Micellar Iron Oxide Nanoparticles Coated with Anti-Tumor Glycosides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and General Procedures
2.2. Synthesis
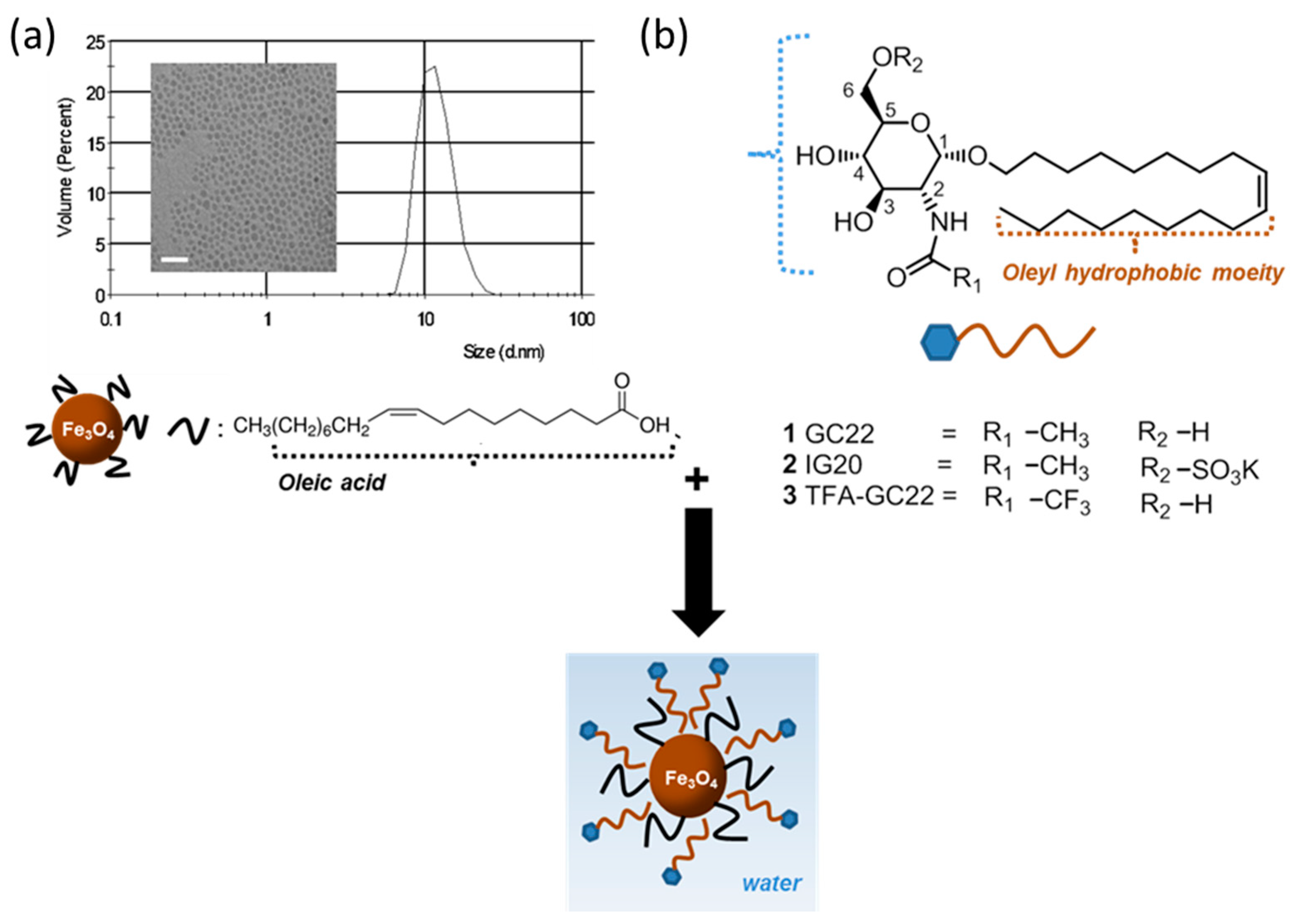
2.2.1. Synthesis of the Oleic Acid-Coated Iron Oxide Nanoparticles, OA-IONP
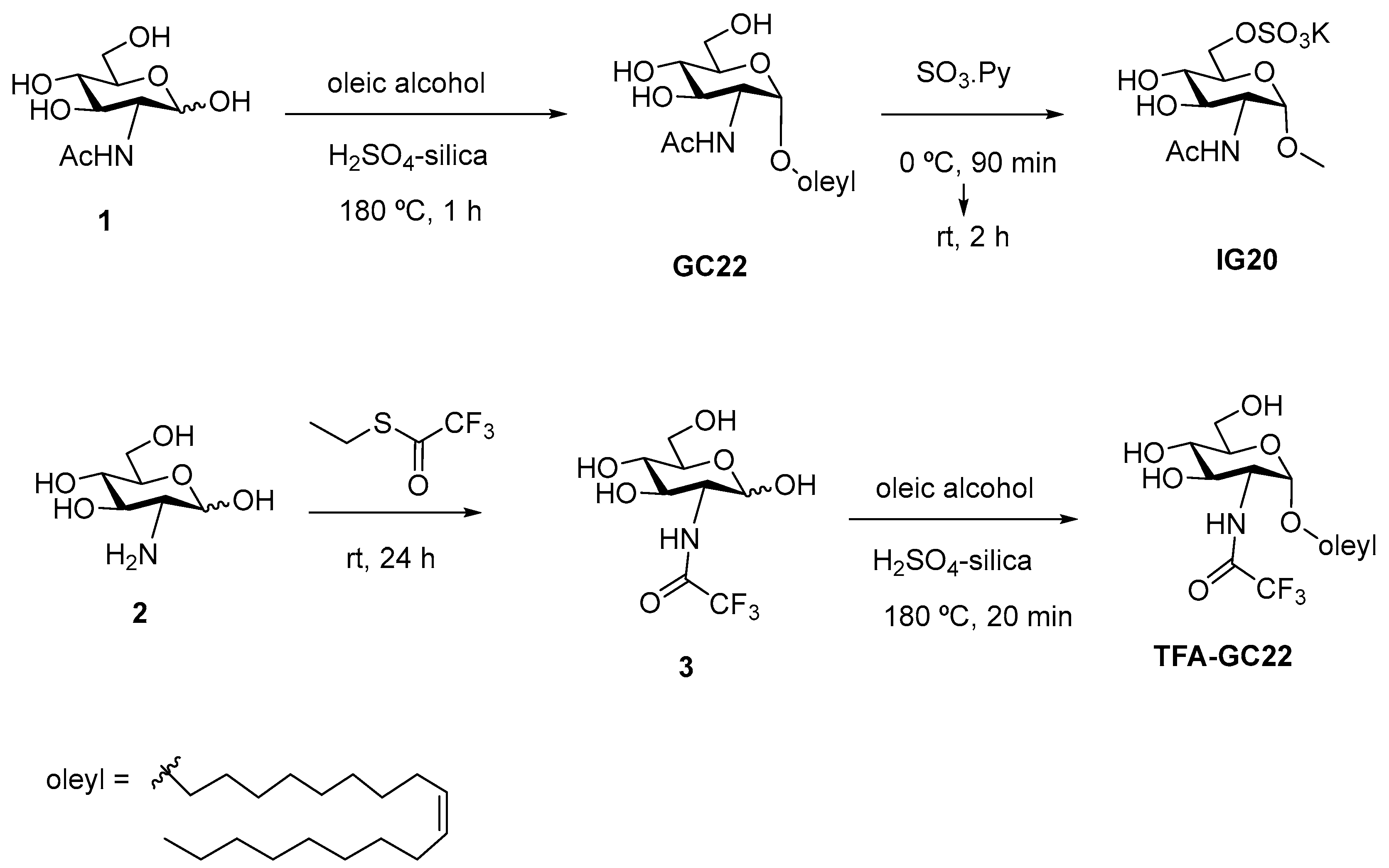
2.2.2. Synthesis of GC22, IG20 and TFA-GC22
2.2.3. Synthesis of Glycosides-Coated IONP Micelles GC22-IONP, IG20-IONP, TFA-GC22-IONP
2.3. Physicochemical Characterization of the Oleic Acid-Coated IONP (OA-IONP) and the Glycosides-Coated IONP Micelles GC22-IONP, IG20-IONP, TFA-GC22-IONP
2.4. Magnetic Characterization of the Oleic Acid-Coated IONP (OA-IONP) and the Glycosides-Coated IONP Micelles GC22-IONP, IG20-IONP, TFA-GC22-IONP
2.5. In Vitro Activity of the Glycosides-Coated IONP Micelles, Inhibition of A549 and C6 Tumor Cell Proliferation
3. Results
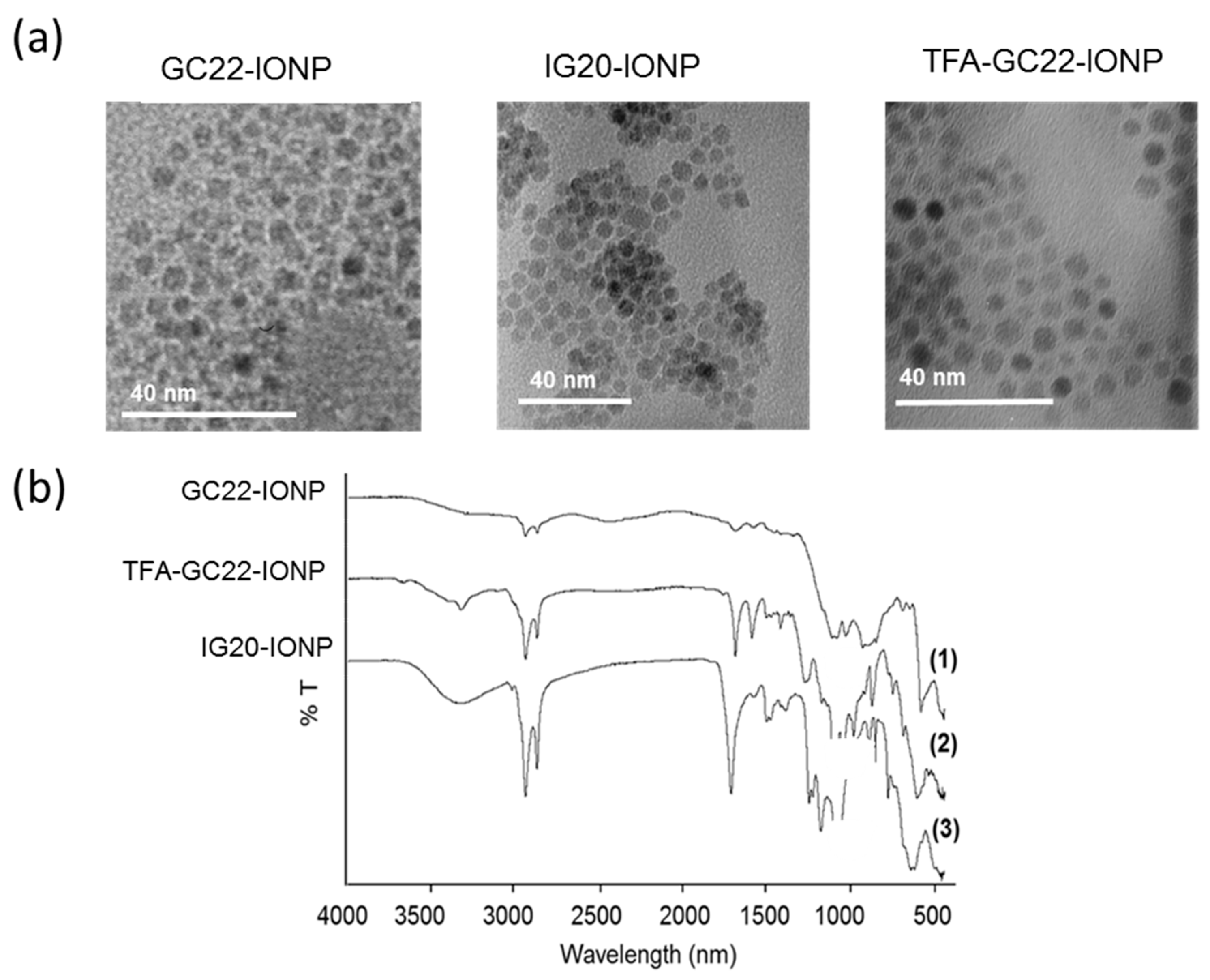
3.1. Synthesis of Glycosides-Coated IONP Micelles
3.2. Physicochemical Characterization of the Glycosides-Coated IONP Micelles
3.3. In Vitro Antitumoral Activities of the Glycosides-Coated IONP Micelles
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, J.; Weissleder, R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008, 60, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Thanos, C.G. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol. Eng. 2006, 23, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Alexiou, C.; Schmid, R.J.; Jurgons, R.; Kremer, M.; Wanner, G.; Bergemann, C.; Huenges, E.; Nawroth, T.; Arnold, W.; Parak, F.G. Targeting cancer cells: Magnetic nanoparticles as drug carriers. Eur. Biophys. J. 2006, 35, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Lübbe, A.S.; Bergemann, C.; Huhnt, W.; Fricke, T.; Riess, H.; Brock, J.W.; Huhn, D. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy. Cancer Res. 1996, 56, 4694–4701. [Google Scholar] [PubMed]
- Lübbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dörken, B.; Herrmann, F.; Gürtler, R.; et al. Clinical experiences with magnetic drug targeting: A phase I study with 4’-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar] [PubMed]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez, I.; Corrales, G.; Doncel-Pérez, E.; Muñoz, A.; Nieto-Sampedro, M.; Fernández-Mayoralas, A. Design and synthesis of glycoside inhibitors of glioma and melanoma growth. J. Med. Chem. 2007, 50, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramírez, L.; García-Álvarez, I.; Casas, J.; Barreda-Manso, M.A.; Yanguas-Casás, N.; Nieto-Sampedro, M.; Fernández-Mayoralas, A. New oleyl glycoside as anti-cancer agent that targets on neutral sphingomyelinase. Biochem. Pharmacol. 2015, 97, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Clavijo, E.; Carmona, A.T.; Vera-Ayoso, Y.; Moreno-Vargas, A.J.; Bello, C.; Vogel, P.; Robina, I. Synthesis of novel pyrrolidine 3,4-diol derivatives as inhibitors of α-L-fucosidases. Org. Biomol. Chem. 2009, 7, 1192. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez, I.; Garrido, L.; Doncel-Pérez, E.; Nieto-Sampedro, M.; Fernández-Mayoralas, A. Detection of metabolite changes in C6 glioma cells cultured with antimitotic oleyl glycoside by 1H MAS NMR. J. Med. Chem. 2009, 52, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, I.; Egido-Gabás, M.; Romero-Ramírez, L.; Doncel-Pérez, E.; Nieto-Sampedro, M.; Casas, J.; Fernández-Mayoralas, A. Lipid and ganglioside alterations in tumor cells treated with antimitotic oleyl glycoside. Mol. Biosyst. 2011, 7, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Birklé, S.; Zeng, G.; Gao, L.; Yu, R.K.; Aubry, J. Role of tumor-associated gangliosides in cancer progression. Biochimie 2003, 85, 455–463. [Google Scholar] [CrossRef]
