Electrospun Oxygen Scavenging Films of Poly(3-hydroxybutyrate) Containing Palladium Nanoparticles for Active Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Characterization
2.3.1. Film Thickness
2.3.2. Scanning Electron Microscopy
2.3.3. Transmission Electronic Microscopy
2.3.4. Differential Scanning Calorimetry
2.3.5. Thermogravimetric Analysis
2.3.6. Infrared Spectroscopy
2.3.7. Mechanical Tests
2.3.8. Water Vapor Permeability
2.3.9. d-Limonene Permeability
2.3.10. Oxygen Scavenging Activity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Optical Properties
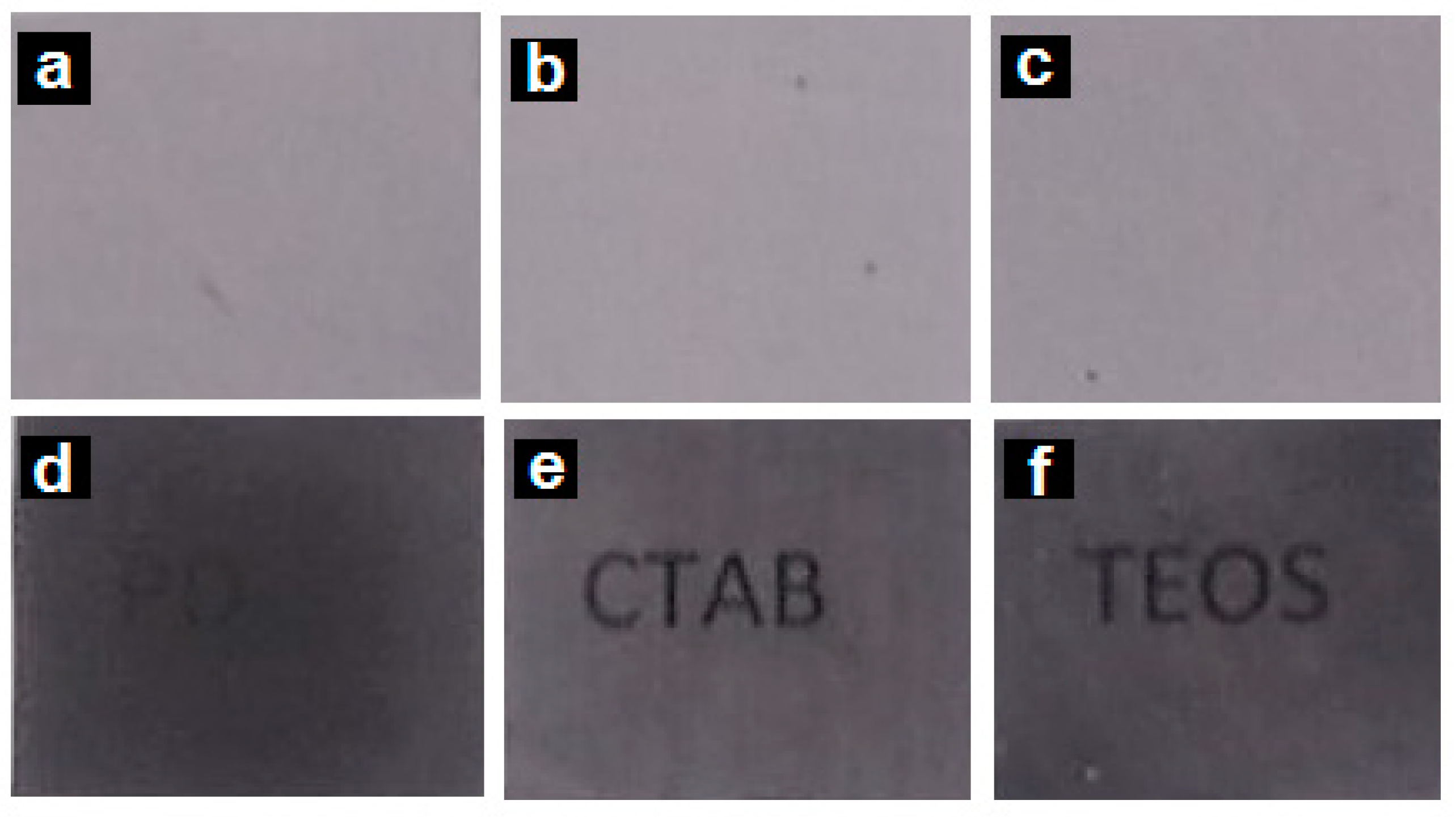
3.1.1. Optical Appearance
3.1.2. Morphology of Electrospun PHB Materials
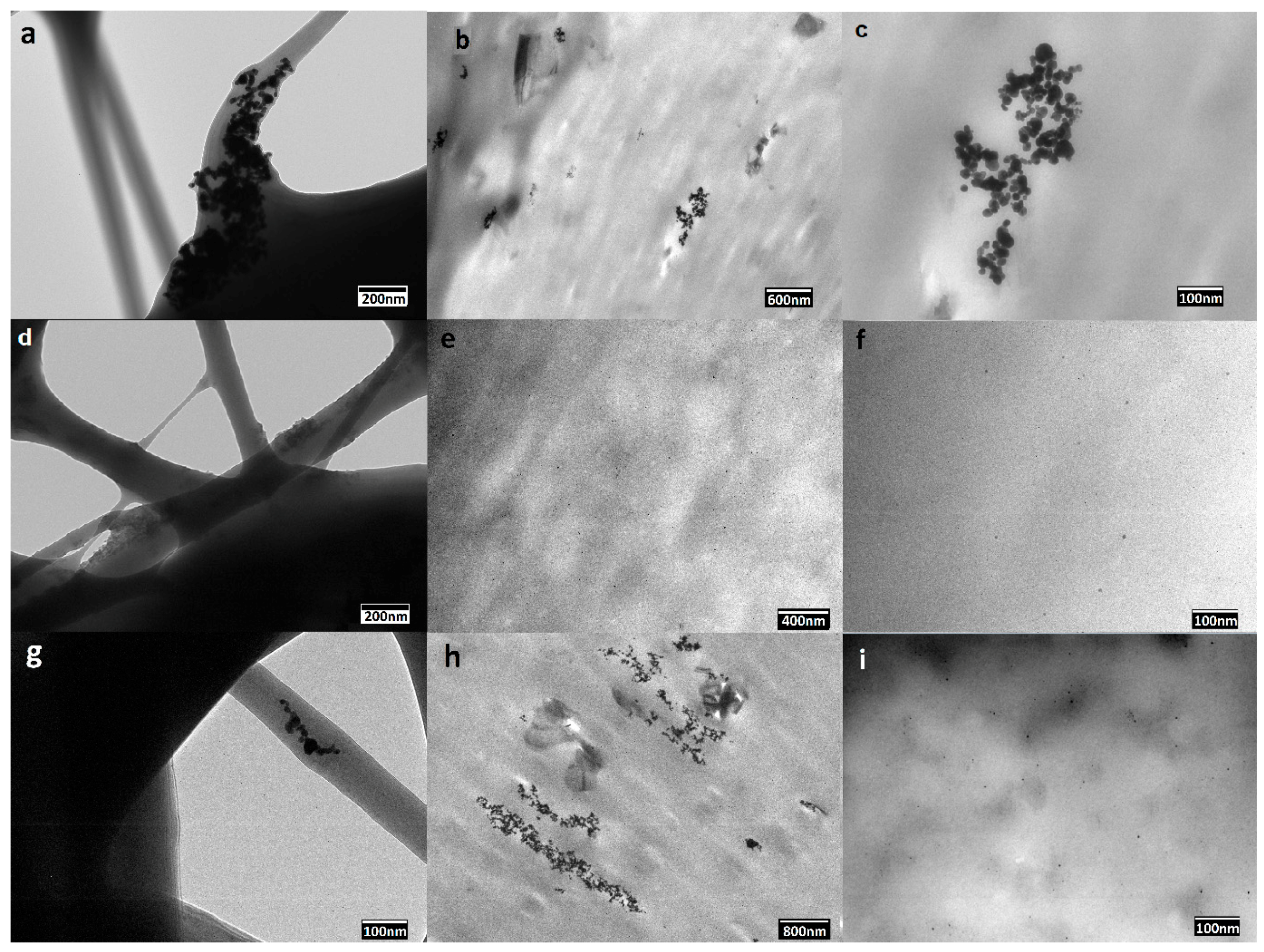
3.1.3. Dispersion of PdNPs
3.2. Thermal Properties
3.2.1. Melting Profile
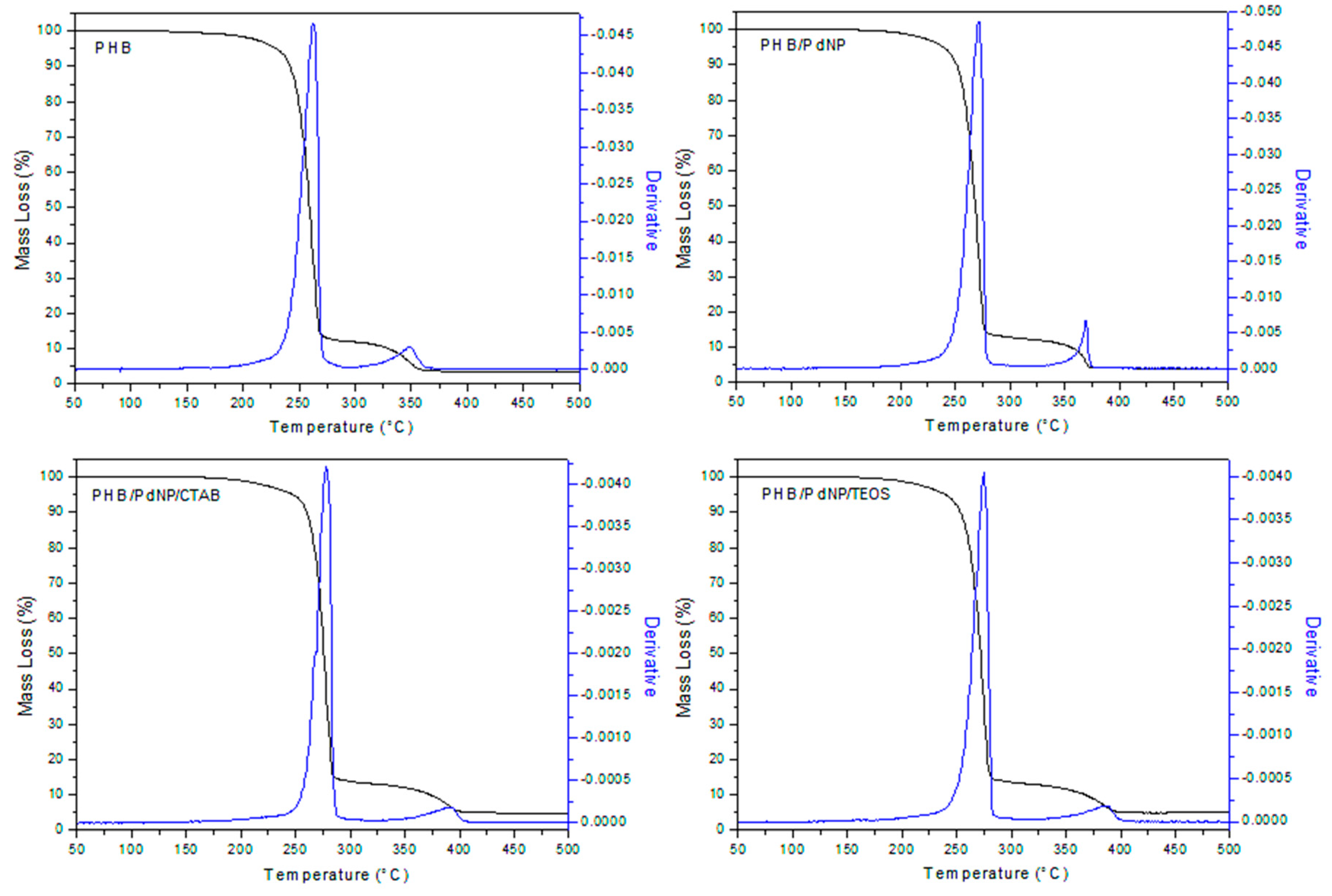
3.2.2. Thermal Stability
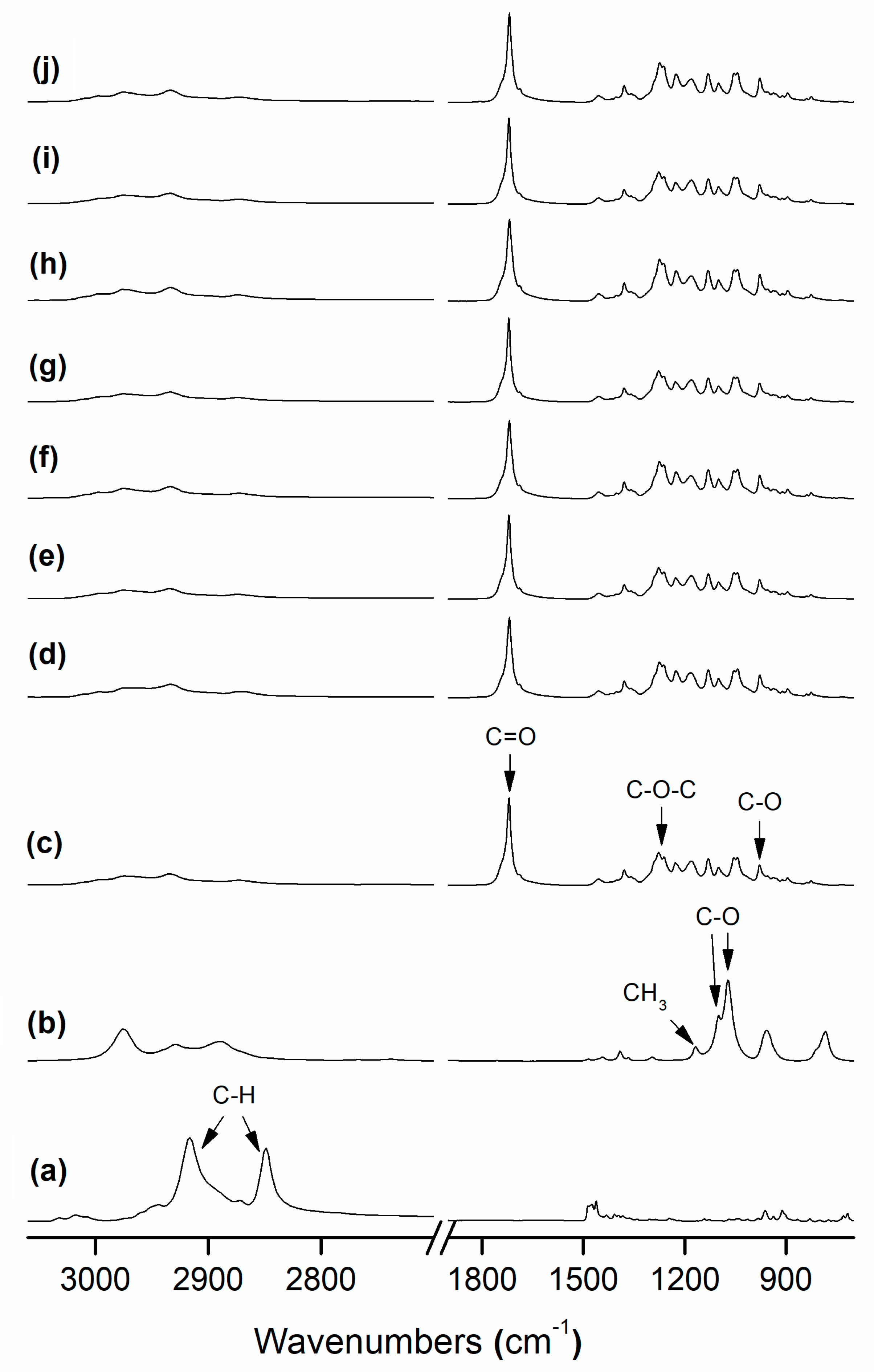
3.3. FTIR Analysis
3.4. Mechanical Properties
3.5. Barrier Properties
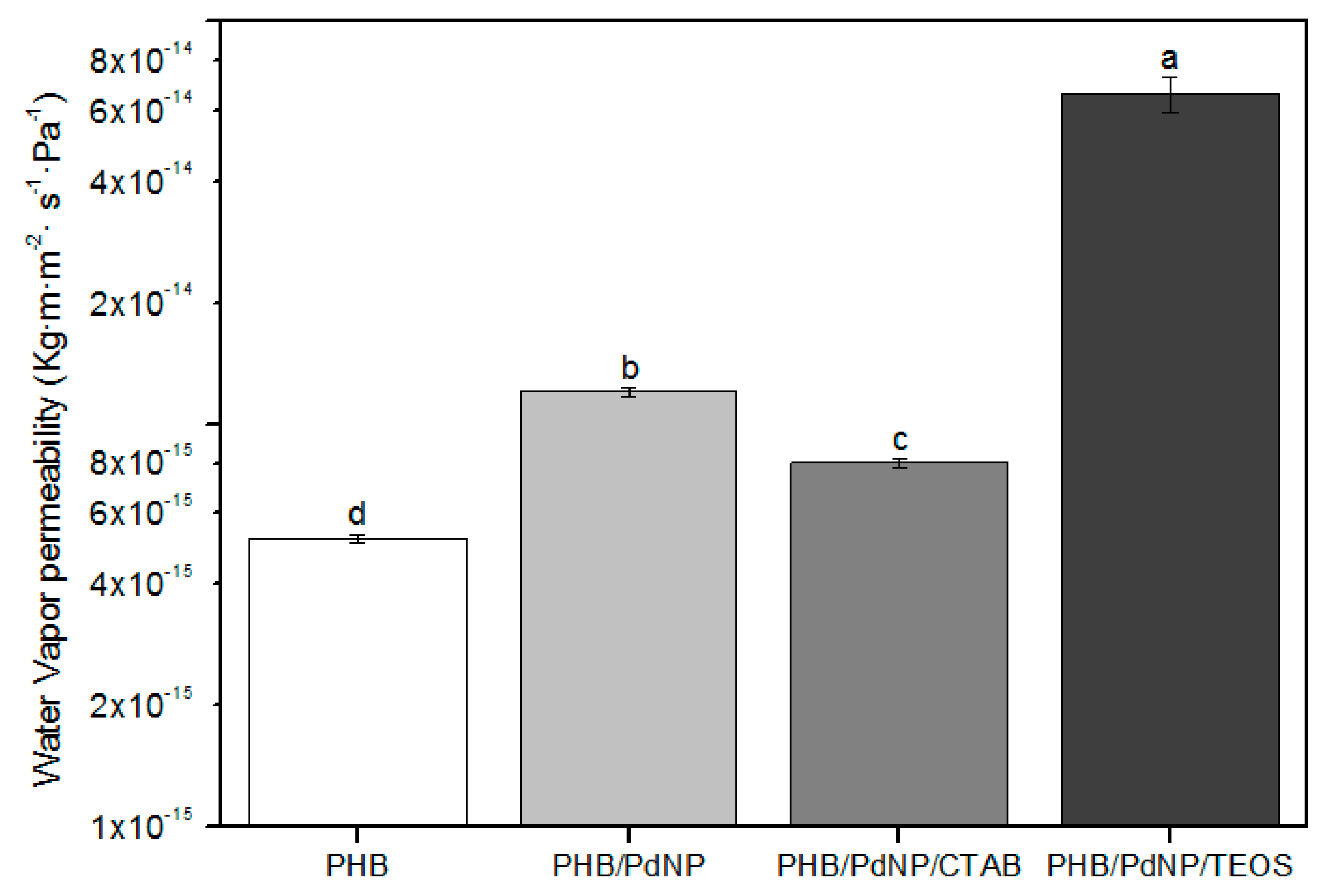
3.5.1. Water Vapor Permeability
3.5.2. d-Limonene Permeability
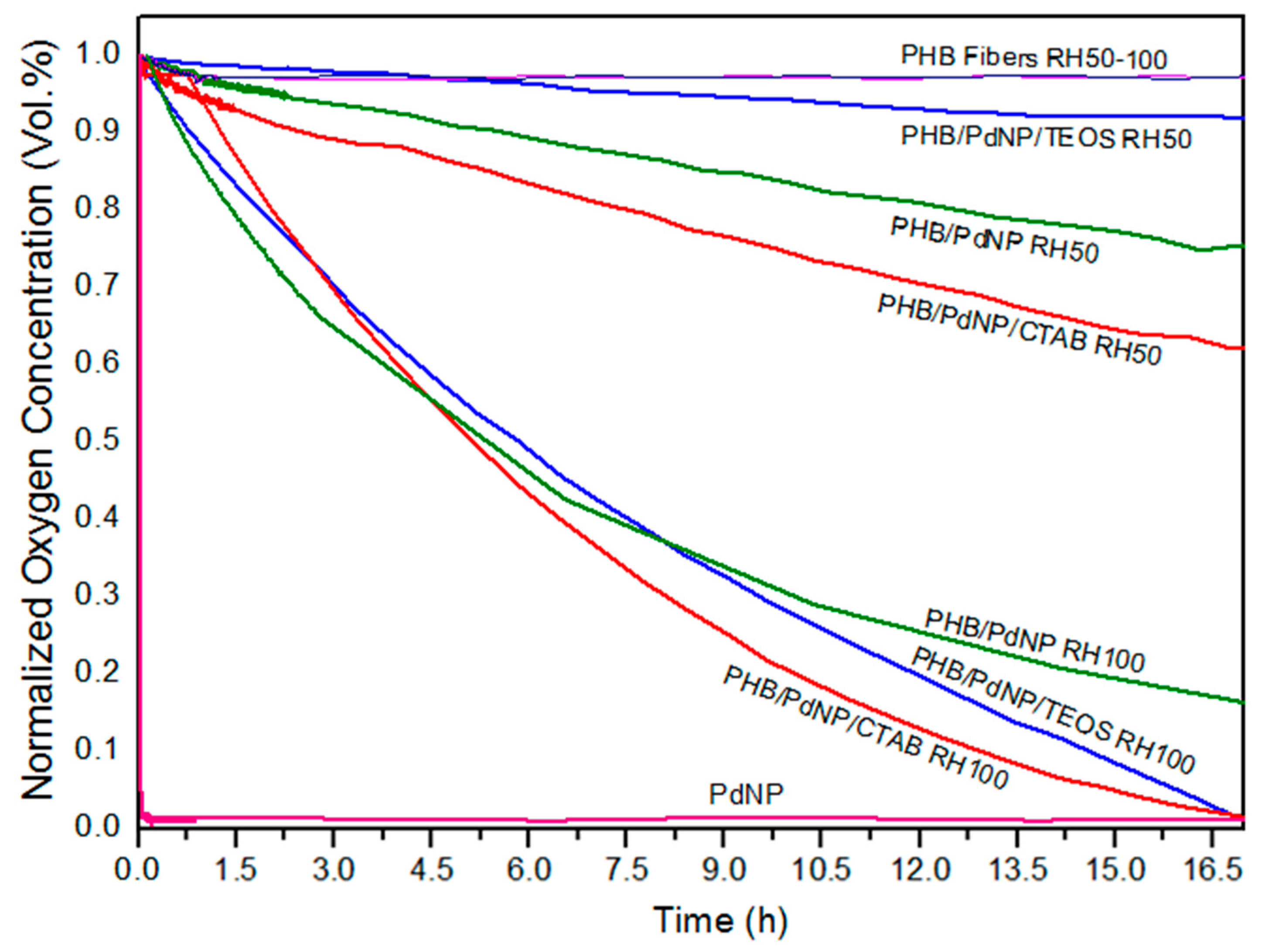
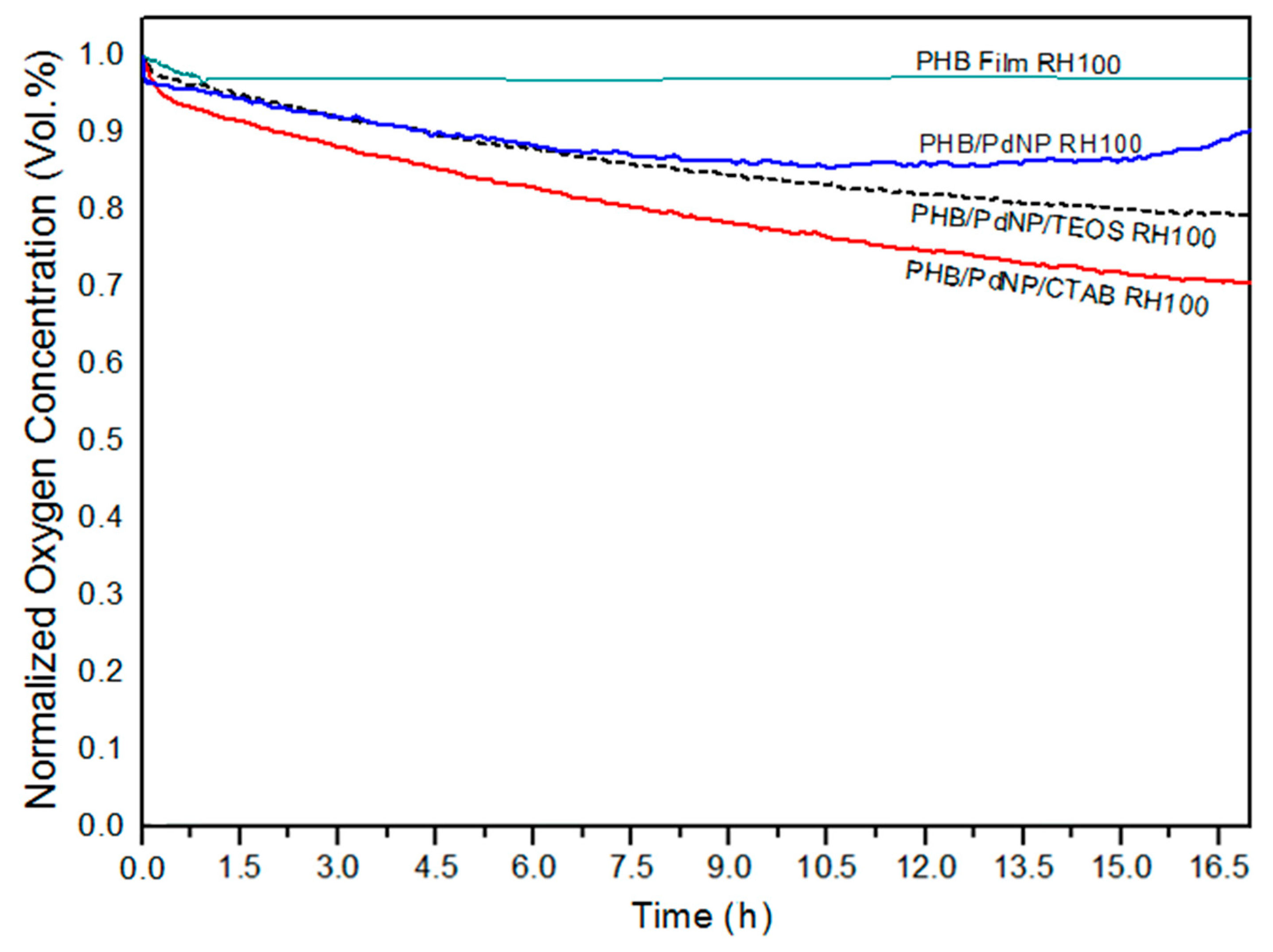
3.5.3. Oxygen Scavenging Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puglia, D.; Fortunati, E.; D’amico, D.A.; Manfredi, L.B.; Cyras, V.P.; Kenny, J. Influence of organically modified clays on the properties and disintegrability in compost of solution cast poly (3-hydroxybutyrate) films. Polym. Degrad. Stab. 2014, 99, 127–135. [Google Scholar] [CrossRef]
