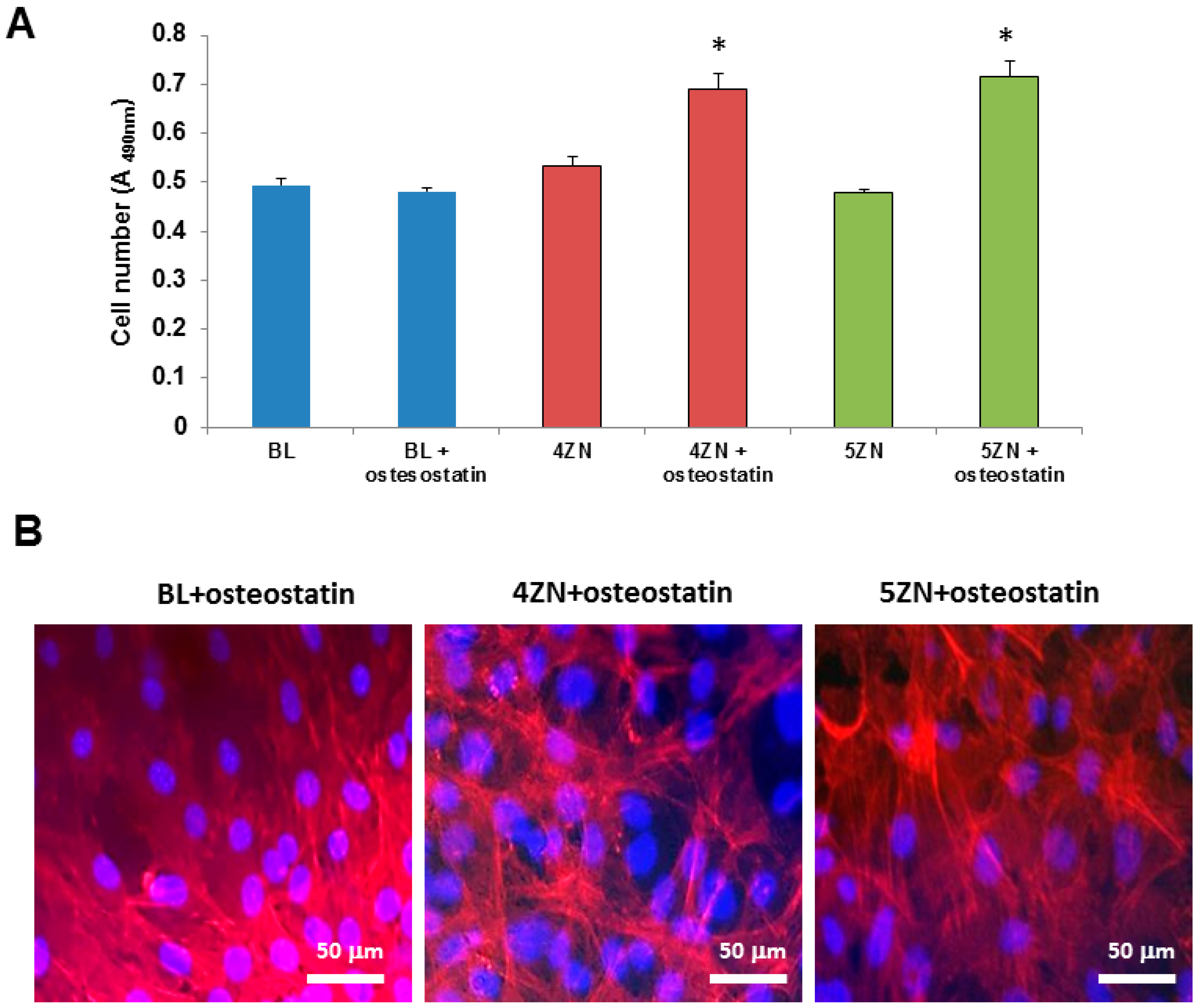
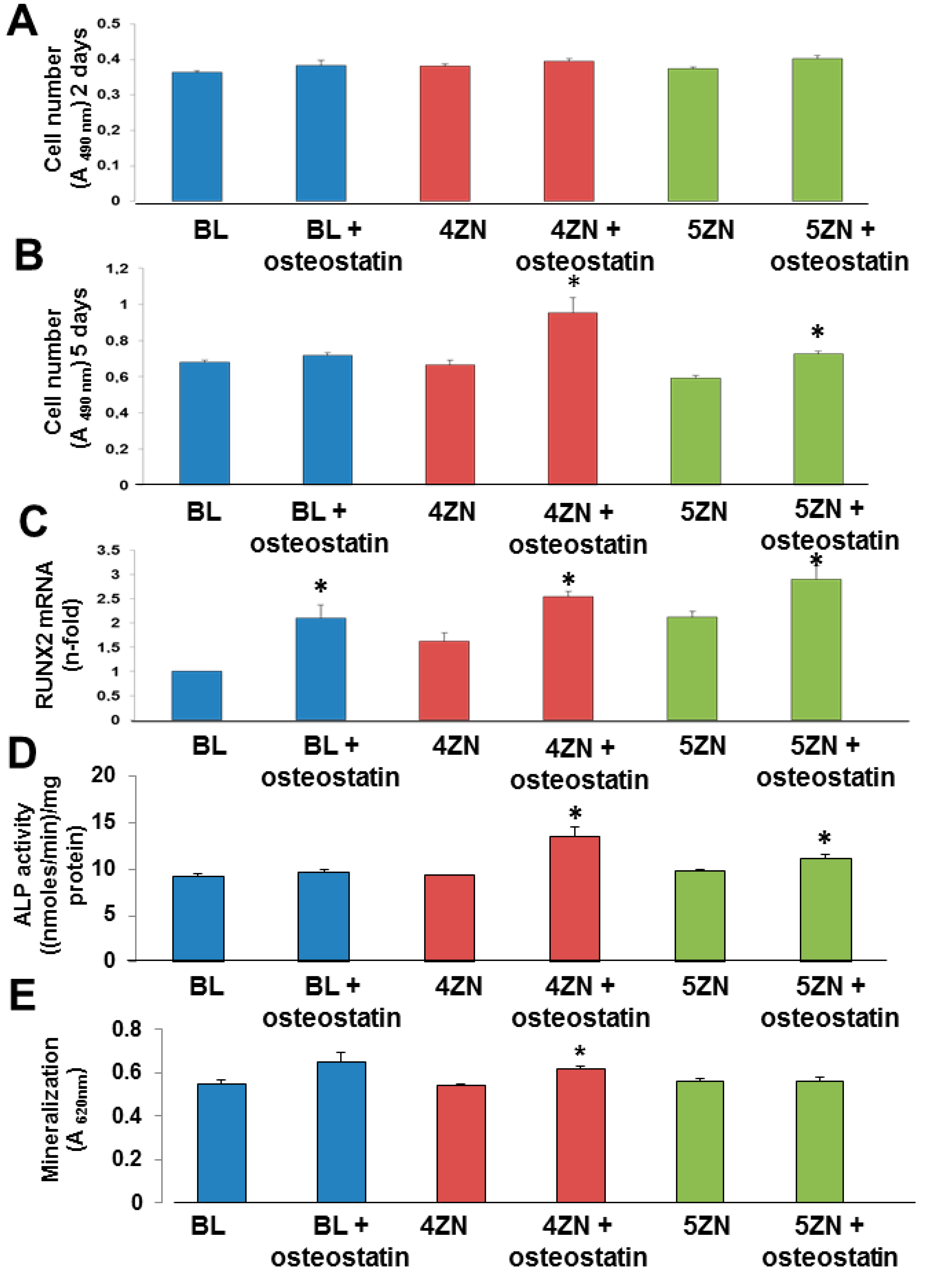
3.6. Cell Culture Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013, 1, 40–51. [Google Scholar] [CrossRef]
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C.; et al. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly Ordered Mesoporous Bioactive Glasses with Superior in Vitro Bone-Forming Bioactivities. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Salinas, A.J.; Arcos, D. Tailoring the Structure of Bioactive Glasses: From the Nanoscale to Macroporous Scaffolds. Int. J. Appl. Glass Sci. 2016, 7, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Barba, I.; Arcos, D.; Sakamoto, Y.; Terasaki, O.; López-Noriega, A. High-Performance Mesoporous Bioceramics Mimicking Bone Mineralization. Chem. Mater. 2008, 20, 3191–3198. [Google Scholar] [CrossRef]
- Ito, A.; Kawamura, H.; Otsuka, M.; Ikeuchi, M.; Ohgushi, H.; Ishikawa, K. Zinc-releasing calcium phosphate for stimulating bone formation. Mater. Sci. Eng. C 2002, 22, 21–25. [Google Scholar] [CrossRef]
- Lansdown, A.B.; Mirastschjski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lu, M.; Rutkowski, B.; Dai, X.; Yang, Y.; Taccardi, N.; Stachewicz, U.; Czyrska-Filemonowicz, A.; Hüser, N.; Boccaccini, A.R. ZnO quantum dots modified bioactive glass nanoparticles with pH-sensitive release of Zn ions, fluorescence, antibacterial and osteogenic properties. J. Mater. Chem. B 2016, 4, 7936–7949. [Google Scholar] [CrossRef] [Green Version]
- Laurenti, M.; Cauda, V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Design and preparation of biocompatible zwitterionic hydroxyapatite. J. Mater. Chem. 2013, 1, 1595–1606. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Kontopidis, I.; Gkegkes, I.D.; Rafailidis, P.I.; Falagas, M.E. Incidence, characteristics, and outcomes of patients with bone and joint infections due to community-associated methicillin-resistant Staphylococcus aureus: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salcedo, S.; Shruti, S.; Salinas, A.J.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. In vitro antibacterial capacity and cytocompatibility of SiO2–CaO–P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. B 2014, 2, 4836–4847. [Google Scholar] [CrossRef]
- Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regí, M.; Gómez-Barrena, E.; Esbrit, P. Osteostatin-loaded bioceramics stimulate osteoblastic growth and differentiation. Acta Biomater. 2010, 6, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Esbrit, P.; Alcaraz, M.J. Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies. Biochem. Pharmacol. 2013, 85, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.S.; Abou-Samra, A.B. PTH and PTHrP signalling in osteoblasts. Cell Signal. 2009, 21, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.J.; Kemp, B.E.; Hammonds, R.G.; Mitchelhill, K.; Moseley, J.M.; Martin, T.J.; Nicholson, G.C. A potent inhibitor of osteoclastic bone resorption within a highly conserved pentapeptide region of parathyroid hormone-related protein; PTHrP. Endocrinology 1991, 129, 3424–3426. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Callon, K.E.; Nicholson, G.C.; Reid, I.R. Parathyroid hormone-related protein-(107–139) inhibits bone resorption in vivo. Endocrinology 1997, 138, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Cornish, J.; Callon, K.E.; Lin, C.; Xiao, C.; Moseley, J.M.; Reid, I.R. Stimulation of osteoblast proliferation by C-terminal fragments of parathyroid hormone-related protein. J. Bone Mine. Res. 1999, 14, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; De Castro, L.F.; Dapía, S.; Andrade-Zapata, I.; Manzarbeitia, F.; Alvarez-Arroyo, M.V.; Gómez-Barrena, E.; Esbrit, P. Role