- Lahiri, S.; Futerman, A.H. The metabolism and function of sphingolipids and glycosphingolipids. Cell. Mol. Life Sci. 2007, 64, 2270–2284. [Google Scholar] [CrossRef] [PubMed]
- Bektas, M.; Spiegel, S. Glycosphingolipids and cell death. Glycoconj. J. 2004, 20, 39–47. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, I.; Groult, H.; Casas, J.; Barreda-Manso, M.A.; Yanguas-Casás, N.; Nieto-Sampedro, M.; Romero-Ramírez, L.; Fernández-Mayoralas, A. Synthesis of Antimitotic Thioglycosides: In Vitro and in vivo Evaluation of Their Anticancer Activity. J. Med. Chem. 2011, 54, 6949–6955. [Google Scholar] [CrossRef] [PubMed]
- Chojnowska, S.; Kępka, A.; Szajda, S.D.; Waszkiewicz, N.; Bierć, M.; Zwierz, K. Exoglycosidase markers of diseases. Biochem. Soc. Trans. 2011, 39, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Driguez, H. Thiooligosaccharides in glycobiology. In Glycoscience Synthesis of Substrate Analogs and Mimetics; Driguez, H., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 187, pp. 85–116. ISBN 978-3-540-62032-7. [Google Scholar]
- Kingsley, J.D.; Dou, H.; Morehead, J.; Rabinow, B.; Gendelman, H.E.; Destache, C.J. Nanotechnology: A focus on nanoparticles as a drug delivery system. J. Neuroimmune Pharmacol. 2006, 1, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Herranz, F.; Salinas, B.; Groult, H.; Pellico, J.; Lechuga-Vieco, A.; Bhavesh, R.; Ruiz-Cabello, J. Superparamagnetic Nanoparticles for Atherosclerosis Imaging. Nanomaterials 2014, 4, 408–438. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, I.; Fernández-Mayoralas, A.; Moreno-Lillo, S.; Sánchez-Sierra, M.; Nieto-Sampedro, M.; Doncel-Pérez, E. Inhibition of glial proliferation, promotion of axonal growth and myelin production by synthetic glycolipid: A new approach for spinal cord injury treatment. Restor. Neurol. Neurosci. 2015, 33, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Wolfrom, M.L.; Conigliaro, P.J. Trifluoroacetyl as an N-protective group in the synthesis of purine nucleosides of 2-amino-2-deoxy saccharides. Carbohydr. Res. 1969, 11, 63–76. [Google Scholar] [CrossRef]