- Ma, P.; Xu, P.; Chen, M.; Dong, W.; Cai, X.; Schmit, P.; Spoelstra, A.; Lemstra, P. Structure–property relationships of reactively compatibilized phb/eva/starch blends. Carbohydr. Polym. 2014, 108, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, S.; Romero, M.C.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and pla optical purity on the characteristics of pla-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Bittmann, B.; Bouza, R.; Barral, L.; Diez, J.; Ramirez, C. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/clay nanocomposites for replacement of mineral oil based materials. Polym. Compos. 2013, 34, 1033–1040. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Fabra, M.; Lagaron, J.M. Stabilized nanosilver based antimicrobial poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites of interest in active food packaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)–morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.-X.; Noda, I.; Ochiai, S.; Ozaki, Y. Structure, dispersibility, and crystallinity of poly (hydroxybutyrate)/poly (l-lactic acid) blends studied by ft-ir microspectroscopy and differential scanning calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Rüegg, N.; Lohwasser, W. Development of palladium-based oxygen scavenger: Optimization of substrate and palladium layer thickness. Packag. Technol. Sci. 2015, 28, 710–718. [Google Scholar] [CrossRef]
- Cernohorsky, O.; Zdansky, K.; Zavadil, J.; Kacerovsky, P.; Piksova, K. Palladium nanoparticles on inp for hydrogen detection. Nanoscale Res. Lett. 2011, 6, 410. [Google Scholar] [CrossRef] [PubMed]
- Damaj, Z.; Joly, C.; Guillon, E. Toward new polymeric oxygen scavenging systems: Formation of poly (vinyl alcohol) oxygen scavenger film. Packag. Technol. Sci. 2015, 28, 293–302. [Google Scholar] [CrossRef]
- Wendorff, J.H.; Agarwal, S.; Greiner, A. Electrospinning: Materials, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Uyar, T.; Kny, E. Electrospun Materials for Tissue Engineering and Biomedical Applications: Research, Design and Commercialization; Woodhead Publishing: New York, NY, USA, 2017; pp. 1–428. [Google Scholar]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Torres-Giner, S. Electrospun nanofibers for food packaging applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: New York, NY, USA, 2011; pp. 108–125. [Google Scholar]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Kundu, S. A new route for the formation of au nanowires and application of shape-selective au nanoparticles in sers studies. J. Mater. Chem. C 2013, 1, 831–842. [Google Scholar] [CrossRef]
- Mayer, A.; Antonietti, M. Investigation of polymer-protected noble metal nanoparticles by transmission electron microscopy: Control of particle morphology and shape. Colloid Polym. Sci. 1998, 276, 769–779. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Post-processing optimization of electrospun submicron poly(3-hydroxybutyrate) fibers to obtain continuous films of interest in food packaging applications. Food Addit. Contam. Part A 2017, 34, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Tekmen, C.; Tsunekawa, Y.; Nakanishi, H. Electrospinning of carbon nanofiber supported fe/co/ni ternary alloy nanoparticles. J. Mater. Process. Technol. 2010, 210, 451–455. [Google Scholar] [CrossRef]
- Sainudeen, S.S.; Asok, L.B.; Varghese, A.; Nair, A.S.; Krishnan, G. Surfactant-driven direct synthesis of a hierarchical hollow mgo nanofiber–nanoparticle composite by electrospinning. RSC Adv. 2017, 7, 35160–35168. [Google Scholar] [CrossRef]
- Sakai, S.; Kawakami, K.; Taya, M. Controlling the diameters of silica nanofibers obtained by sol–gel/electrospinning methods. J. Chem. Eng. Jpn. 2012, 45, 436–440. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Cabedo, L.; Lagaron, J.M. On the use of the electrospinning coating technique to produce antimicrobial polyhydroxyalkanoate materials containing in situ-stabilized silver nanoparticles. Nanomaterials 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abad, A.; Sanchez, G.; Lagaron, J.M.; Ocio, M.J. Influence of speciation in the release profiles and antimicrobial performance of electrospun ethylene vinyl alcohol copolymer (evoh) fibers containing ionic silver ions and silver nanoparticles. Colloid Polym. Sci. 2013, 291, 1381–1392. [Google Scholar] [CrossRef]
