Effect of Growth Rate on the Crystal Orientation and Magnetization Performance of Cobalt Nanocrystal Arrays Electrodeposited from Aqueous Solution
Abstract
1. Introduction
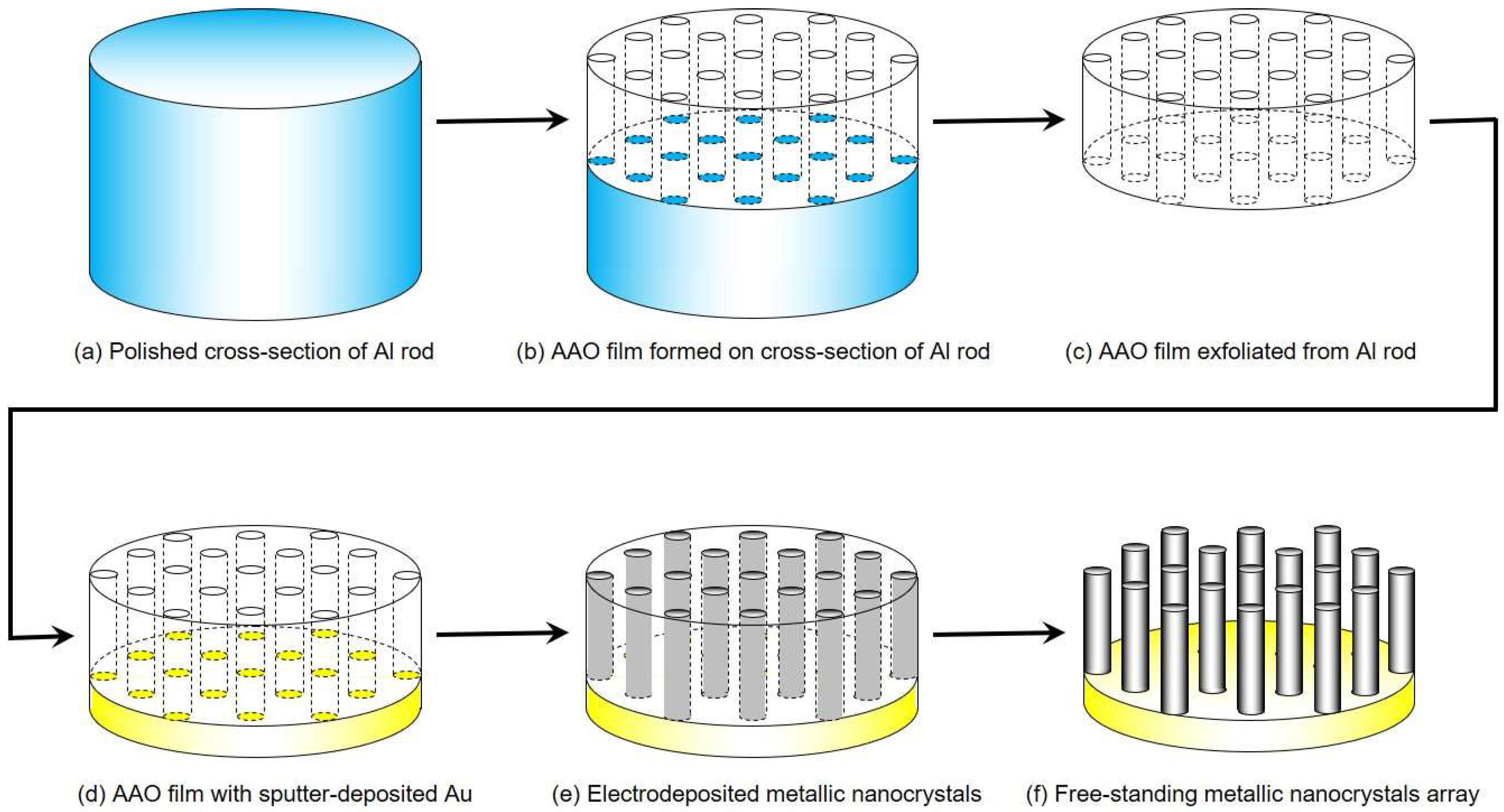
2. Materials and Methods
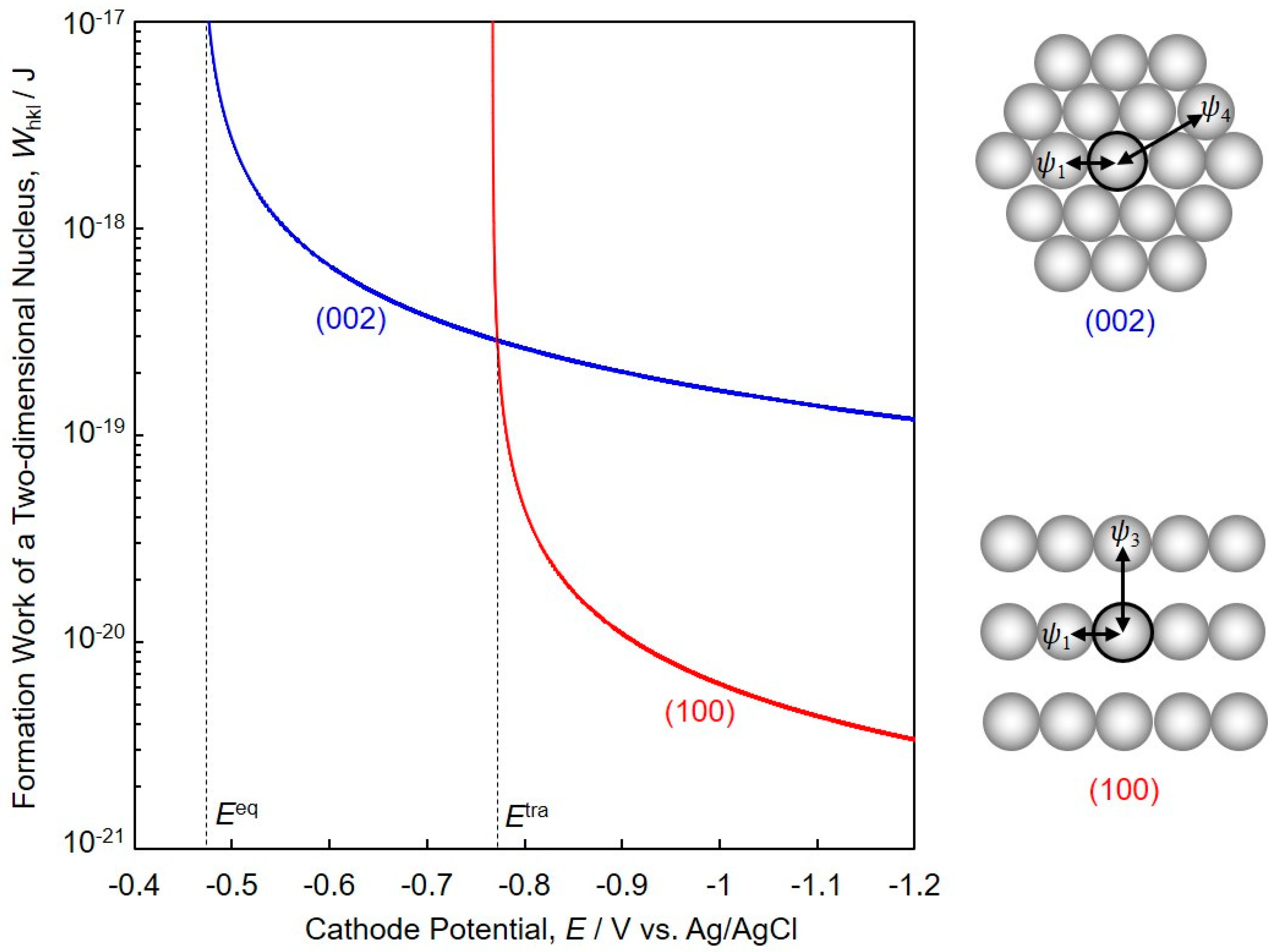
3. Formation Work of a Two-Dimensional hcp-Co Nucleus Based on Pangarov’s Theory
4. Results and Discussion
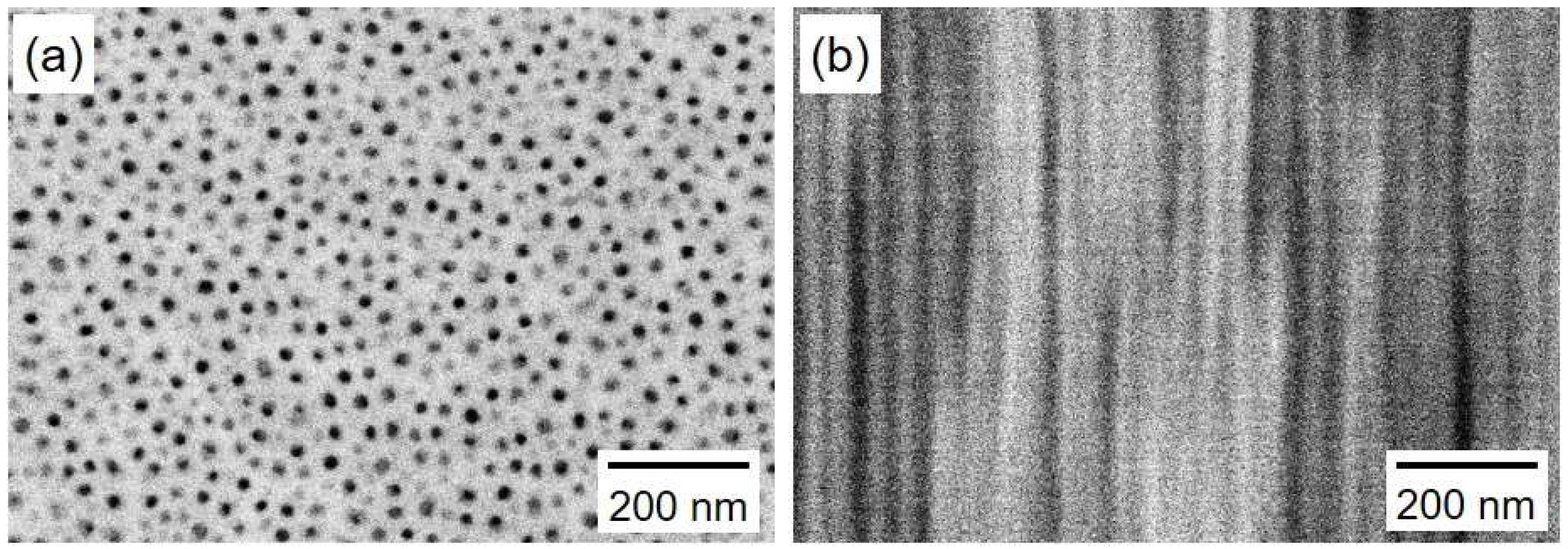
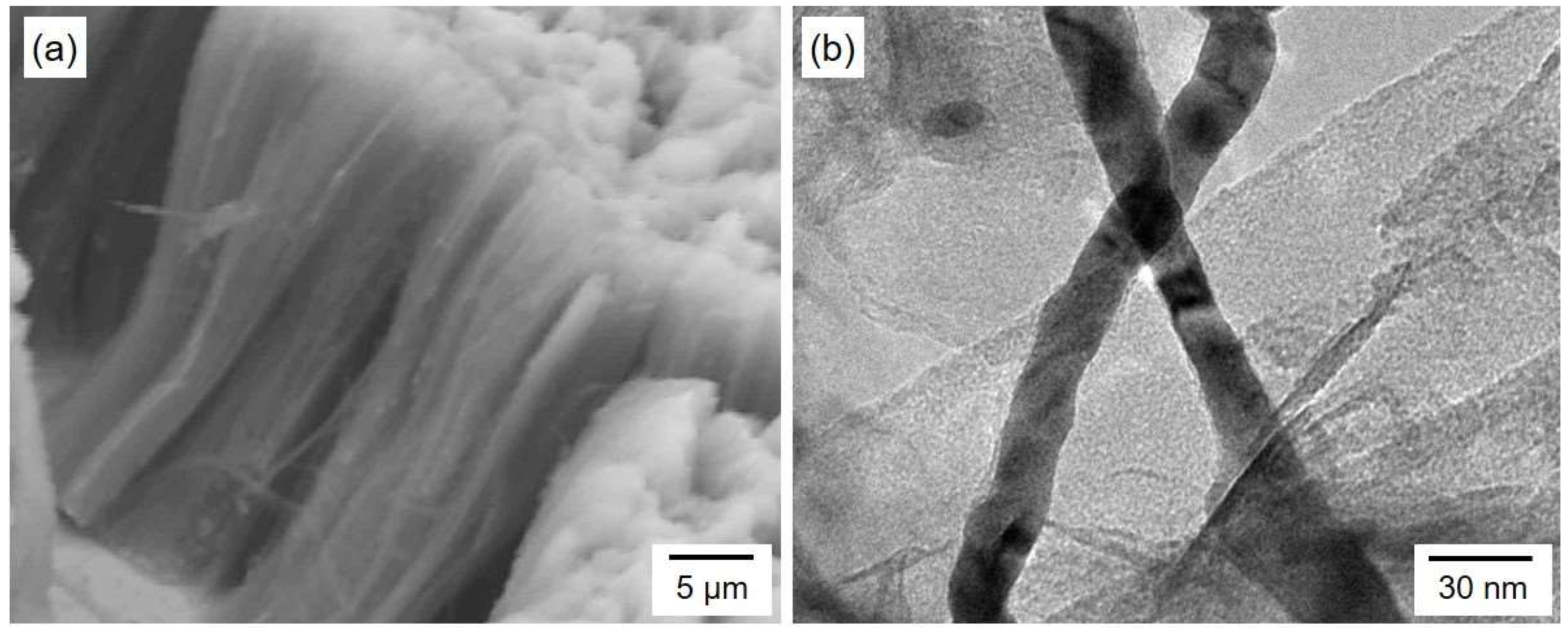
4.1. Fabrication of AAO Nanochannel Templates
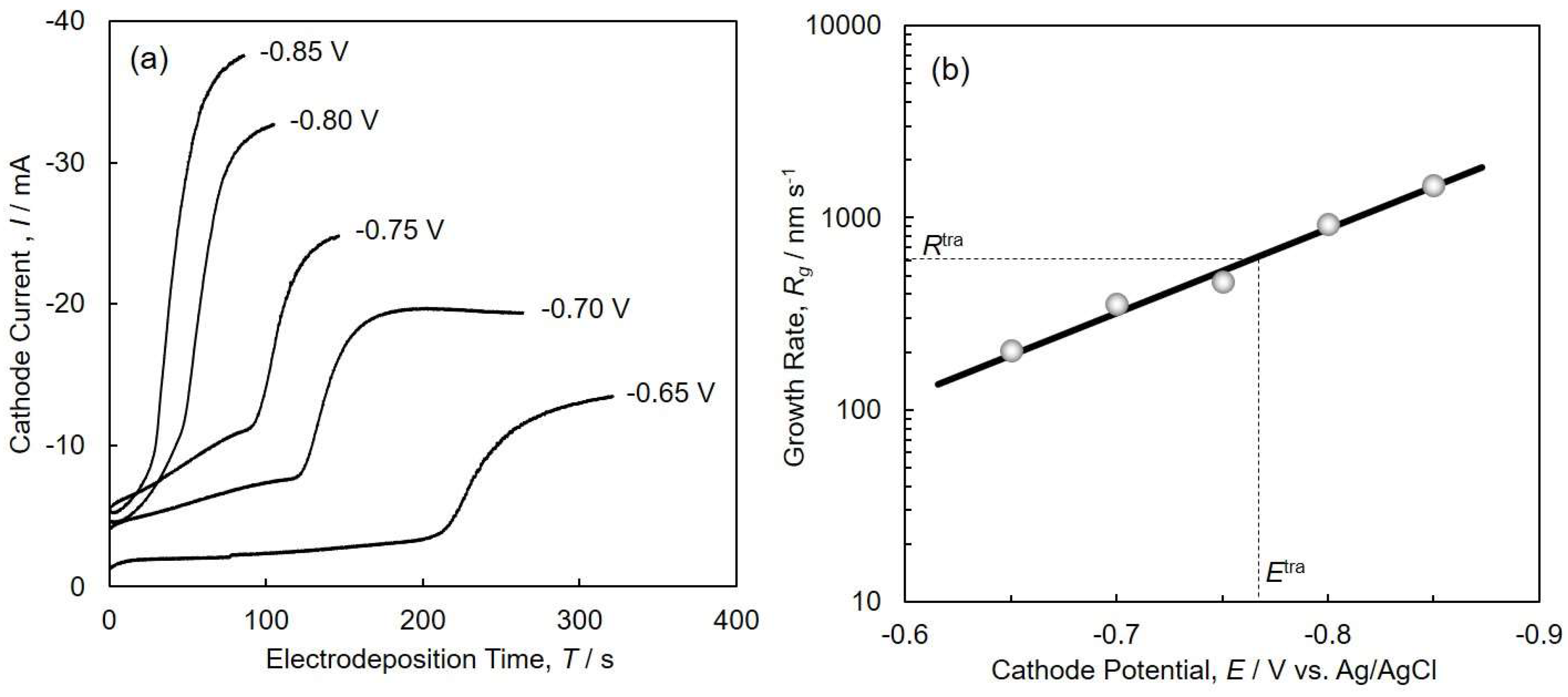
4.2. Electrodeposition of Cobalt Nanocrystal Arrays
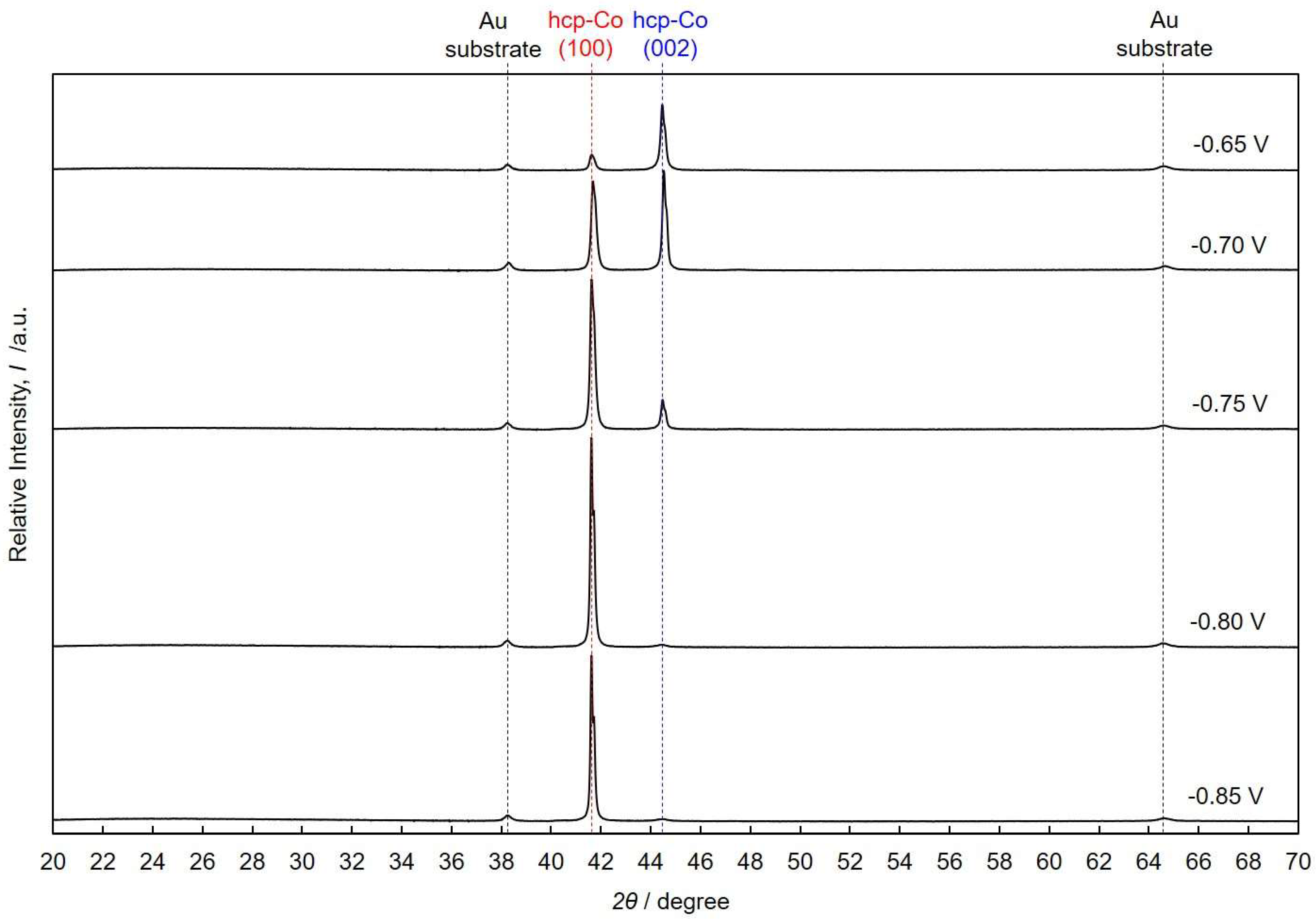
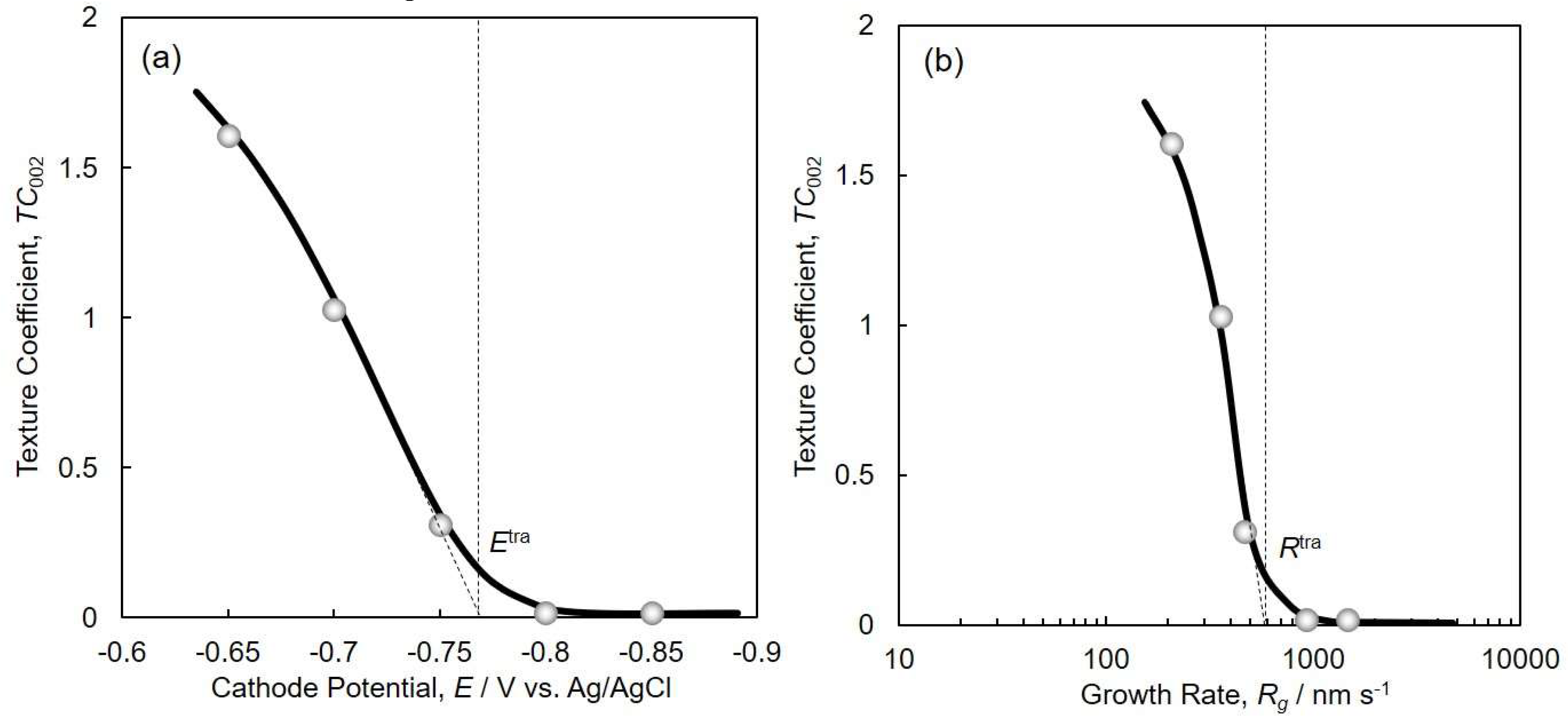
4.3. Crystallographic Orientation of Co Nanocrystal Arrays
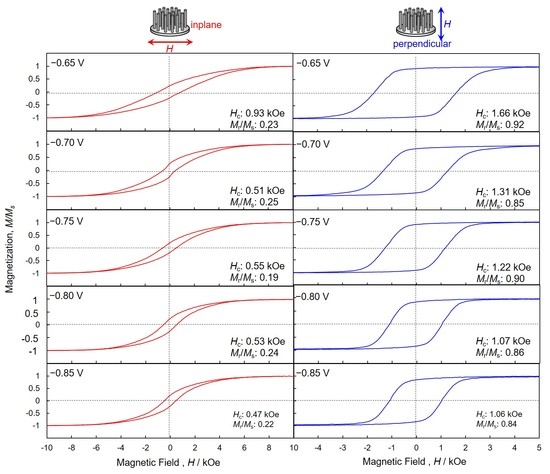
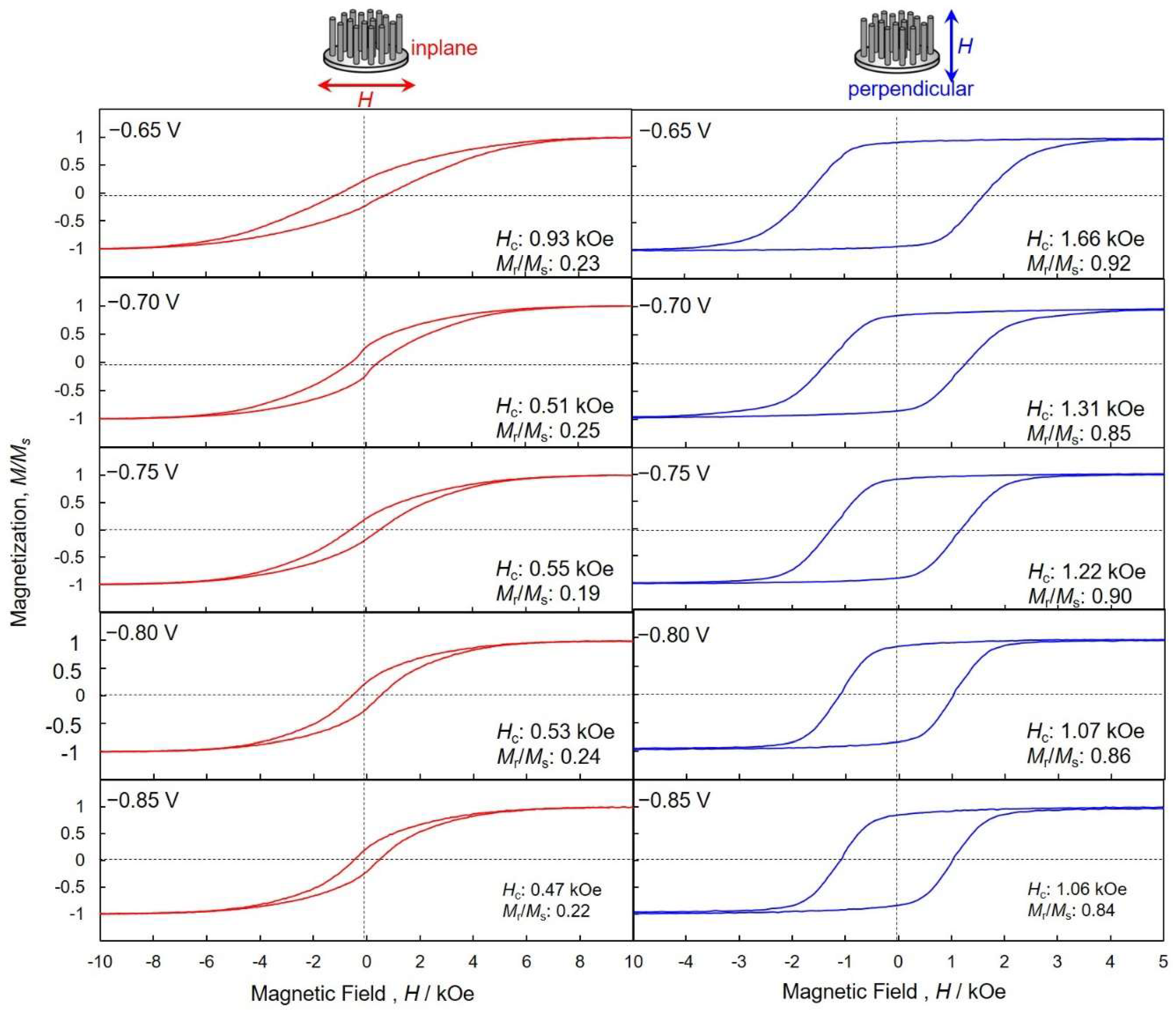
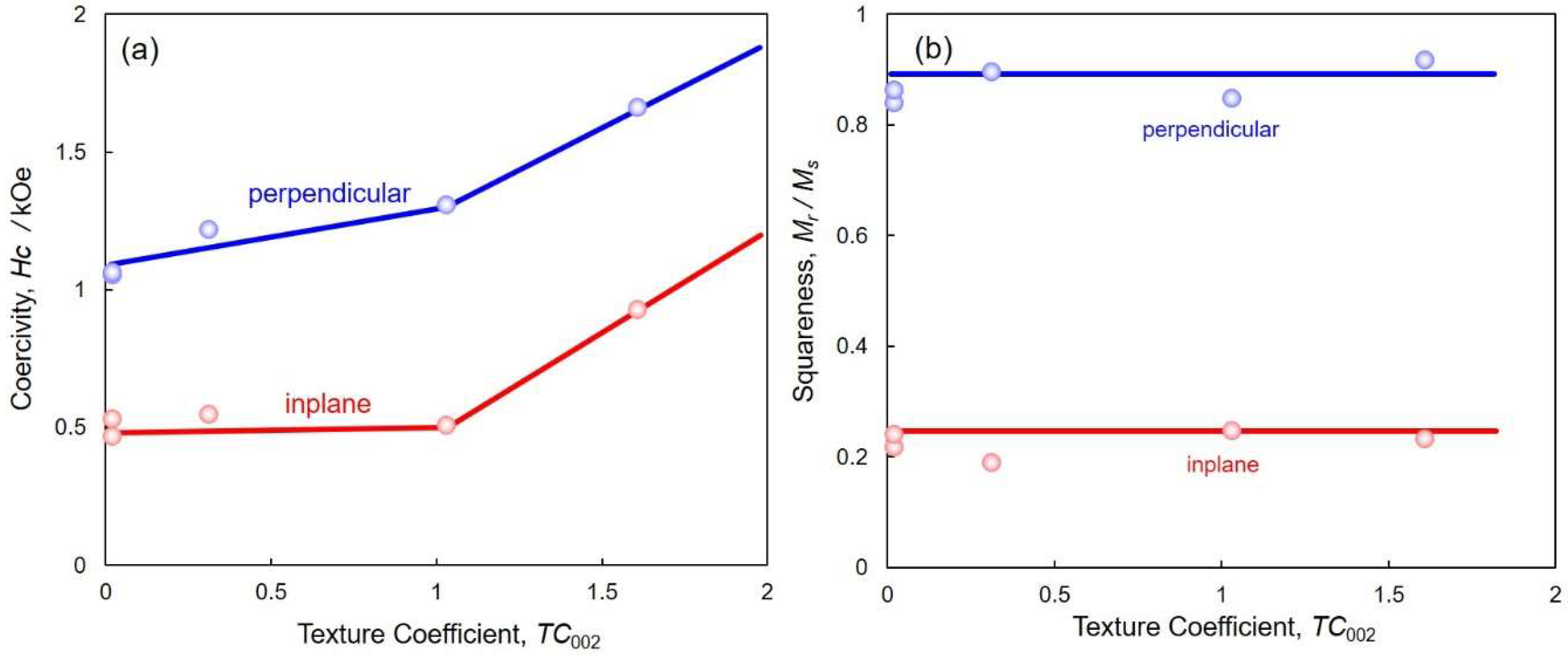
4.4. Perpendicular Magnetization of hcp-Co Nanocrystal Arrays
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pajda, M.; Kudrnovský, J.; Turek, I.; Drchal, V.; Bruno, P. Ab initio calculations of exchange interactions, spin-wave stiffness constants, and curie temperatures of Fe, Co, and Ni. Phys. Rev. B 2001, 64, 1744021–1744029. [Google Scholar] [CrossRef]