of Parathyroid Hormone-Related Protein in the Decreased Osteoblast Function in Diabetes-Related Osteopenia. Endocrinology 2009, 150, 2027–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, D.; Fernández-de-Castro, L.; Portal-Núñez, S.; López-Herradón, A.; Dapía, S.; Gómez-Barrena, E.; Esbrit, P. The C-terminal fragment of parathyroid hormone-related peptide promotes bone formation in diabetic mice with low-turnover osteopenia. Br. J. Pharmacol. 2011, 162, 1424–1438. [Google Scholar] [CrossRef] [PubMed]
- Rihani-Basharat, S.; Lewinson, D. PTHrP(107–111) Inhibits In Vivo Resorption that was Stimulated by PTHrP(1–34) When Applied Intermittently to Neonatal Mice. Calcif. Tissue Int. 1997, 61, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.J.; Kemp, B.E.; Kent, G.N.; Moseley, J.M.; Zheng, M.H.; Rowe, D.J.; Britto, J.M.; Martin, T.J.; Nicholson, G.C. A Carboxyl-Terminal Peptide from the Parathyroid Hormone-Related Protein Inhibits Bone Resorption by Osteoclasts. Endocrinology 1991, 129, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- De Gortázar, A.R.; Alonso, V.; Alvarez-Arroyo, M.V.; Esbrit, P. Transient Exposure to PTHrP (107–139) Exerts Anabolic Effects through Vascular Endothelial Growth Factor Receptor 2 in Human Osteoblastic Cells in Vitro. Calcif. Tissue Int. 2006, 79, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Trejo, C.G.; Gómez-Barrena, E.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regí, M.; García-Honduvilla, N.; Esbrit, P.; Buján, J. Osteostatin-loaded onto mesoporous ceramics improves the early phase of bone regeneration in a rabbit osteopenia model. Acta Biomater. 2012, 8, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regí, M.; García-Honduvilla, N.; Buján, J.; et al. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573. [Google Scholar] [CrossRef] [PubMed]