- Shaukat, M.S.; Zulfiqar, S.; Sarwar, M.I. Incorporation of palladium nanoparticles into aromatic polyamide/clay nanocomposites through facile dry route. Polym. Sci. Ser. B 2015, 57, 380–386. [Google Scholar] [CrossRef]
- Yeo, S.; Tan, W.; Bakar, M.A.; Ismail, J. Silver sulfide/poly (3-hydroxybutyrate) nanocomposites: Thermal stability and kinetic analysis of thermal degradation. Polym. Degrad. Stab. 2010, 95, 1299–1304. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly (3-hydroxybutyrate)/ZnO bionanocomposites with improved mechanical, barrier and antibacterial properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Montanes, N.; Boronat, T.; Quiles-Carrillo, L.; Balart, R. Melt grafting of sepiolite nanoclay onto poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by reactive extrusion with multi-functional epoxy-based styrene-acrylic oligomer. Eur. Polym. J. 2016, 84, 693–707. [Google Scholar] [CrossRef]
- Primeau, N.; Vautey, C.; Langlet, M. The effect of thermal annealing on aerosol-gel deposited SiO2 films: A ftir deconvolution study. Thin Solid Films 1997, 310, 47–56. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. The influence of the industrial processing on the degradation of poly(hidroxybutyrate)—PHB. Mat. Res. 2013, 16, 237–332. [Google Scholar] [CrossRef]
- Mottin, A.C.; Ayres, E.; Oréfice, R.L.; Câmara, J.J.D. What changes in poly(3-hydroxybutyrate) (PHB) when processed as electrospun nanofibers or thermo-compression molded film? Mater. Res. 2016, 19, 57–66. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Terada, M.; Marchessault, R.H. Determination of solubility parameters for poly(3-hydroxyalkanoates). Int. J. Biol. Macromol. 1999, 25, 207–215. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef]