- Erdem, E. Electron beam curing of CoFe2O4 nanoparticles. Hybrid Mater. 2014, 1, 62–70. [Google Scholar] [CrossRef]
- Böttcher, R.; Erdem, E.; Langhammer, H.T.; Müller, T.; Abicht, H.P. Incorporation of chromium into hexagonal barium titanate: an electron paramagnetic resonance study. J. Phys. Condens. Matter 2005, 17, 2763. [Google Scholar] [CrossRef]
- Virot, F.; Favre, L.; Hayn, R.; Kuz’Min, M.D. Theory of magnetic domains in uniaxial thin films. J. Phys. D Appl. Phys. 2012, 45, 405003. [Google Scholar] [CrossRef]
- Gambardella, P.; Rusponi, S.; Veronese, M.; Dhesi, S.S.; Grazioli, C.; Dallmeyer, A.; Cabria, I.; Zeller, R.; Dederichs, P.H.; Kern, K.; et al. Giant Magnetic Anisotropy of Single Cobalt Atoms and Nanoparticles. Science 2003, 300, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Barriga-Castro, E.D.; Garcıa, J.; Mendoza-Resendez, R.; Prida, V.M.; Luna, C. Pseudo-monocrystalline properties of cylindrical nanowires confinedly grown by electrodeposition in nanoporous alumina templates. RSC Adv. 2017, 7, 13817. [Google Scholar] [CrossRef]
- Song, J.; Xiang, J.; Mu, C.; Wang, B.; Wen, F.; Su, C.; Wang, C.; Liu, Z. Facile synthesis and excellent electrochemical performance of CoP nanowire on carbon cloth as bifunctional electrode for hydrogen evolution reaction and supercapacitor. Sci. China Mater. 2017, 60, 1179–1186. [Google Scholar] [CrossRef]