- Lozano, D.; Sánchez-Salcedo, S.; Portal-Nuñez, S.; Vila, M.; López-Herradón, A.; Ardura, J.A.; Mulero, F.; Gomez-Barrena, E.; Vallet-Regi, M.; Esbrit, P. Parathyroid hormone-related protein (107–111) improves the bone regeneration potential of gelatin–glutaraldehyde biopolymer-coated hydroxyapatite. Acta Biomater. 2014, 10, 3307–3316. [Google Scholar] [CrossRef] [PubMed]
- Ardura, J.A.; Portal-Núñez, S.; Lozano, D.; Gutiérrez-Rojas, I.; Sánchez-Salcedo, S.; López-Herradón, A.; Mulero, F.; Villanueva-Peñacarrillo, M.L.; Vallet-Regí, M.; Esbrit, P. Local delivery of parathyroid hormone-related protein-derived peptides coated onto a hydroxyapatite-based implant enhances bone regeneration in old and diabetic rats. J. Biomed. Mater. Res. A 2016, 104, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Haimi, S.; Gorianc, G.; Moimas, L.; Lindroos, B.; Huhtala, H.; Raty, S.; Kuokkanen, H.; Sandor, G.K.; Schmid, C.; Miettinen, S.; et al. Characterization of zinc-releasing three-dimensional bioactive glass scaffolds and their effect on human adipose stem cell proliferation and osteogenic differentiation. Acta Biomater. 2009, 5, 3122–3131. [Google Scholar] [CrossRef] [PubMed]
- Salih, V.; Patel, A.; Knowles, J.C. Zinc-containing phosphate-based glasses for tissue engineering. Biomed. Mater. 2007, 2, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Aina, V.; Malavasi, G.; Fiorio, P.A.; Munaron, L.; Morterra, C. Zinc-containing bioactive glasses: Surface reactivity and behaviour towards endothelial cells. Acta Biomater. 2009, 5, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Maçon, A.L.