- Busolo, M.A.; Fernandez, P.; Ocio, M.J.; Lagaron, J.M. Novel silver-based nanoclay as an antimicrobial in polylactic acid food packaging coatings. Food Addit. Contam. Part A 2010, 27, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.; Wang, L.; Hong, S. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and barrier properties of solvent cast composites of thermoplastic biopolymers and purified cellulose fibers. Carbohydr. Polym. 2008, 71, 235–244. [Google Scholar] [CrossRef]
- Nyberg, C.; Tengstål, C. Adsorption and reaction of water, oxygen, and hydrogen on pd (100): Identification of adsorbed hydroxyl and implications for the catalytic H2–O2 reaction. J. Chem. Phys. 1984, 80, 3463–3468. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Mishra, G.; Ahalawat, S. Functional role of cationic surfactant to control the nano size of silica powder. Appl. Nanosci. 2011, 1, 117–122. [Google Scholar] [CrossRef]
- Nestorson, A.; Neoh, K.G.; Kang, E.T.; Järnström, L.; Leufvén, A. Enzyme immobilization in latex dispersion coatings for active food packaging. Packag. Technol. Sci. 2008, 21, 193–205. [Google Scholar] [CrossRef]


| Sample | Tm1 (°C) | Tm2 (°C) | ΔHm (J/g) | Tc (J/g) | ΔHc (J/g) |
|---|---|---|---|---|---|
| PHB Fibers | - | 169.1 ± 0.9 a | 64.1 ± 1.1 a | 110.2 ± 0.9 a | 59.3 ± 2.0 b,c |