- Herranz, F.; Morales, M.P.; Roca, A.G.; Desco, M.; Ruiz-Cabello, J. A New Method for the Rapid Synthesis of Water Stable Superparamagnetic Nanoparticles. Chem. Eur. J. 2008, 14, 9126–9130. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.G.; Morales, M.P.; O’Grady, K.; Serna, C.J. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783–2788. [Google Scholar] [CrossRef]
- Ahmad, S.; Riaz, U.; Kaushik, A.; Alam, J. Soft Template Synthesis of Super Paramagnetic Fe3O4 Nanoparticles a Novel Technique. J. Inorg. Organomet. Polym. Mater. 2009, 19, 355–360. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N.; et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Groult, H.; Ruiz-Cabello, J.; Lechuga-Vieco, A.V.; Mateo, J.; Benito, M.; Bilbao, I.; Martínez-Alcázar, M.P.; Lopez, J.A.; Vázquez, J.; Herranz, F.F. Phosphatidylcholine-Coated Iron Oxide Nanomicelles for in vivo Prolonged Circulation Time with an Antibiofouling Protein Corona. Chem. Eur. J. 2014, 20, 16662–16671. [Google Scholar] [CrossRef] [PubMed]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Pellico, J.; Lechuga-Vieco, A.V.; Benito, M.; García-Segura, J.M.; Fuster, V.; Ruiz-Cabello, J.; Herranz, F. Microwave-driven synthesis of bisphosphonate nanoparticles allows in vivo visualisation of atherosclerotic plaque. RSC Adv. 2015, 5, 1661–1665. [Google Scholar] [CrossRef]
- Mejías, R.; Pérez-Yagüe, S.; Gutiérrez, L.; Cabrera, L.I.; Spada, R.; Acedo, P.; Serna, C.J.; Lázaro, F.J.; Villanueva, A.; Morales, M.D.P.; et al. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials 2011, 32, 2938–2952. [Google Scholar] [CrossRef] [PubMed]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.