- Khan, H.R.; Petrikowski, K. Magnetic and structural properties of the electrochemically deposited arrays of Co and CoFe nanowires. J. Magn. Magn. Mater. 2002, 249, 458–461. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Shekhar, R. Crystal anisotropy induced temperature dependent magnetization in cobalt nanowires electrodeposited within alumina template. J. Magn. Magn. Mater. 2014, 349, 21–26. [Google Scholar] [CrossRef]
- Kaur, D.; Chaudhary, S.; Pandya, D.K.; Gupta, R.; Kotnala, R.K. Magnetization reversal studies in structurally tailored cobalt nanowires. J. Magn. Magn. Mater. 2013, 344, 72–78. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Soltanian, S.; Koohbor, M.; Salimi, A.; Servati, P. Modification of microstructure and magnetic properties of electrodeposited Co nanowire arrays: A study of the effect of external magnetic field, electrolyte acidity and annealing process. Mater. Chem. Phys. 2015, 160, 389–397. [Google Scholar] [CrossRef]
- Yang, P.; An, M.; Su, C.; Wang, F. Fabrication of cobalt nanowires from mixture of 1-ethyl-3-methylimidazolium chloride ionic liquid and ethylene glycol using porous anodic alumina template. Electrochim. Acta 2008, 54, 763–767. [Google Scholar] [CrossRef]