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, A.J.; Vallet-Regí, M. Glasses in bone regeneration: A multiscale issue. J. Non-Cryst. Sol. 2016, 432, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Reddi, A.H.; Huggins, C.B. Citrate and alkaline phosphatase during transformation of fibroblasts by the matrix and minerals of bone. Proc. Soc. Exp. Biol. Med. 1972, 140, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Shruti, S.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Substitutions of cerium, gallium and zinc in ordered mesoporous bioactive glasses. Acta Biomater. 2011, 7, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Shruti, S.; Salinas, A.J.; Malavasi, G.; Lusvardi, G.; Menabue, L.; Ferrara, G.; Mustarelli, P.; Vallet-Regi, M. Structural and in vitro study of cerium, gallium and zinc containing sol–gel bioactive glasses. J. Mater. Chem. 2012, 22, 13698–13706. [Google Scholar] [CrossRef]
- Serra, J.; Gonzalez, P.; Liste, S.; Chiussi, S.; Leon, B.; Perez-Amor, M.; Ylanen, H.O.; Hupa, M. Influence of the non-bridging oxygen groups on the bioactivity of silicate glasses. J. Mater. Sci. Mater. Med. 2002, 13, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Turdean-Ionescu, C.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Surface Reactions of Mesoporous Bioactive Glasses Monitored by Solid-State NMR: Concentration Effects in Simulated Body Fluid. J. Phys. Chem. C 2016, 120, 4961–4974. [Google Scholar] [CrossRef]
- Tsai, T.W.T.; Chan, J.C.C. Recent Progress in the Solid-State NMR Studies of Biomineralization. Ann. Rep. NMR 2011, 73, 1–61. [Google Scholar]
- Leonova, E.; Izquierdo-Barba, I.; Arcos, D.; Lopez-Noriega, A.; Hedin, N.; Vallet-Regi, M.; Eden, M. Multinuclear Solid-State NMR Studies of Ordered Mesoporous Bioactive Glasses. J. Phys. Chem. C 2008, 112, 5552–5562. [Google Scholar] [CrossRef]
- Garcıa, A.; Cicuendez, M.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. Essential Role of Calcium Phosphate Heterogeneities in 2D-Hexagonal and 3D-Cubic SiO2−CaO−P2O5 Mesoporous Bioactive Glasses. Chem. Mater. 2009, 21, 5474–5484. [Google Scholar] [CrossRef]