-F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional Polymeric Micelles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Castrillo, A.; Punzón, E.; de Pascual, R.; Maroto, M.; Padín, J.F.; García-Álvarez, I.; Nanclares, C.; Ruiz-Pascual, L.; Gandía, L.; Fernández-Mayoralas, A.; et al. Novel synthetic sulfoglycolipid IG20 facilitates exocytosis in chromaffin cells through the regulation of sodium channels. J. Neurochem. 2015, 135, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Schreier, S.; Malheiros, S.V.P.; de Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef]
- Liu, Y.; Miyoshi, H.; Nakamura, M. Nanomedicine for drug delivery and imaging: A promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int. J. Cancer 2007, 120, 2527–2537. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Wientjes, M.G.; Lu, D.; Au, J.L.S. Drug delivery and transport to solid tumors. Pharm. Res. 2003, 20, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Sfara, C.; Battistelli, S.; Canonico, B.; Arcangeletti, M.; Manuali, E.; Salamida, S.; Papa, S.; Magnani, M. New Strategies to Prolong the in vivo Life Span of Iron-Based Contrast Agents for MRI. PLoS ONE 2013, 8, e78542. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Clinical relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Wáng, Y.X.J.; Idée, J.-M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [PubMed]
- Cortajarena, A.L.; Ortega, D.; Ocampo, S.M.; Gonzalez-García, A.; Couleaud, P.; Miranda, R.; Belda-Iniesta, C.; Ayuso-Sacido, A. Engineering Iron Oxide Nanoparticles for Clinical Settings. Nanobiomedicine 2014, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, T.-I.; Lee, J.; Lim, E.-K.; Hyung, W.; Lee, C.-H.; Song, Y.J.; Suh, J.-S.; Yoon, H.-G.; Huh, Y.-M.; et al. Synthesis of Ultrasensitive Magnetic Resonance Contrast Agents for Cancer Imaging Using PEG-Fatty Acid. Chem. Mater. 2007, 19, 3870–3876. [Google Scholar] [CrossRef]