- Cattaneo, L.; Franz, S.; Albertini, F.; Ranzieri, P.; Vincenzo, A.; Bestetti, M.; Cavallotti, P.L. Electrodeposition of hexagonal Co nanowires with large magnetocrystalline anisotropy. Electrochim. Acta 2012, 85, 57–65. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Altimari, P.; Rubino, A.; Pagnanelli, F. Electrodeposition of cobalt nanowires into alumina templates generated by one-step anodization. Electrochim. Acta 2018, 259, 711–722. [Google Scholar] [CrossRef]
- Neetzel, C.; Ohgai, T.; Yanai, T.; Nakano, M.; Fukunaga, H. Uniaxial magnetization performance of Co-Al2O3 nano-composite films electrochemically synthesized from acidic aqueous solution. J. Solid State Electrochem. 2016, 20, 1665–1672. [Google Scholar] [CrossRef]
- Garcia, C.; Rosa, W.O.; Garcia, J.; Prida, V.M.; Hernando, B.; López, J.A.; Vargas, P.; Ross, C.A. Magnetization Reversal in Radially Distributed Nanowire Arrays. J. Phys. Chem. C 2018, 122, 5124–5130. [Google Scholar] [CrossRef]
- Garcia, J.; Prida, V.M.; Vega, V.; Rosa, W.O.; Flores, R.C.; Iglesias, L.; Hernando, B. 2D and 3D ordered arrays of Co magnetic nanowires. J. Magn. Magn. Mater. 2015, 383, 88–93. [Google Scholar] [CrossRef]
