Effect of the Sodium Polyacrylate on the Magnetite Nanoparticles Produced by Green Chemistry Routes: Applicability in Forward Osmosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MNC and MNP
2.2.1. Coprecipitation
2.2.2. Oxidative Precipitation
2.2.3. Coating of MNP: A Two-Step Process
2.3. Characterization of MNC’s
3. Results and Discussion
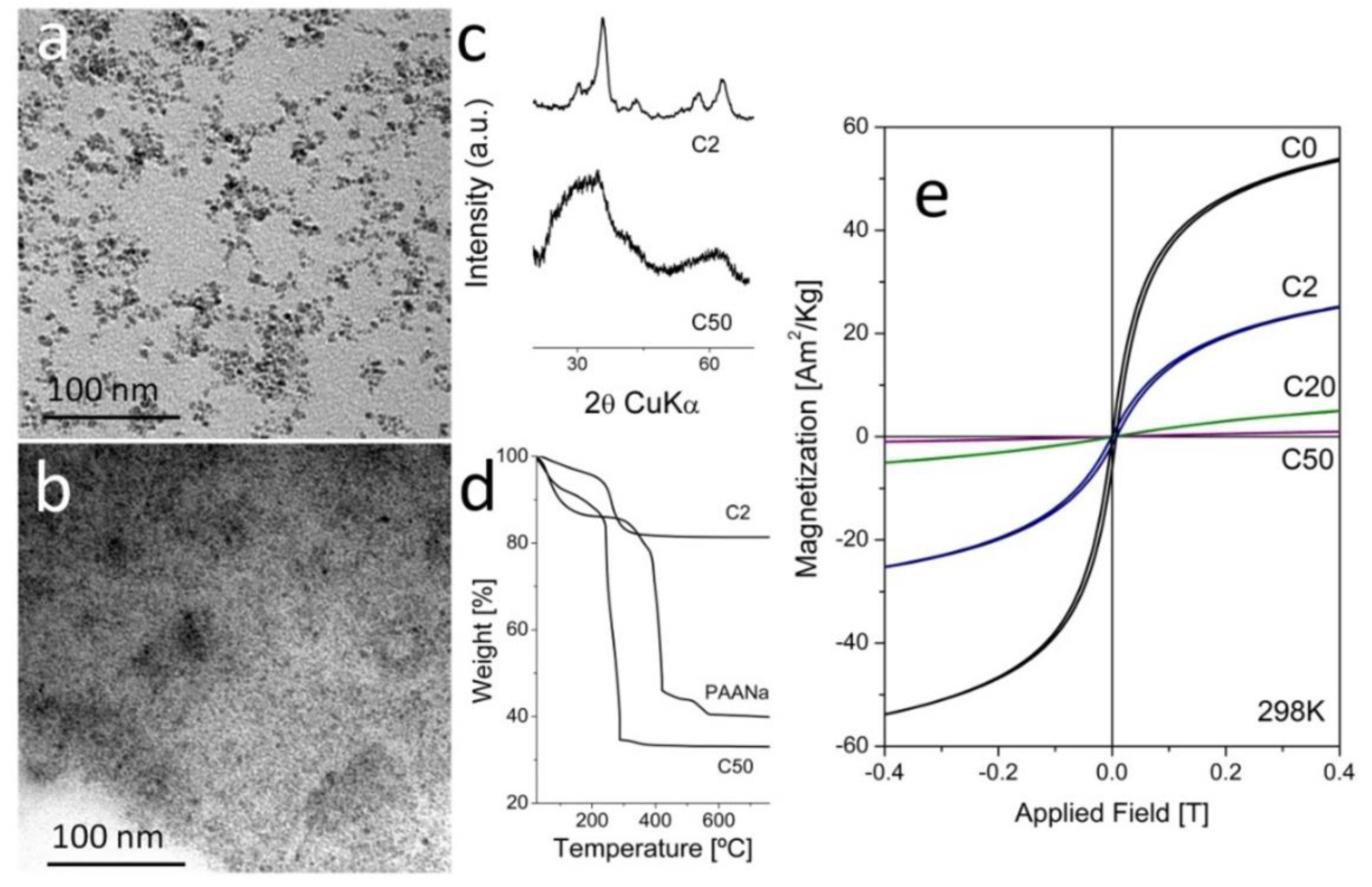
3.1. Characterization of MNCs Prepared by a Two-Step Process
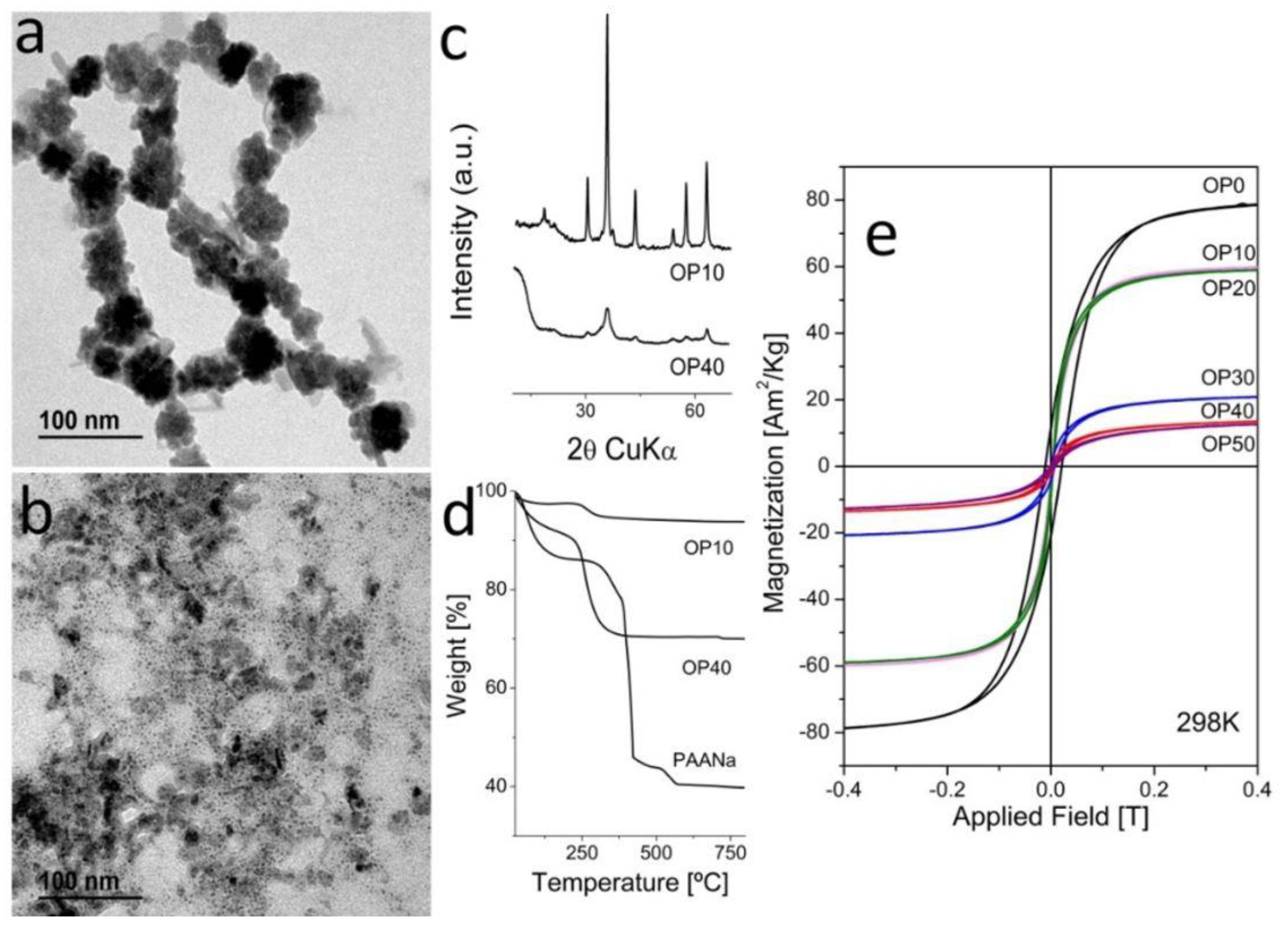
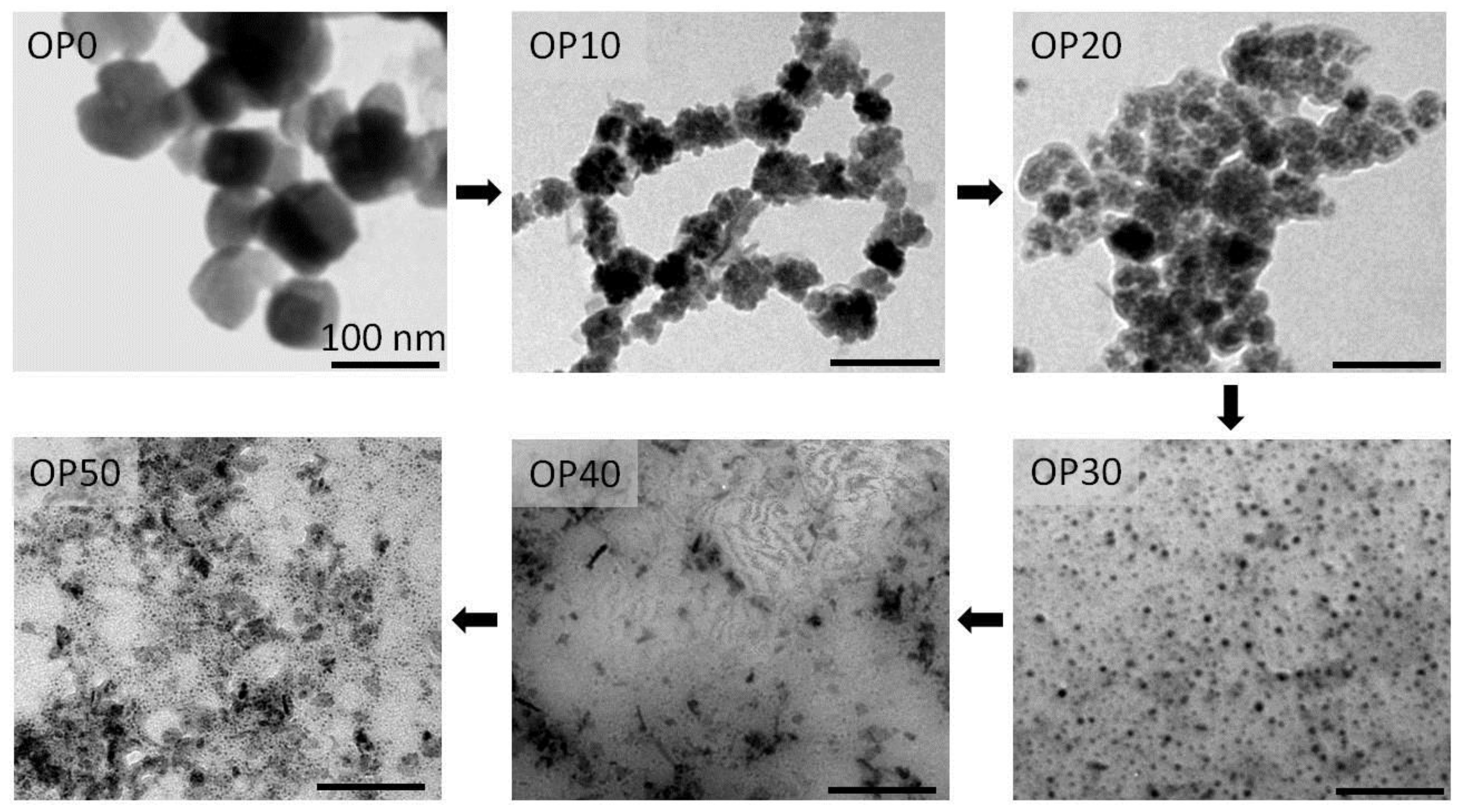
3.2. Characterization of MNCs Prepared by a One-Step Process
3.2.1. Samples Prepared by Coprecipitation in Presence of PAA
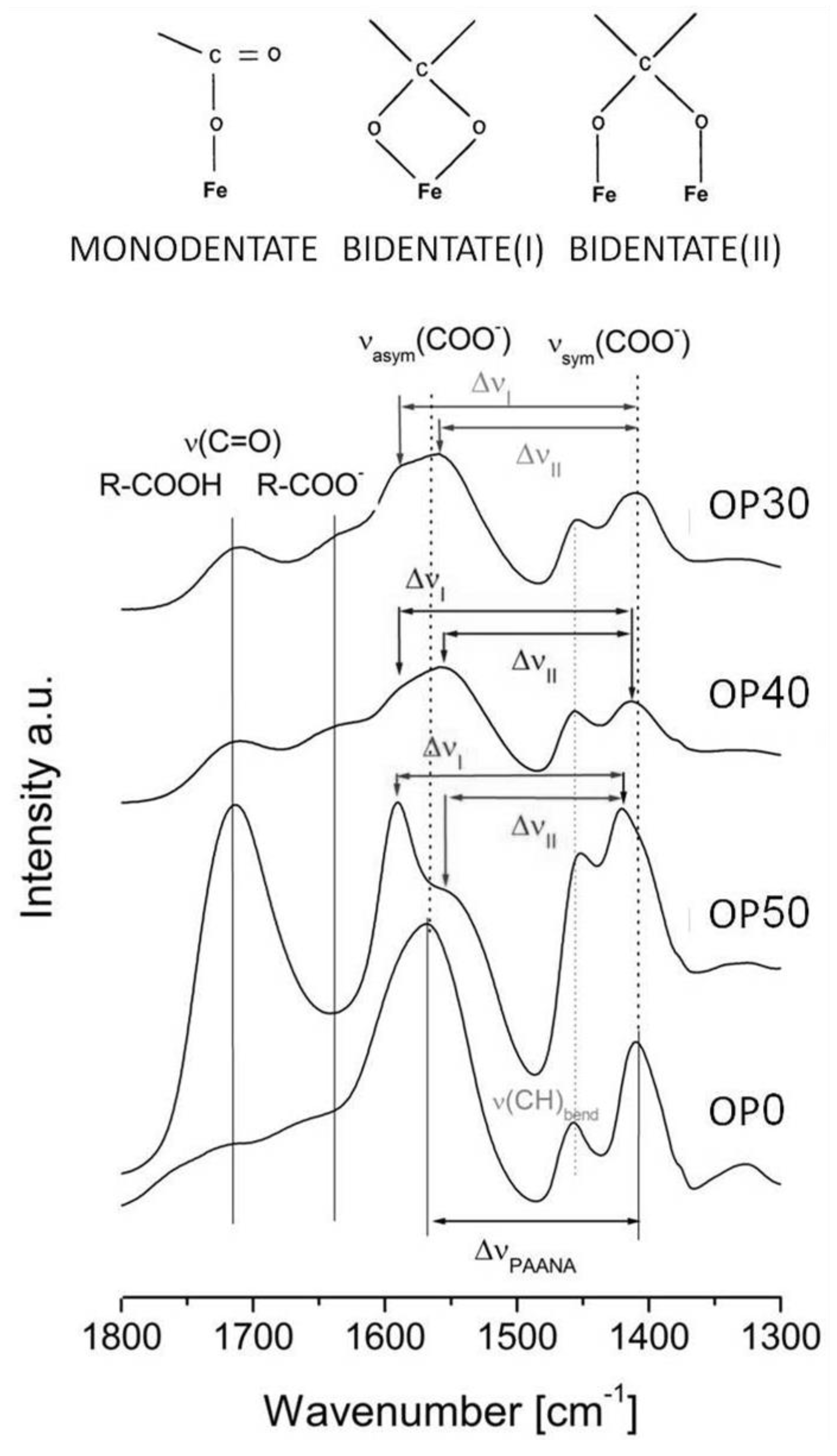
3.2.2. Samples Prepared by Oxidative Precipitation in Presence of PAANa
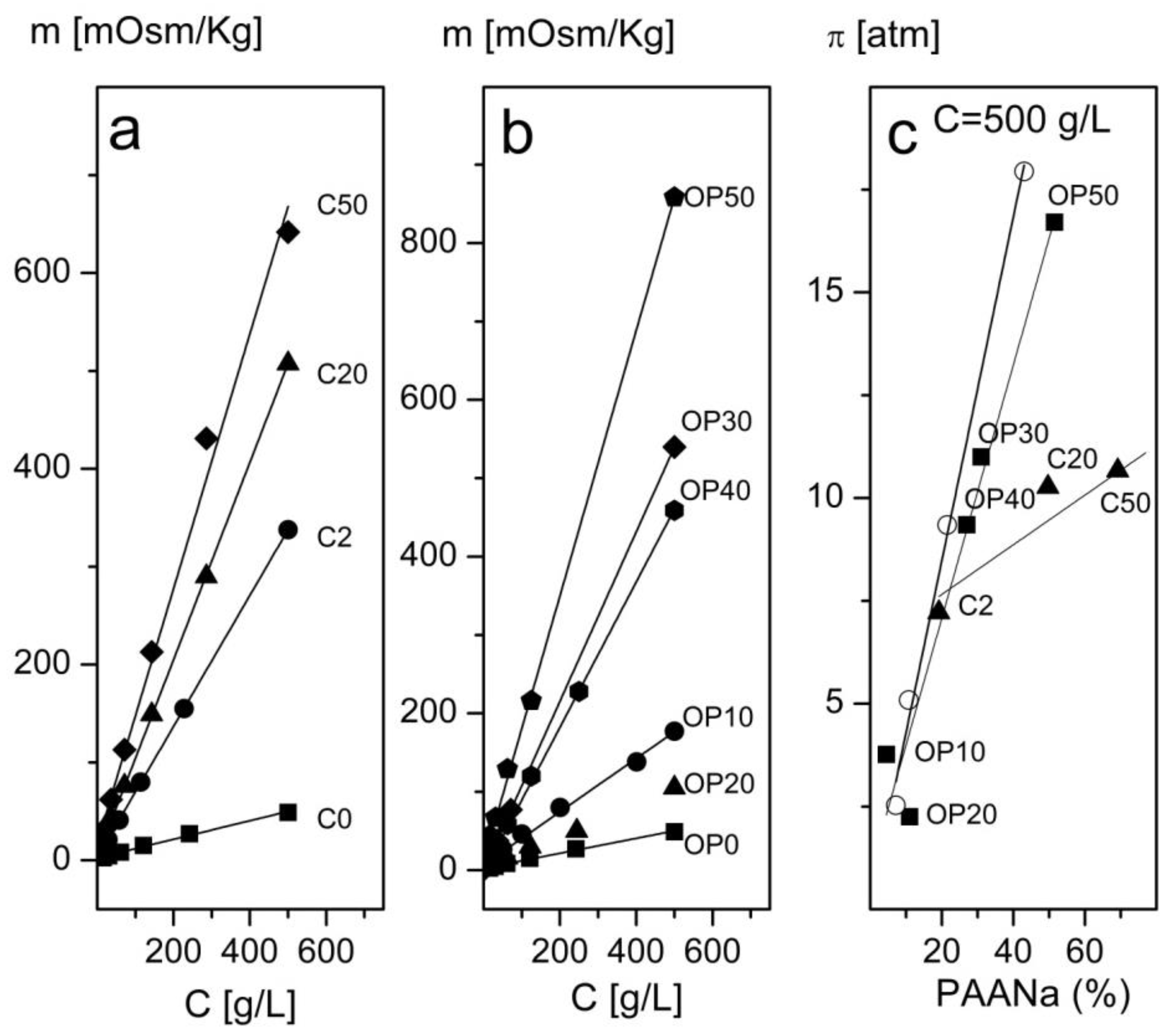
3.2.3. Osmolality and Osmotic Pressure as a Function of the Concentration
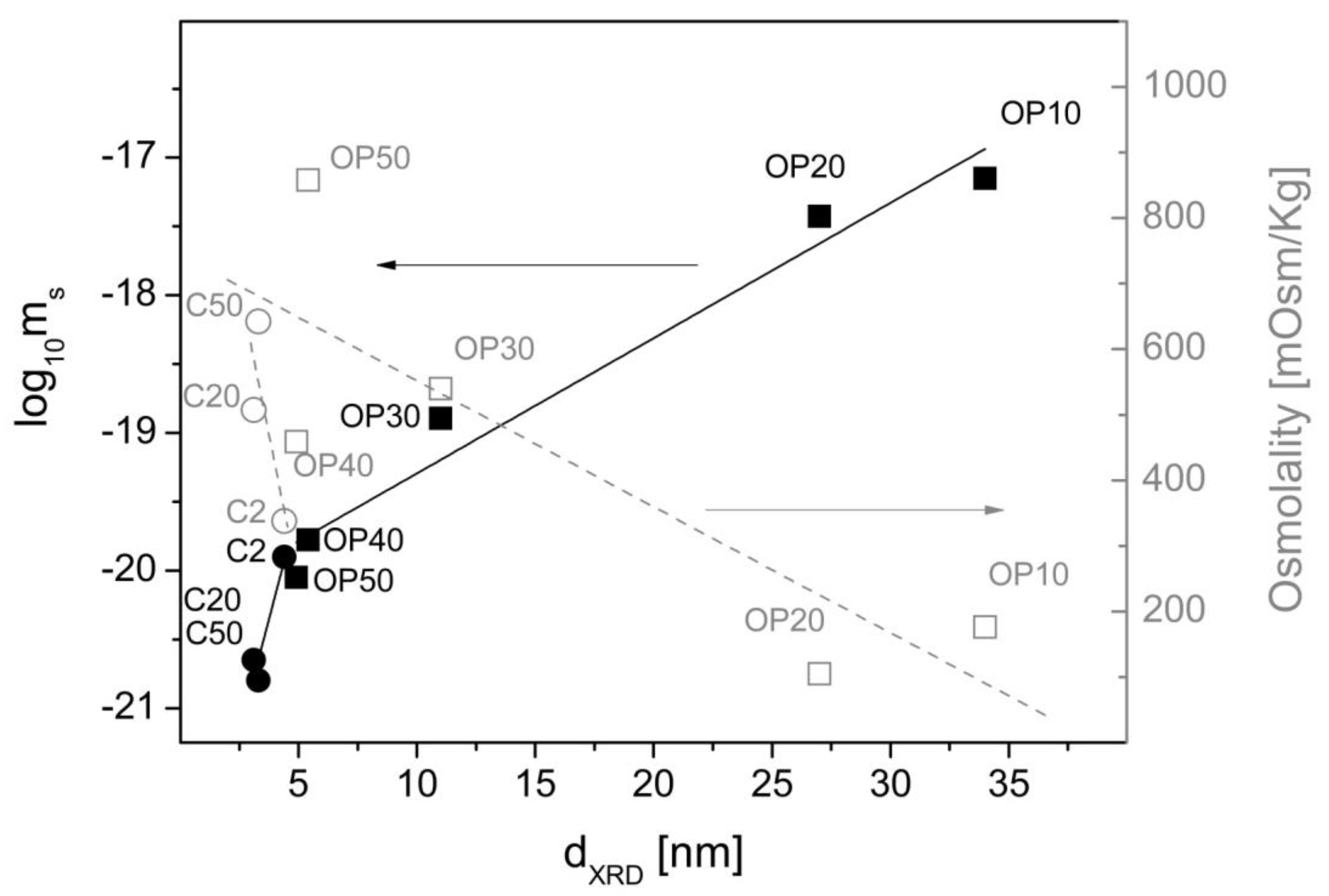
3.2.4. Magnetic Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klaysom, C.; Cath, T.Y.; Depuydt, T.; Vankelecom, I.F.J. Forward and pressure retarded osmosis: Potential solutions for global challenges in energy and water supply. Chem. Soc. Rev. 2013, 42, 6959–6989. [Google Scholar] [CrossRef] [PubMed]
- McGovern, R.K.; Lienhard, J.H. On the potential of forward osmosis to energetically outperform reverse osmosis desalination. J. Membr. Sci. 2014, 469, 245–250. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Sarp, S.; Bucs, S.S.; Amy, G.; Vrouwenvelder, J.S. Forward osmosis niches in seawater desalination and wastewater reuse. Water Res. 2014, 66, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Darwish, N.A.; Sarp, S.; Hilal, N. Water desalination by forward (direct) osmosis phenomenon: A comprehensive review. Desalination 2015, 374, 47–69. [Google Scholar] [CrossRef]