| Compound | IC50 (μM) C6 | IC50 (μM) A549 |
|---|---|---|
| GC22 | 15.5 ± 0.3 | 10 |
| GC22-IONP w/o magnet | 55.0 | 100.5 |
| GC22-IONP w/ magnet | 70.0 | 95.0 |
| IG20 | >100 | 97 |
| IG20-IONP w/o magnet | 68.5 | 64.4 |
| IG20-IONP w/ magnet | 57.2 | 91.0 |
| TFA-GC22 | 14.2 ± 0.3 | 8.6 |
| TFA-GC22-IONP w/o magnet | 24.4 | 40.3 |
| TFA-GC22-IONP w/ magnet | 49.8 | 42.0 |
| Glycosidic IONP Micelles | Size (nm) | Pdi | Zeta Potential (mV) | [Fe] (mg/mL) | C° [glyco] (mg/mL) | Relaxometric Parameters (s−1·mM−1) |
|---|---|---|---|---|---|---|
| GC22-IONP | 40.5 | 0.24 | −27 | 0.6 | 2.5 | r1 2.7 r2 140 |
| IG20-IONP | 52.2 | 0.15 | −42 | 1.1 | 3.1 | r1 4.4 r2 195 |
| TFA-GC22-IONP | 49.1 | 0.17 | +53 | 0.3 | 2.5 | r1 3.6 r2 137 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groult, H.; García-Álvarez, I.; Romero-Ramírez, L.; Nieto-Sampedro, M.; Herranz, F.; Fernández-Mayoralas, A.; Ruiz-Cabello, J. Micellar Iron Oxide Nanoparticles Coated with Anti-Tumor Glycosides. Nanomaterials 2018, 8, 567. https://doi.org/10.3390/nano8080567
Groult H, García-Álvarez I, Romero-Ramírez L, Nieto-Sampedro M, Herranz F, Fernández-Mayoralas A, Ruiz-Cabello J. Micellar Iron Oxide Nanoparticles Coated with Anti-Tumor Glycosides. Nanomaterials. 2018; 8(8):567. https://doi.org/10.3390/nano8080567
Chicago/Turabian StyleGroult, Hugo, Isabel García-Álvarez, Lorenzo Romero-Ramírez, Manuel Nieto-Sampedro, Fernando Herranz, Alfonso Fernández-Mayoralas, and Jesús Ruiz-Cabello. 2018. "Micellar Iron Oxide Nanoparticles Coated with Anti-Tumor Glycosides" Nanomaterials 8, no. 8: 567. https://doi.org/10.3390/nano8080567
APA StyleGroult, H., García-Álvarez, I., Romero-Ramírez, L., Nieto-Sampedro, M., Herranz, F., Fernández-Mayoralas, A., & Ruiz-Cabello, J. (2018). Micellar Iron Oxide Nanoparticles Coated with Anti-Tumor Glycosides. Nanomaterials, 8(8), 567. https://doi.org/10.3390/nano8080567