- Samardak, A.S.; Ognev, A.V.; Samardak, A.Y.; Stebliy, E.V.; Modin, E.B.; Chebotkevich, L.A.; Komogortsev, S.V.; Stancu, A.; Panahi-Danaei, E.; Fardi-Ilkhichy, A.; et al. Variation of magnetic anisotropy and temperature-dependent FORC probing of compositionally tuned Co-Ni alloy nanowires. J. Alloys Compd. 2018, 732, 683–693. [Google Scholar] [CrossRef]
- Ghemes, A.; Dragos-Pinzaru, O.; Chiriac, H.; Lupu, N.; Grigoras, M.; Shore, D.; Stadler, B.; Tabakovic, I. Controlled Electrodeposition and Magnetic Properties of Co35Fe65 Nanowires with High Saturation Magnetization. J. Electrochem. Soc. 2017, 164, D13–D22. [Google Scholar] [CrossRef]
- Neetzel, C.; Ohgai, T.; Yanai, T.; Nakano, M.; Fukunaga, H. Uniaxial Magnetization Performance of Textured Fe Nanowire Arrays Electrodeposited by a Pulsed Potential Deposition Technique. Nanoscale Res. Lett. 2017, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Hasegawa, F.; Ono, S. Self-Ordering of Cell Arrangement of Anodic Porous Alumina Formed in Sulfuric Acid Solution. J. Electrochem. Soc. 1997, 144, L127–L130. [Google Scholar] [CrossRef]
- Pangarov, N.A. The crystal orientation of electrodeposited metals. Electrochim. Acta 1962, 7, 139–146. [Google Scholar] [CrossRef]
