Scalable Exfoliation of Bulk MoS2 to Single- and Few-Layers Using Toroidal Taylor Vortices
Abstract
:1. Introduction
2. Materials and Methods
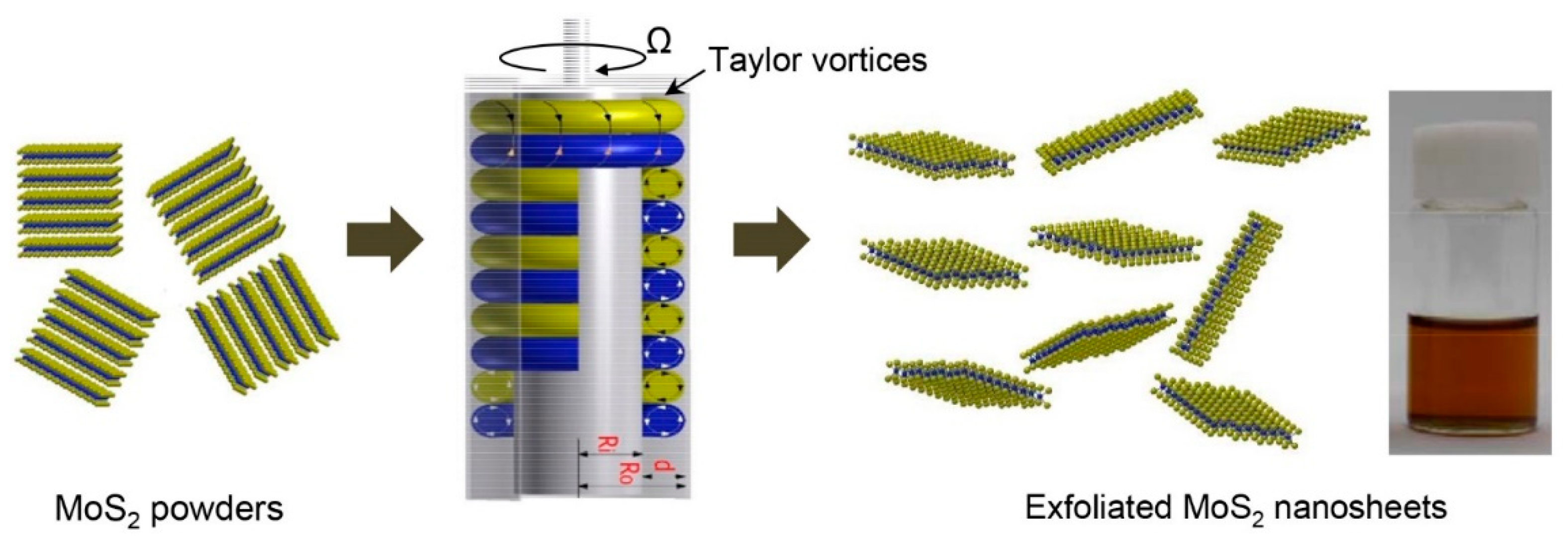
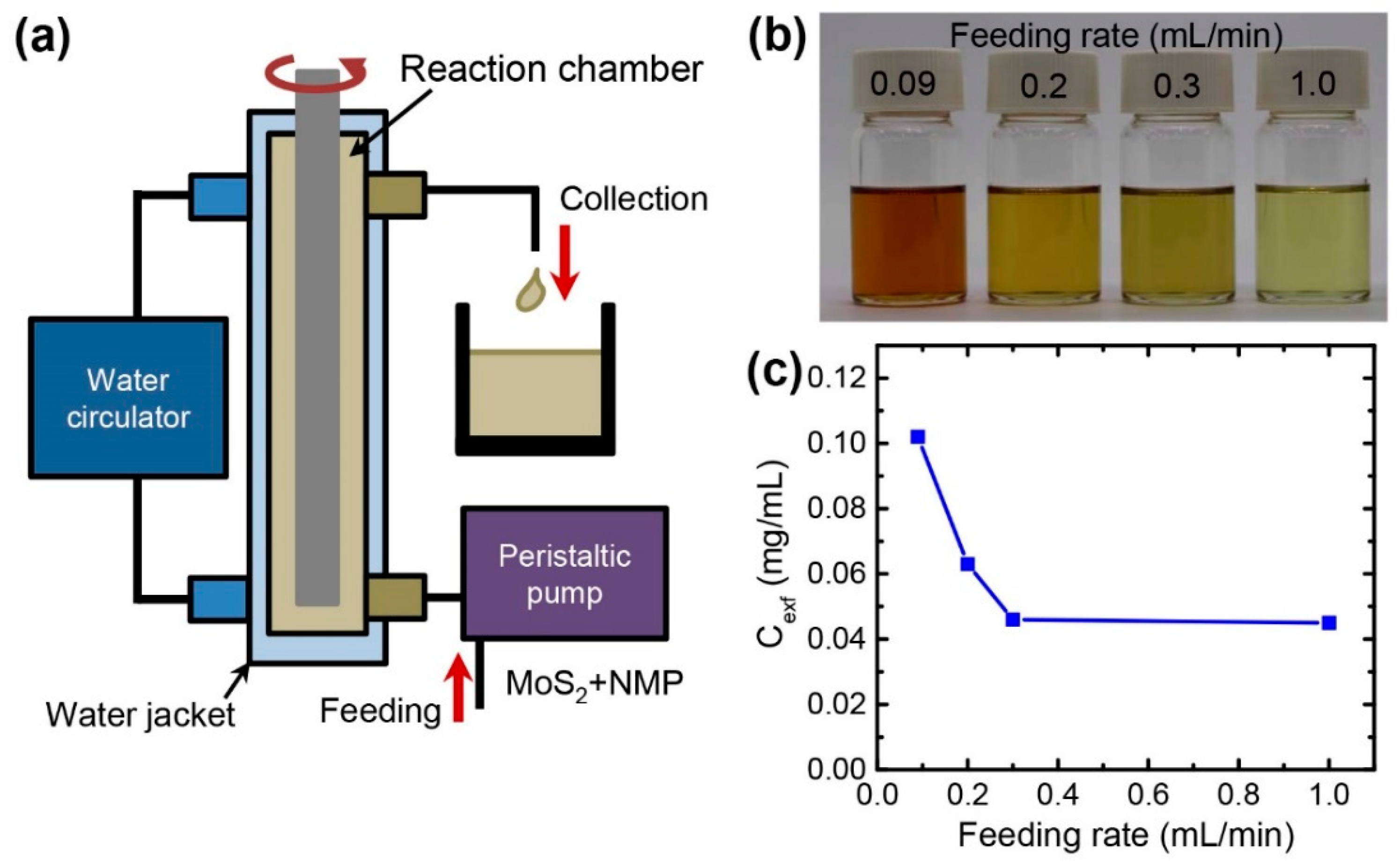
2.1. Exfoliation of MoS2 Layers with Taylor-Couette Flow
2.2. Characterization
3. Results and Discussion
3.1. Exfoliation of Bulk MoS2 in NMP
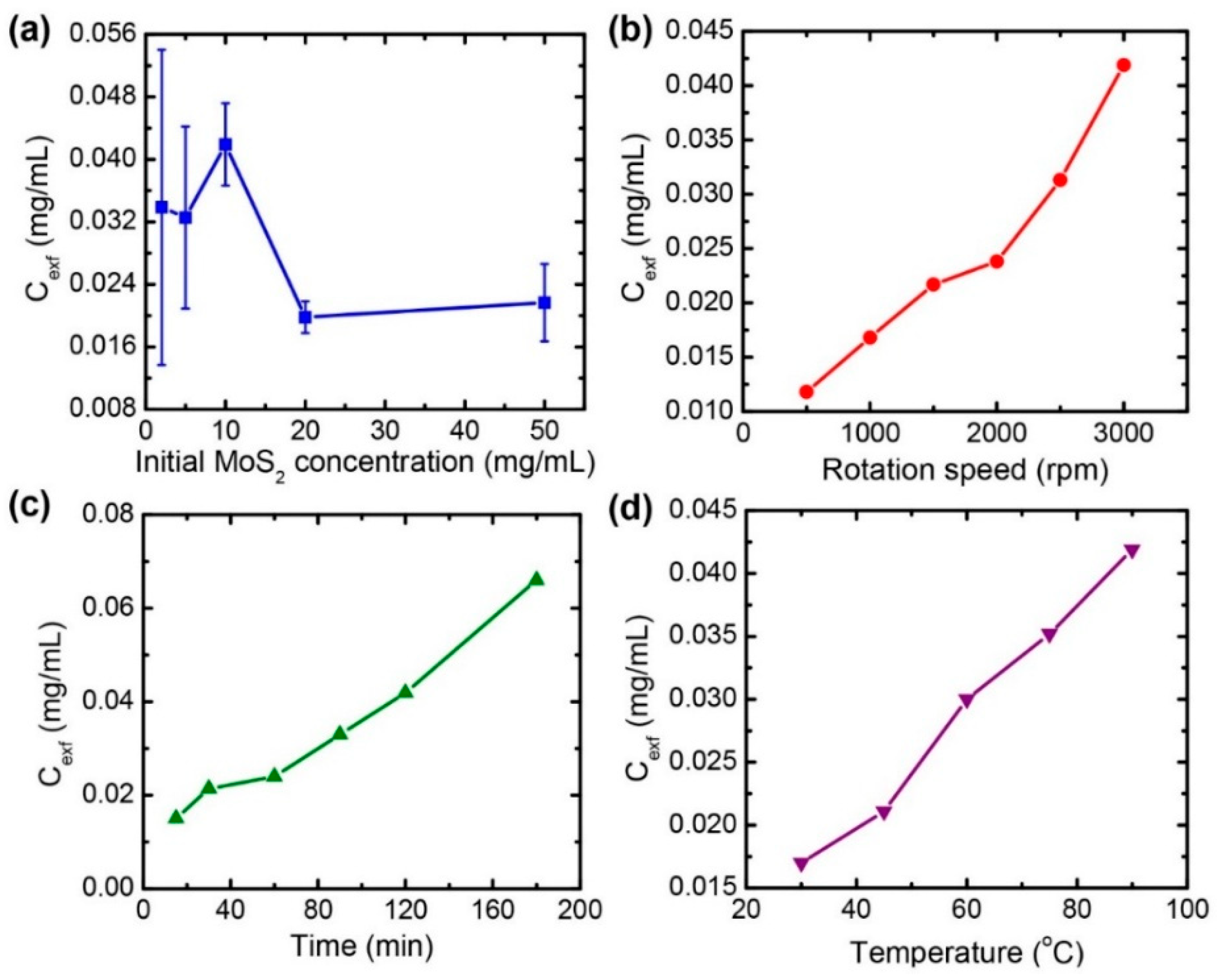
3.2. Parametric Study for Optimizing the Exfoliation Process
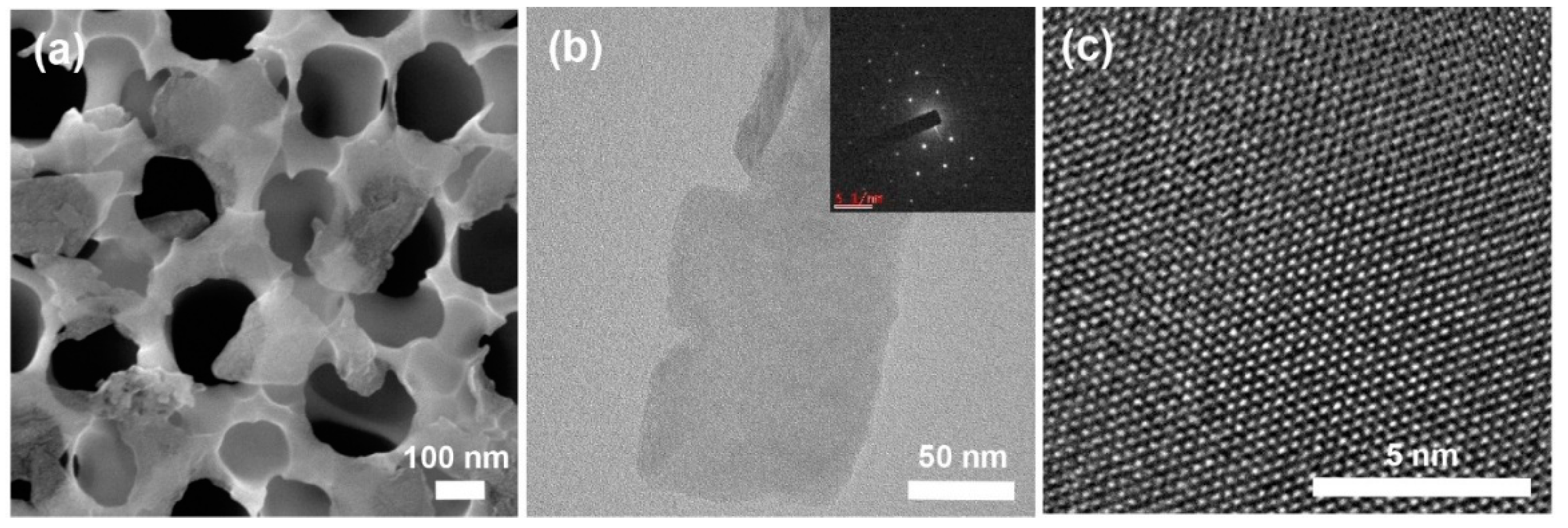
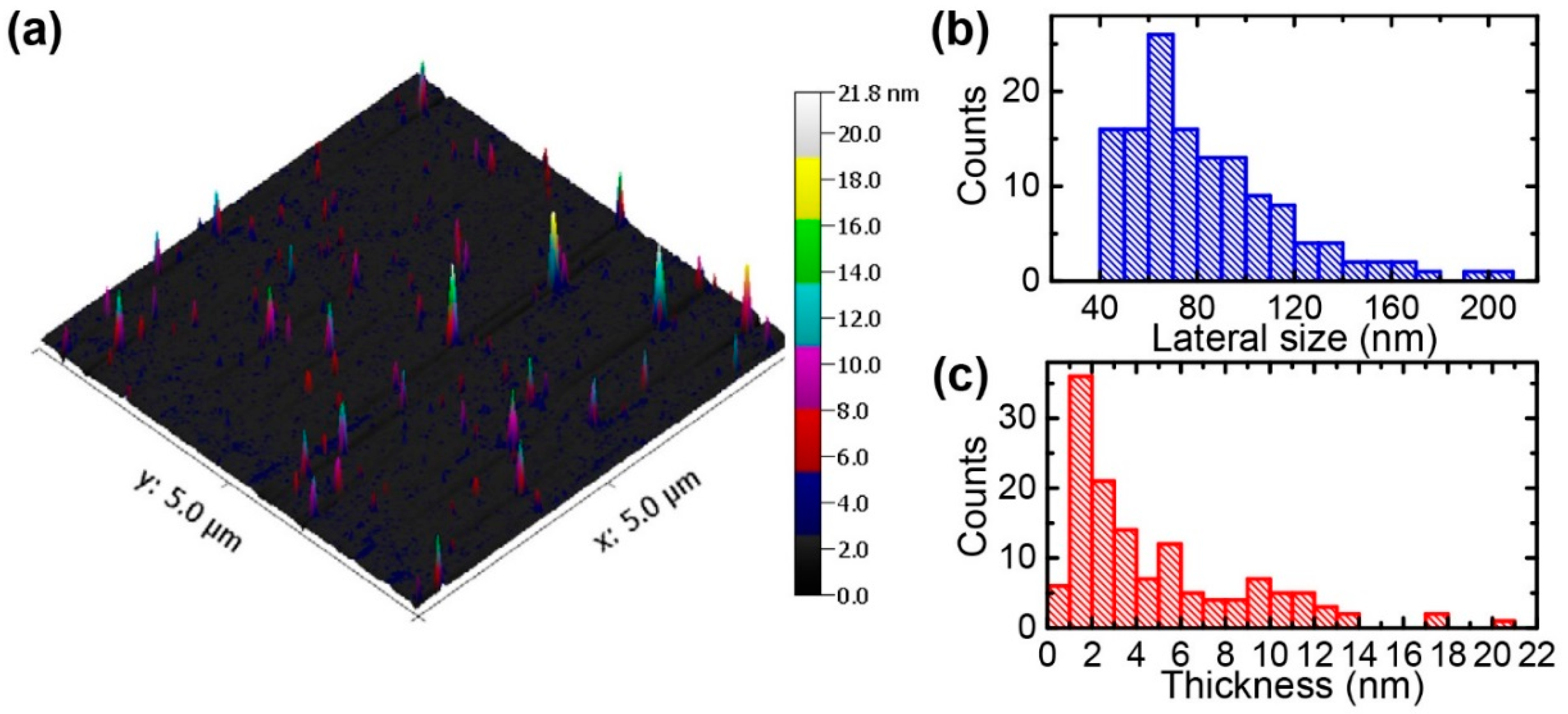
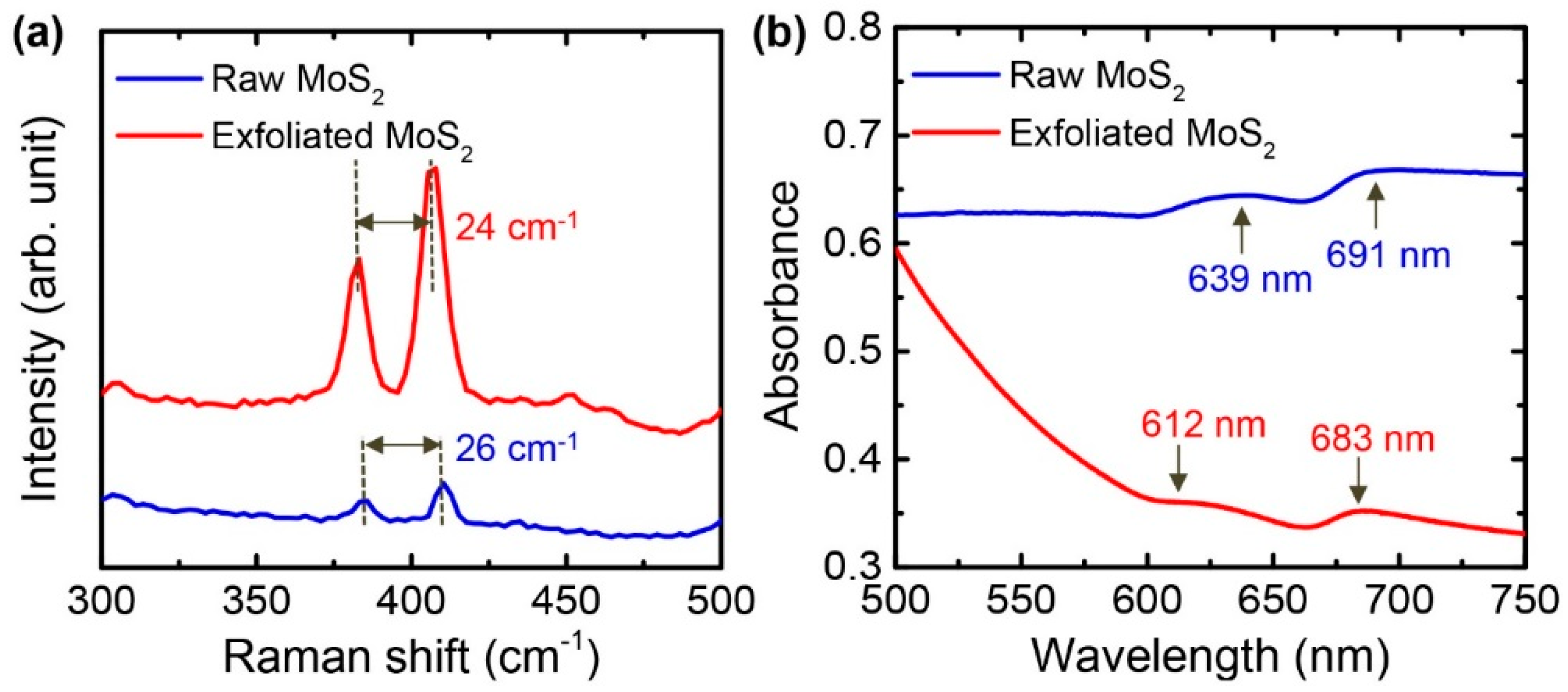
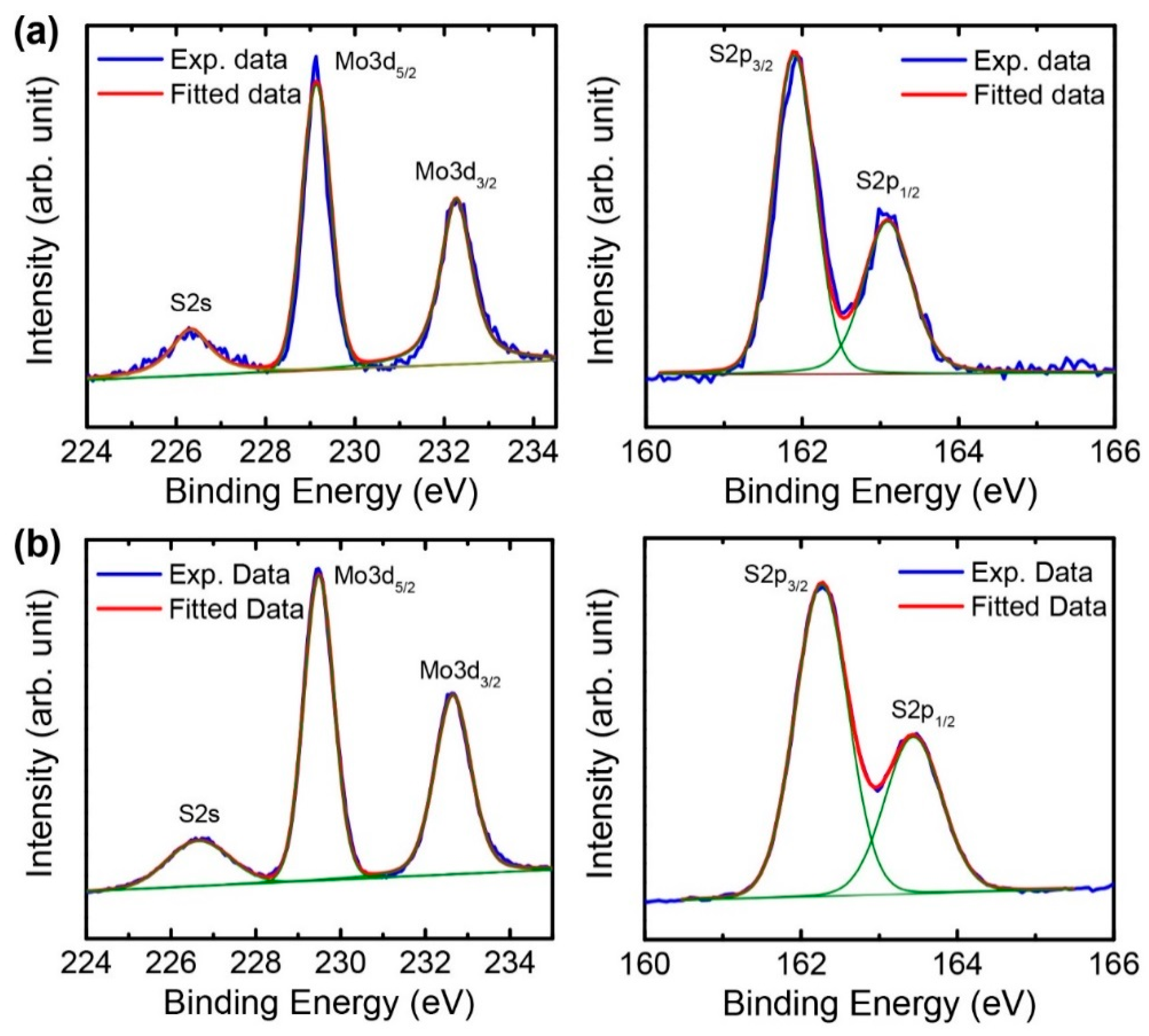
3.3. Characterization of Exfoliated MoS2 Nanosheets
3.4. Continuous Production of Exfoliated MoS2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-dimensional transition metal dichalcogenide (tmd) nanosheets. Chem. Soc. Rev. 2015, 44, 2584–2586. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Duan, X.; Wang, C.; Pan, A.; Yu, R.; Duan, X. Two-dimensional transition metal dichalcogenides as atomically thin semiconductors: Opportunities and challenges. Chem. Soc. Rev. 2015, 44, 8859–8876. [Google Scholar] [CrossRef] [PubMed]
- Tedstone, A.A.; Lewis, D.J.; O’Brien, P. Synthesis, properties, and applications of transition metal-doped layered transition metal dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shin, J.H.; Lee, G.H.; Lee, C.H. Two-dimensional semiconductor optoelectronics based on van der waals heterostructures. Nanomaterials 2016, 6, 193. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Sun, L.; Zhang, Y.B.; Li, T.S.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, B.; Ryu, S.H.; Jung, S.W.; Kim, J.; Moreschini, L.; Jozwiak, C.; Rotenberg, E.; Bostwick, A.; Kim, K.S. Universal mechanism of band-gap engineering in transition-metal dichalcogenides. Nano Lett. 2017, 17, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Mi, Z.; Song, J. Bandgap transition of 2h transition metal dichalcogenides: Predictive tuning via inherent interface coupling and strain. J. Phys. Chem. C 2016, 120, 8927–8935. [Google Scholar] [CrossRef]
- Su, X.; Ju, W.; Zhang, R.; Guo, C.; Yong, Y.; Cui, H.; Li, X. Band gap modulation of transition-metal dichalcogenide mx2 nanosheets by in-plane strain. Physica E 2016, 84, 216–222. [Google Scholar] [CrossRef]
- Lin, Z.; Karthik, P.S.; Hada, M.; Nishikawa, T.; Hayashi, Y. Simple technique of exfoliation and dispersion of multilayer graphene from natural graphite by ozone-assisted sonication. Nanomaterials 2017, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Wang, H.; Liu, X.; Sun, J.; Brongersma, M.; Pop, E.; Cui, Y. Li intercalation in MoS2: In situ observation of its dynamics and tuning optical and electrical properties. Nano Lett. 2015, 15, 6777–6784. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, V.; Zhang, R.Y.; Backstrom, J.; Dahlstrom, C.; Andres, B.; Norgren, M.; Andersson, M.; Hummelgard, M.; Olin, H. Exfoliated MoS2 in water without additives. PLoS ONE 2016, 11, e0154522. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.B.; Xu, P.T.; Zhou, D.K.; Sun, Y.F.; Li, Y.G.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and efficient preparation of exfoliated 2h MoS2 nanosheets by sonication-assisted lithium intercalation and infrared laser-induced 1t to 2h phase reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Large-scale production of size-controlled MoS2 nanosheets by shear exfoliation. Chem. Mater. 2015, 27, 1129–1139. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, T.; Kind, M. Continuous polymerization of methyl methacrylate in a taylor-couette reactor. I. Influence of fluid dynamics on monomer conversion. Polym. Eng. Sci. 2013, 53, 96–104. [Google Scholar] [CrossRef]
- Thai, D.K.; Mayra, Q.-P.; Kim, W.-S. Agglomeration of ni-rich hydroxide crystals in taylor vortex flow. Powder Technol. 2015, 274, 5–13. [Google Scholar] [CrossRef]
- Kim, M.; Park, K.J.; Lee, K.U.; Kim, M.J.; Kim, W.-S.; Kwon, O.J.; Kim, J.J. Preparation of black pigment with the couette-taylor vortex for electrophoretic displays. Chem. Eng. Sci. 2014, 119, 245–250. [Google Scholar] [CrossRef]
- Sanchez Fellay, L.; Vanni, M. The effect of flow configuration on hydrodynamic stresses and dispersion of low density rigid aggregates. J. Colloid Interface Sci. 2012, 388, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Park, W.K.; Kim, H.; Kim, T.; Kim, Y.; Yoo, S.; Kim, S.; Yoon, D.H.; Yang, W.S. Facile synthesis of graphene oxide in a couette-taylor flow reactor. Carbon 2015, 83, 217–223. [Google Scholar] [CrossRef]
- Park, W.K.; Yoon, Y.; Kim, S.; Choi, S.Y.; Yoo, S.; Do, Y.; Jung, S.; Yoon, D.H.; Park, H.; Yang, W.S. Toward green synthesis of graphene oxide using recycled sulfuric acid via couette-taylor flow. ACS Omega 2017, 2, 186–192. [Google Scholar] [CrossRef]