- Linati, L.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Mustarell, P.; Segre, U. Qualitative and Quantitative Structure−Property Relationships Analysis of Multicomponent Potential Bioglasses. J. Phys. Chem. B 2005, 109, 4989–4998. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Salinas, A.J.; Vallet-Regí, M. Bioactive glasses: From macro to Nano. Int. J. Appl. Glass Sci. 2013, 4, 149–161. [Google Scholar] [CrossRef]
- Balas, F.; Manzano, M.; Horcajada, P.; Vallet-Regí, M. Confinement and controlled release of bisphosphonates on ordered mesoporous silica-based materials. J. Am. Chem. Soc. 2006, 128, 8116–8117. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Springer: New York, NY, USA, 1975. [Google Scholar]
- Manzano, M.; Aina, V.; Arean, C.O.; Balas, F.; Cauda, V.; Colilla, M.; Delgado, M.R.; Vallet-Regi, M. Studies on MCM-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalisation. Chem. Eng. J. 2008, 137, 30–37. [Google Scholar] [CrossRef]
- Elliott, J.C. Structures and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994; Volume 1, pp. 1–201. [Google Scholar]
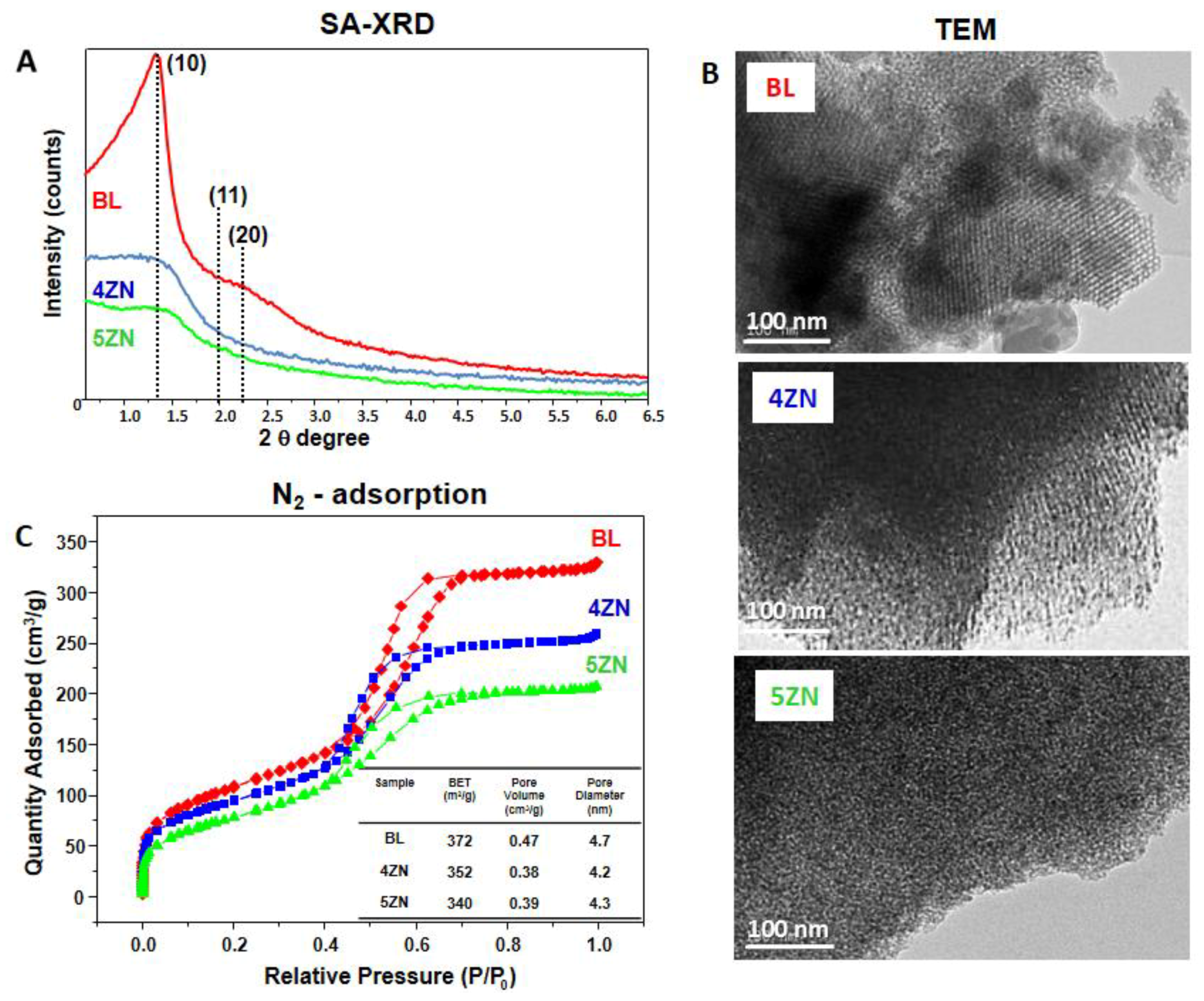
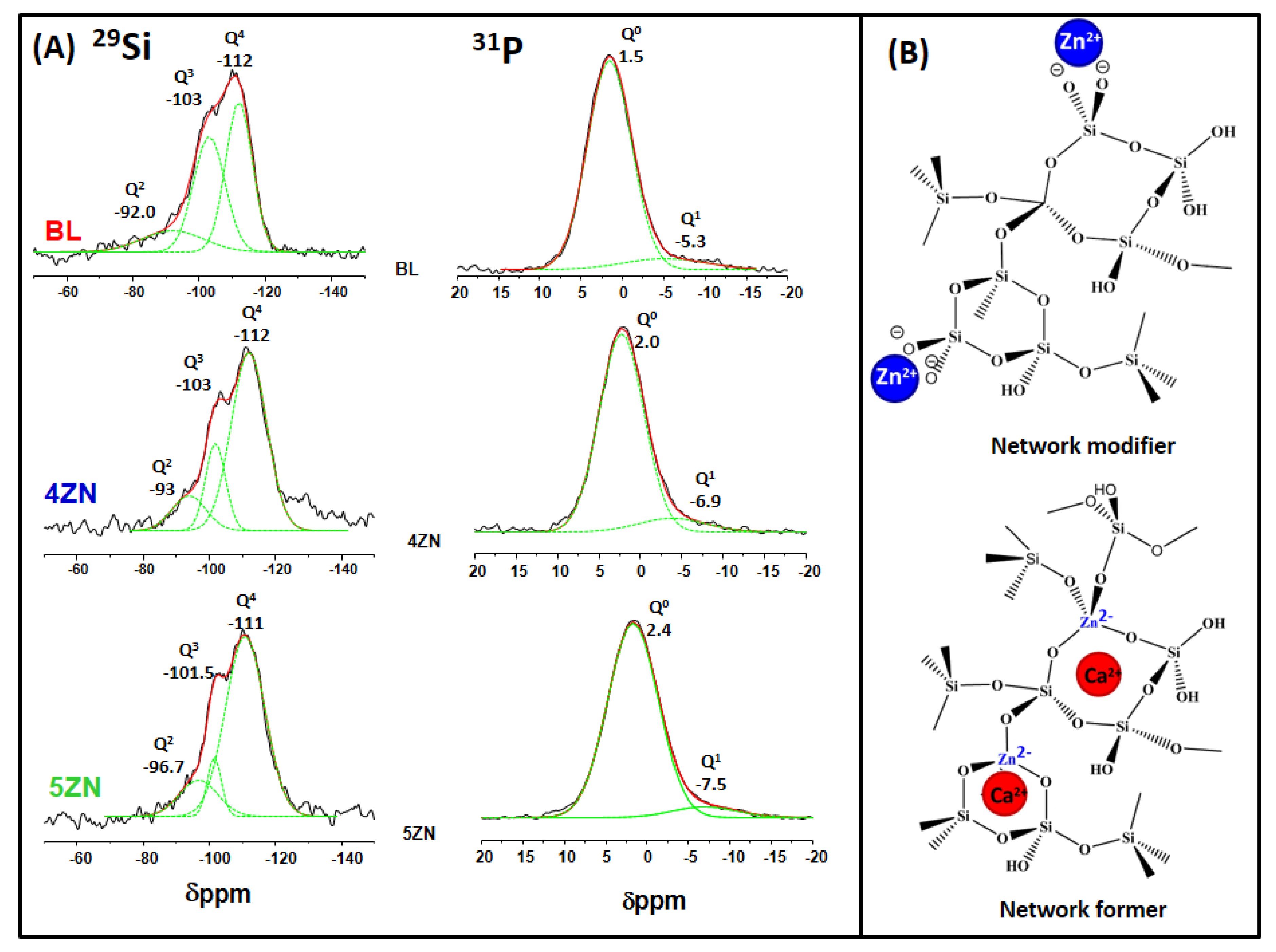

| Sample | 29Si | 31P | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q4 | Q3 | Q2 | Q0 | Q1 | ||||||||||||
| CS ppm | Area (%) | FWHM ppm | CS ppm | Area (%) | FWHM ppm | CS ppm | Area (%) | FWHM ppm | <Qn> | CS ppm | Area (%) | FWHM ppm | CS ppm | Area (%) | FWHM ppm | |
| BL | −112 | 61.7 | 8.04 | −103 | 33.8 | 9.4 | −92 | 4.4 | 18.5 | 3.57 | 1.5 | 91.2 | 5.7 | −5.3 | 8.8 | 13.0 |
| 4ZN | −112 | 68 | 10.6 | −102 | 18.9 | 6.0 | −94 | 12.9 | 10.3 | 3.55 | 2.0 | 93.7 | 7.7 | −6.9 | 6.3 | 6.1 |
| 5ZN | −110 | 75.7 | 11.8 | −103 | 15.4 | 4.3 | −93.5 | 8.92 | 11.6 | 3.67 | 2.4 | 92.9 | 5.8 | −7.5 | 7.0 | 7.4 |