- Pangarov, N.A. On the crystal orientation of electrodeposited metals. Electrochim. Acta 1964, 9, 721–726. [Google Scholar] [CrossRef]
- Pangarov, N.A. Preferred orientations in electro-deposited metals. J. Electroanal. Chem. 1965, 9, 70–85. [Google Scholar] [CrossRef]
- Pangarov, N.A.; Vitkova, S.D. Preferred orientation of electrodeposited cobalt crystallites. Electrochim. Acta 1966, 11, 1733–1745. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. On the determination of molecular fields. -II. From the equation of state of a gas. Proc. Royal Soc. Lond. A 1924, 106, 463–477. [Google Scholar] [CrossRef]
- Smith, W.F. Principles of Materials Science and Engineering, 2nd ed.; McGraw-Hill: New York, NY, USA, 1990; pp. 53–56. [Google Scholar]
- Ohgai, T.; Tanaka, Y.; Washio, R. Nanocrystalline structure and soft magnetic properties of nickel–molybdenum alloy thin films electrodeposited from acidic and alkaline aqueous solutions. J. Solid. State. Electrochem. 2013, 17, 743–750. [Google Scholar] [CrossRef]
- Ohgai, T.; Tanaka, Y.; Fujimaru, T. Soft magnetic properties of Ni–Cr and Co–Cr alloy thin films electrodeposited from aqueous solutions containing trivalent chromium ions and glycine. J. Appl. Electrochem. 2012, 42, 893–899. [Google Scholar] [CrossRef]