- Tran, T.S.; Park, S.J.; Yoo, S.S.; Lee, T.-R.; Kim, T. High shear-induced exfoliation of graphite into high quality graphene by taylor-couette flow. RSC Adv. 2016, 6, 12003–12008. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.Y.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.P.; Coleman, J.N. Solvent exfoliation of transition metal dichalcogenides: Dispersibility of exfoliated nanosheets varies only weakly between compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, Y.; Wu, J.; Gao, C.; Keyshar, K.; Zhang, X.; Yang, Y.; Ye, M.; Vajtai, R.; Lou, J.; et al. Liquid phase exfoliation of two-dimensional materials by directly probing and matching surface tension components. Nano Lett. 2015, 15, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wu, J.; Wang, M.; Dong, P.; Xu, J.; Li, X.; Zhang, X.; Yuan, J.; Wang, X.; Ye, M.; et al. Surface tension components based selection of cosolvents for efficient liquid phase exfoliation of 2d materials. Small 2016, 12, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, A.; Nepal, D.; Park, K.; Jespersen, M.; Qualley, A.; Mirau, P.; Drummy, L.F.; Vaia, R.A. Mechanism for liquid phase exfoliation of MoS2. Chem. Mater. 2016, 28, 337–348. [Google Scholar] [CrossRef]
- White, F.M. Fluid Mechanics, 7th ed.; McGraw Hill: New York, NY, USA, 2011; 862p. [Google Scholar]
- Dumont, E.; Fayolle, F.; Sobolik, V.; Legrand, J. Wall shear rate in the taylor-couette-poiseuille flow at low axial reynolds number. Int. J. Heat Mass Trans. 2002, 45, 679–689. [Google Scholar] [CrossRef]
- Liu, N.; Kim, P.; Kim, J.H.; Ye, J.H.; Kim, S.; Lee, C.J. Large-area atomically thin MoS2 nanosheets prepared using electrochemical exfoliation. ACS Nano 2014, 8, 6902–6910. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Sohn, W.; Oh, J.H.; Jang, H.W.; Kim, S.Y. Size-dependent properties of two-dimensional MoS2 and WS2. J. Phys. Chem. C 2016, 120, 10078–10085. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.Y.; Yin, Z.Y.; Huang, X.; Li, H.; He, Q.Y.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushik, V.; Wu, S.; Jang, H.; Kang, J.; Kim, K.; Suk, J.W. Scalable Exfoliation of Bulk MoS2 to Single- and Few-Layers Using Toroidal Taylor Vortices. Nanomaterials 2018, 8, 587. https://doi.org/10.3390/nano8080587
Kaushik V, Wu S, Jang H, Kang J, Kim K, Suk JW. Scalable Exfoliation of Bulk MoS2 to Single- and Few-Layers Using Toroidal Taylor Vortices. Nanomaterials. 2018; 8(8):587. https://doi.org/10.3390/nano8080587
Chicago/Turabian StyleKaushik, Vishakha, Shunhe Wu, Hoyoung Jang, Je Kang, Kyunghoon Kim, and Ji Won Suk. 2018. "Scalable Exfoliation of Bulk MoS2 to Single- and Few-Layers Using Toroidal Taylor Vortices" Nanomaterials 8, no. 8: 587. https://doi.org/10.3390/nano8080587