| Powders | Disks | Disks + Osteostatin | Composition (EDX) Atomic % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBET (m2/g) | VT (m3/g) | DP (nm) | SBET (m2/g) | VT (m3/g) | DP (nm) | SBET (m2/g) | VT (m3/g) | DP (nm) | SiO2 | CaO | P2O5 | ZnO | |
| BL | 372 | 0.47 | 4.7 | 287 | 0.38 | 4.7 | 244 | 0.32 | 4.6 | 77.0 (80) | 6.1 (5) | 16.8 (15) | -- |
| 4ZN | 352 | 0.38 | 4.2 | 280 | 0.30 | 3.7 | 221 | 0.24 | 3.6 | 74.3 (77) | 7.0 (4.8) | 14.3 (14.4) | 4.2 (4) |
| 5ZN | 340 | 0.39 | 4.3 | 285 | 0.22 | 3.3 | 208 | 0.17 | 3.1 | 68.1 (76) | 9.2 (4.8) | 17.3 (14.3) | 5.3 (5) |
| W0 (g/g) | A (%) | k1(*103) (h−1) | R | ||
|---|---|---|---|---|---|
| BL | 0.80 | 99.3 ± 3.3 | 37.7 ± 3 | 0.14 ± 0.04 | 0.996 |
| 4ZN | 0.90 | 99.7 ± 3.9 | 31.5 ± 3 | 0.13 ± 0.04 | 0.998 |
| 5ZN | 0.95 | 99.6 ± 3.9 | 31.5 ± 4 | 0.12 ± 0.07 | 0.996 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, R.; Sanchez-Salcedo, S.; Lozano, D.; Heras, C.; Esbrit, P.; Vallet-Regí, M.; Salinas, A.J. Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin. Nanomaterials 2018, 8, 592. https://doi.org/10.3390/nano8080592
Pérez R, Sanchez-Salcedo S, Lozano D, Heras C, Esbrit P, Vallet-Regí M, Salinas AJ. Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin. Nanomaterials. 2018; 8(8):592. https://doi.org/10.3390/nano8080592
Chicago/Turabian StylePérez, Rebeca, Sandra Sanchez-Salcedo, Daniel Lozano, Clara Heras, Pedro Esbrit, María Vallet-Regí, and Antonio J. Salinas. 2018. "Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin" Nanomaterials 8, no. 8: 592. https://doi.org/10.3390/nano8080592
APA StylePérez, R., Sanchez-Salcedo, S., Lozano, D., Heras, C., Esbrit, P., Vallet-Regí, M., & Salinas, A. J. (2018). Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin. Nanomaterials, 8(8), 592. https://doi.org/10.3390/nano8080592