- Subramani, S.; Panda, R.C.; Panda, B. Studies on performances of membrane, draw solute and modeling of forward osmosis process in desalination—A review. Desalination Water Treat. 2017, 70, 46–63. [Google Scholar] [CrossRef]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.A.; Hasan, S.W. Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, X.M. A critical review on draw solutes development for forward osmosis. Desalination 2016, 391, 16–29. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of inorganic-based draw solutions for forward osmosis applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Hau Thi, N.; Chen, S.-S.; Nguyen Cong, N.; Huu Hao, N.; Guo, W.; Li, C.-W. Exploring an innovative surfactant and phosphate-based draw solution for forward osmosis desalination. J. Membr. Sci. 2015, 489, 212–219. [Google Scholar]
- Long, Q.W.; Wang, Y. Sodium tetraethylenepentamine heptaacetate as novel draw solute for forward osmosis-synthesis, application and recovery. Energies 2015, 8, 12917–12928. [Google Scholar] [CrossRef]
- Long, Q.; Qi, G.; Wang, Y. Synthesis and application of ethylenediamine tetrapropionic salt as a novel draw solute for forward osmosis application. Aiche J. 2015, 61, 1309–1321. [Google Scholar] [CrossRef]
- Nguyen Cong, N.; Hau Thi, N.; Ho, S.-T.; Chen, S.-S.; Huu Hao, N.; Guo, W.; Ray, S.S.; Hsu, H.-T. Exploring high charge of phosphate as new draw solute in a forward osmosis-membrane distillation hybrid system for concentrating high-nutrient sludge. Sci. Total Environ. 2016, 557, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Su, J.; Amy, G.L.; Chung, T.