- Blanco, S.; Vargas, R.; Mostany, J.; Borrás, C.; Scharifker, B.R. Modeling the Growth of Nanowire Arrays in Porous Membrane Templates. J. Electrochem. Soc. 2014, 161, E3341–E3347. [Google Scholar] [CrossRef]
- Ohgai, T.; Enculescu, I.; Zet, C.; Westerberg, L.; Hjort, K.; Spohr, R.; Neumann, R. Magneto-sensitive nickel nanowires fabricated by electrodeposition into multi- and single-ion track templates. J. Appl. Electrochem. 2006, 36, 1157–1162. [Google Scholar] [CrossRef]
- Duan, J.; Liu, J.; Cornelius, T.W.; Yao, H.; Mo, D.; Chen, Y.; Zhang, L.; Sun, Y.; Hou, M.; Trautmann, C.; et al. Magnetic and optical properties of cobalt nanowires fabricated in polycarbonate ion-track templates. Nucl. Instr. Meth. Phys. Res. B 2009, 267, 2567–2570. [Google Scholar] [CrossRef]
- Kac, M.; Zarzycki, A.; Kac, S.; Kopec, M.; Perzanowski, M.; Dutkiewicz, E.M.; Suchanek, K.; Maximenko, A.; Marszalek, M. Effect of the template-assisted electrodeposition parameters on the structure and magnetic properties of Co nanowire arrays. Mater. Sci. Eng. B 2016, 211, 75–84. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Q.F.; Li, S.L.; Wang, J.B.; Han, X.H. The effect of structure on magnetic properties of Co nanowire arrays. J. Magn. Magn. Mater. 2009, 321, 226–230. [Google Scholar] [CrossRef]