| PHB Film | - | 168.4 ± 1.3 a | 71.8 ± 1.3 e | 110.5 ± 1.2 a | 61.1 ± 0.4 a,b |
| PHB/PdNP Fibers | 156.4 ± 2.1 a,b | 168.3 ± 1.1 a,b | 59.4 ± 0.9 c,d | 108.4 ± 0.5 a,b | 63.1 ± 1.5 a |
| PHB/PdNP Film | 155.0 ± 1.2 a | 168.4 ± 1.2 a,b | 58.5 ± 0.8 d | 109.5 ± 2.1 a | 56.3 ± 1.0 c,d |
| PHB/PdNP/CTAB Fibers | 156.7 ± 0.5 a,b | 167.7 ± 0.9 a,b | 62.2 ± 1.4 b | 109.1 ± 0.9 a,b | 60.3 ± 0.8 a,b |
| PHB/PdNP/CTAB Film | 158.6 ± 1.4 c | 165.1 ± 1.4 b | 60.3 ± 1.5 c | 106.8 ± 0.3 b | 55.1 ± 0.7 d |
| PHB/PdNP/TEOS Fibers | 155.2 ± 0.7 a | 169.9 ± 0.5 a | 59.7 ± 0.9 d | 110.8 ± 1.0 a | 60.1 ± 1.1 a,b |
| PHB/PdNP/TEOS Film | 159.4 ± 1.9 c | 165.5 ± 1.2 b | 62.9 ± 0.8 a,b | 110.0 ± 2.3 a | 51.4 ± 1.8 e |
| Film Sample | T5% (°C) | Tdeg1 (°C) | Tdeg2 (°C) | R500 (%) |
|---|---|---|---|---|
| PHB | 207.0 ± 4.4 | 262.0 ± 3.8 | 348.0 ± 6.4 | 3.08 ± 0.04 |
| PHB/PdNP | 224.1 ± 3.6 | 271.3 ± 2.6 | 368.2 ± 3.8 | 3.17 ± 0.03 |
| PHB/PdNP/CTAB | 220.0 ± 8.1 | 278.1 ± 3.4 | 392.2 ± 5.4 | 3.81 ± 0.04 |
| PHB/PdNP/TEOS | 230.2 ± 2.5 | 275.2 ± 4.1 | 386.1 ± 2.3 | 4.44 ± 0.04 |
| Sample | FWHM1722 (cm−1) | A1230:A1453 |
|---|---|---|
| PHB Fibers | 16.20 | 4.28 |
| PHB Film | 16.35 | 4.03 |
| PHB/PdNP Fibers | 15.10 | 4.24 |
| PHB/PdNP Film | 15.60 | 4.86 |
| PHB/PdNP/CTAB Fibers | 14.44 | 3.58 |
| PHB/PdNP/CTAB Film | 14.23 | 4.28 |
| PHB/PdNP/TEOS Fibers | 15.89 | 4.25 |
| PHB/PdNP/TEOS Film | 16.00 | 4.28 |
| Sample | E (MPa) | σb (MPa) | εb (%) | T (mJ/m3) |
|---|---|---|---|---|