-S. Exploration of polyelectrolytes as draw solutes in forward osmosis processes. Water Res. 2012, 46, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Gwak, G.; Jung, B.; Han, S.; Hong, S. Evaluation of poly (aspartic acid sodium salt) as a draw solute for forward osmosis. Water Res. 2015, 80, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Hu, C.; Qin, Y.; Ren, Y.; Wang, X.; Wang, X.; Xiao, P.; Yang, X. A study of poly (sodium 4-styrenesulfonate) as draw solute in forward osmosis. Desalination 2015, 360, 130–137. [Google Scholar] [CrossRef]
- Nguyen Thi, H.; Chen, S.-S.; Nguyen Cong, N.; Huang, K.Z.; Huu Hao, N.; Guo, W. Exploration of EDTA sodium salt as novel draw solution in forward osmosis process for dewatering of high nutrient sludge. J. Membr. Sci. 2014, 455, 305–311. [Google Scholar]
- Zhao, D.; Chen, S.; Wang, P.; Zhao, Q.; Lu, X. A dendrimer-based forward osmosis draw solute for seawater desalination. Ind. Eng. Chem. Res. 2014, 53, 16170–16175. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, Q.; Chen, N.; Lu, X. Thermoresponsive copolymer-based draw solution for seawater desalination in a combined process of forward osmosis and membrane distillation. Desalination 2014, 348, 26–32. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kang, H.; Choi, Y.-S.; Yu, Y.A.; Lee, J.-C. Thermo-responsive oligomeric poly(tetrabutylphosphonium styrenesulfonate)s as draw solutes for forward osmosis (FO) applications. Desalination 2016, 381, 84–94. [Google Scholar] [CrossRef]