- Han, X.; Liu, Q.; Wang, J.; Li, S.; Ren, Y.; Liu, R.; Li, F. Influence of crystal orientation on magnetic properties of hcp Co nanowire arrays. J. Phys. D Appl. Phys. 2009, 42, 095005–095010. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, R.; Ohgai, T. Effect of Growth Rate on the Crystal Orientation and Magnetization Performance of Cobalt Nanocrystal Arrays Electrodeposited from Aqueous Solution. Nanomaterials 2018, 8, 566. https://doi.org/10.3390/nano8080566
Saeki R, Ohgai T. Effect of Growth Rate on the Crystal Orientation and Magnetization Performance of Cobalt Nanocrystal Arrays Electrodeposited from Aqueous Solution. Nanomaterials. 2018; 8(8):566. https://doi.org/10.3390/nano8080566
Chicago/Turabian StyleSaeki, Ryusei, and Takeshi Ohgai. 2018. "Effect of Growth Rate on the Crystal Orientation and Magnetization Performance of Cobalt Nanocrystal Arrays Electrodeposited from Aqueous Solution" Nanomaterials 8, no. 8: 566. https://doi.org/10.3390/nano8080566
APA StyleSaeki, R., & Ohgai, T. (2018). Effect of Growth Rate on the Crystal Orientation and Magnetization Performance of Cobalt Nanocrystal Arrays Electrodeposited from Aqueous Solution. Nanomaterials, 8(8), 566. https://doi.org/10.3390/nano8080566