| PHB film * | 1104 ± 74 a | 17.8 ± 1.8 a | 2.9 ± 1.0 a | 0.3 ± 0.1 a |
| PHB/PdNP | 1255 ± 15 b | 22.5 ± 4.2 b | 3.1 ± 1.0 a | 0.4 ± 0.1 a |
| PHB/PdNP/CTAB | 1262 ± 14 b | 23.3 ± 1.4 c | 3.0 ± 0.1 a | 0.4 ± 0.1 a |
| PHB/PdNP/TEOS | 1288 ± 230 c | 21.7 ± 4.1 b | 2.7 ± 1.0 a | 0.3 ± 0.2 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherpinski, A.; Gozutok, M.; Sasmazel, H.T.; Torres-Giner, S.; Lagaron, J.M. Electrospun Oxygen Scavenging Films of Poly(3-hydroxybutyrate) Containing Palladium Nanoparticles for Active Packaging Applications. Nanomaterials 2018, 8, 469. https://doi.org/10.3390/nano8070469
Cherpinski A, Gozutok M, Sasmazel HT, Torres-Giner S, Lagaron JM. Electrospun Oxygen Scavenging Films of Poly(3-hydroxybutyrate) Containing Palladium Nanoparticles for Active Packaging Applications. Nanomaterials. 2018; 8(7):469. https://doi.org/10.3390/nano8070469
Chicago/Turabian StyleCherpinski, Adriane, Melike Gozutok, Hilal Turkoglu Sasmazel, Sergio Torres-Giner, and Jose M. Lagaron. 2018. "Electrospun Oxygen Scavenging Films of Poly(3-hydroxybutyrate) Containing Palladium Nanoparticles for Active Packaging Applications" Nanomaterials 8, no. 7: 469. https://doi.org/10.3390/nano8070469
APA StyleCherpinski, A., Gozutok, M., Sasmazel, H. T., Torres-Giner, S., & Lagaron, J. M. (2018). Electrospun Oxygen Scavenging Films of Poly(3-hydroxybutyrate) Containing Palladium Nanoparticles for Active Packaging Applications. Nanomaterials, 8(7), 469. https://doi.org/10.3390/nano8070469