- Zhong, Y.; Feng, X.; Chen, W.; Wang, X.; Huang, K.-W.; Gnanou, Y.; Lai, Z. Using UCST ionic liquid as a draw solute in forward osmosis to treat high-salinity water. Environ. Sci. Technol. 2016, 50, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Hartanto, Y.; Zargar, M.; Wang, H.; Jin, B.; Dai, S. Thermoresponsive acidic microgels as functional draw agents for forward osmosis desalination. Environ. Sci. Technol. 2016, 50, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Hartanto, Y.; Zargar, M.; Cui, X.; Shen, Y.; Jin, B.; Dai, S. Thermoresponsive cationic copolymer microgels as high performance draw agents in forward osmosis desalination. J. Membr. Sci. 2016, 518, 273–281. [Google Scholar] [CrossRef]
- Wei, J.; Low, Z.-X.; Ou, R.; Simon, G.P.; Wang, H. Hydrogel-polyurethane interpenetrating network material as an advanced draw agent for forward osmosis process. Water Res. 2016, 96, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kaneti, Y.V.; Tang, J.; Salunkhe, R.R.; Jiang, X.; Yu, A.; Wu, K.C.W.; Yamauchi, Y. Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Shirai, H.; Mai Thanh, N.; Cempel, D.; Tsukamoto, H.; Tokunaga, T.; Liao, Y.-C.; Yonezawa, T. Preparation of Au/Pd bimetallic nanoparticles by a microwave-induced plasma in liquid process. Bull. Chem. Soc. Jpn. 2017, 90, 279–285. [Google Scholar] [CrossRef]
- Guo, C.X.; Zhao, D.; Zhao, Q.; Wang, P.; Lu, X. Na+-functionalized carbon quantum dots: A new draw solute in forward osmosis for seawater desalination. Chem. Commun. 2014, 50, 7318–7321. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Izake, E.L. Magnetic nanoparticles boosting the osmotic efficiency of a polymeric FO draw agent: Effect of polymer conformation. Desalination 2015, 373, 79–85. [Google Scholar] [CrossRef]
- Ling, M.M.; Wang, K.Y.; Chung, T.-S. Highly water-soluble magnetic nanoparticles as novel draw solutes in forward osmosis for water reuse. Ind. Eng. Chem. Res. 2010, 49, 5869–5876. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Sun, D.D. Highly water soluble and recovered dextran coated Fe3O4 magnetic nanoparticles for brackish water desalination. Sep. Purif. Technol. 2011, 81, 392–399. [Google Scholar] [CrossRef]
- Shabani, Z.; Rahimpour, A. Chitosan- and dehydroascorbic acid-coated Fe3O4 nanoparticles: Preparation, characterization and their potential as draw solute in forward osmosis process. Iran. Polym. J. 2016, 25, 887–895. [Google Scholar] [CrossRef]
- Bai, J.; Wang, X.; Fu, P.; Cui, Z.; Zhao, Q.; Pang, X.; Liu, M. Highly water-dispersed superparamagnetic magnetite colloidal nanocrystal clusters from multifunctional polymeric nanoreactors: Synthesis and properties. RSC Adv. 2016, 6, 9429–9435. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, H.-W.; Chung, J.W.; Kwak, S.-Y. Magnetic core-hydrophilic shell nanosphere as stability-enhanced draw solute for forward osmosis (FO) application. Desalination 2016, 397, 22–29. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, N.; Zhao, D.; Lu, X. Thermoresponsive magnetic nanoparticles for seawater desalination. ACS Appl. Mater. Interfaces 2013, 5, 11453–11461. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.M.; Chung, T.-S.; Lu, X. Facile synthesis of thermosensitive magnetic nanoparticles as “smart” draw solutes in forward osmosis. Chem. Commun. 2011, 47, 10788–10790. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, S.; Guo, C.X.; Zhao, Q.; Lu, X. Multi-functional forward osmosis draw solutes for seawater desalination. Chin. J. Chem. Eng. 2016, 24, 23–30. [Google Scholar] [CrossRef]
- Alejo, T.; Arruebo, M.; Carcelen, V.; Monsalvo, V.M.; Sebastian, V. Advances in draw solutes for forward osmosis: Hybrid organic-inorganic nanoparticles and conventional solutes. Chem. Eng. J. 2017, 309, 738–752. [Google Scholar] [CrossRef]
- Bee, A.; Massart, R.; Neveu, S. Synthesis of very fine maghemite particles. J. Magn. Magn. Mater. 1995, 149, 6–9. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matijevic, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid Interface Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Andres Verges, M.; Costo, R.; Roca, A.G.; Marco, J.F.; Goya, G.F.; Serna, C.J.; Morales, M.P. Uniform and water stable magnetite nanoparticles with diameters around the monodomain-multidomain limit. J. Phys. D-Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Costo, R. Synthesis and Characterization of Ultrasmall Iron Oxide Magnetic Colloids for Biomedical Applications; Autonomous University of Madrid: Madrid, Spain, 2010. [Google Scholar]
- Veintemillas, V.S.; Morales, H.M.P.; Serna, P.C.; Marsans, A.C.; Lopez, H.V. Electrolito Nanoestructurado útil Para Desalinización por Osmosis Directa, Procedimiento de Obtención del Electrolito y usos del Mismo. WO2015177391 A1, 26 November 2015. [Google Scholar]
- Costo, R.; Bello, V.; Robic, C.; Port, M.; Marco, J.F.; Puerto Morales, M.; Veintemillas-Verdaguer, S. Ultrasmall iron oxide nanoparticles for biomedical applications: Improving the colloidal and magnetic properties. Langmuir 2012, 28, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Luengo, Y.; Morales, M.P.; Gutierrez, L.; Veintemillas-Verdaguer, S. Counterion and solvent effects on the size of magnetite nanocrystals obtained by oxidative precipitation. J. Mater. Chem. C 2016, 4, 9482–9488. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.; Martinez, B.; Sandiumenge, F. Surface and internal spin canting in gamma-Fe2O3 nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Jones, F.; Farrow, J.B.; van Bronswijk, W. An infrared study of a polyacrylate flocculant adsorbed on hematite. Langmuir 1998, 14, 6512–6517. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Scales, P.J. Adhesive forces between adsorbed anionic polyelectrolyte layers in high ionic strength solutions. Colloids Surf.-Physicochem. Eng. Asp. 2004, 247, 19–25. [Google Scholar] [CrossRef]
- De la Presa, P.; Luengo, Y.; Velasco, V.; Morales, M.P.; Iglesias, M.; Veintemillas-Verdaguer, S.; Crespo, P.; Hernando, A. Particle interactions in liquid magnetic colloids by zero field cooled measurements: Effects on heating efficiency. J. Phys. Chem. C 2015, 119, 11022–11030. [Google Scholar] [CrossRef]
- Odenbach, S. Chapter 3 ferrofluids. In Handbook of Magnetic Materials, 1st ed.; Elsevier Science (North Holland Publishing Co.): Amsterdam, The Netherlands, 2006; Volume 16, pp. 127–208. [Google Scholar]
- Ling, M.M.; Chung, T.-S. Desalination process using super hydrophilic nanoparticles via forward osmosis integrated with ultrafiltration regeneration. Desalination 2011, 278, 194–202. [Google Scholar] [CrossRef]
- Mino, Y.; Ogawa, D.; Matsuyama, H. Functional magnetic particles providing osmotic pressure as reusable draw solutes in forward osmosis membrane process. Adv. Powder Technol. 2016, 27, 2136–2144. [Google Scholar] [CrossRef]
| Sample | dTEM (nm) | dXRD (nm) | DHYD (Zave) (nm) | Fe3O4 ICP wt % | PAANa EA wt % | Ms 298 K Am2/kg | Χ 298 K Am2/kgT |
|---|---|---|---|---|---|---|---|
| C0 | 10 ± 2 | 7.5 | 170 | 98 | 0 | 71 | 758 |
| C2 | 4 ± 1 | 4.4 | 95 | 74.0 | 19.2 | 43.4 | 192 |
| C20 | 3 ± 0.8 | 3.1 | 170 | 50.7 | 49.7 | 16.7 | 19 |
| C50 | 3 ± 1 | 3.3 | 170 | 21.7 | 77.0 | 5.6 | 4 |
| OP0 | 50 ± 20 | 67.3 | 560 | 98.7 | 0 | 82.5 | * |
| OP10 | 28 ± 5 | 34.2 | 270 | 72.9 | 4.5 | 63 | 1700 |
| OP20 | 32 ± 10 | 27.5 | 140 | 75.5 | 11.0 | 62 | 11,900 |
| OP30 | 7 ± 1 | 10.9 | 150 | 53.0 | 31.0 | 24 | 463 |
| OP40 | 4 ± 1 | 4.9 | 220 | 52.6 | 27.0 | 20 | 219 |
| OP50 | 3 ± 0.6 | 5.4 | 170 | 41.1 | 51.5 | 19 | 186 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zufía-Rivas, J.; Morales, P.; Veintemillas-Verdaguer, S. Effect of the Sodium Polyacrylate on the Magnetite Nanoparticles Produced by Green Chemistry Routes: Applicability in Forward Osmosis. Nanomaterials 2018, 8, 470. https://doi.org/10.3390/nano8070470
Zufía-Rivas J, Morales P, Veintemillas-Verdaguer S. Effect of the Sodium Polyacrylate on the Magnetite Nanoparticles Produced by Green Chemistry Routes: Applicability in Forward Osmosis. Nanomaterials. 2018; 8(7):470. https://doi.org/10.3390/nano8070470
Chicago/Turabian StyleZufía-Rivas, Juan, Puerto Morales, and Sabino Veintemillas-Verdaguer. 2018. "Effect of the Sodium Polyacrylate on the Magnetite Nanoparticles Produced by Green Chemistry Routes: Applicability in Forward Osmosis" Nanomaterials 8, no. 7: 470. https://doi.org/10.3390/nano8070470
APA StyleZufía-Rivas, J., Morales, P., & Veintemillas-Verdaguer, S. (2018). Effect of the Sodium Polyacrylate on the Magnetite Nanoparticles Produced by Green Chemistry Routes: Applicability in Forward Osmosis. Nanomaterials, 8(7), 470. https://doi.org/10.3390/nano8070470





