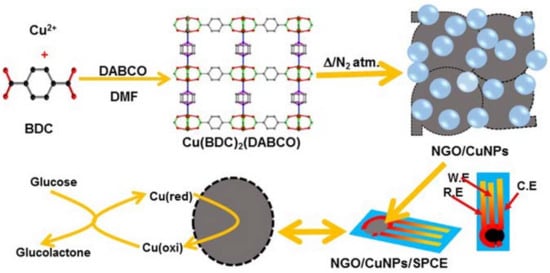
Copper Nanoparticle and Nitrogen Doped Graphite Oxide Based Biosensor for the Sensitive Determination of Glucose
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Apparatus
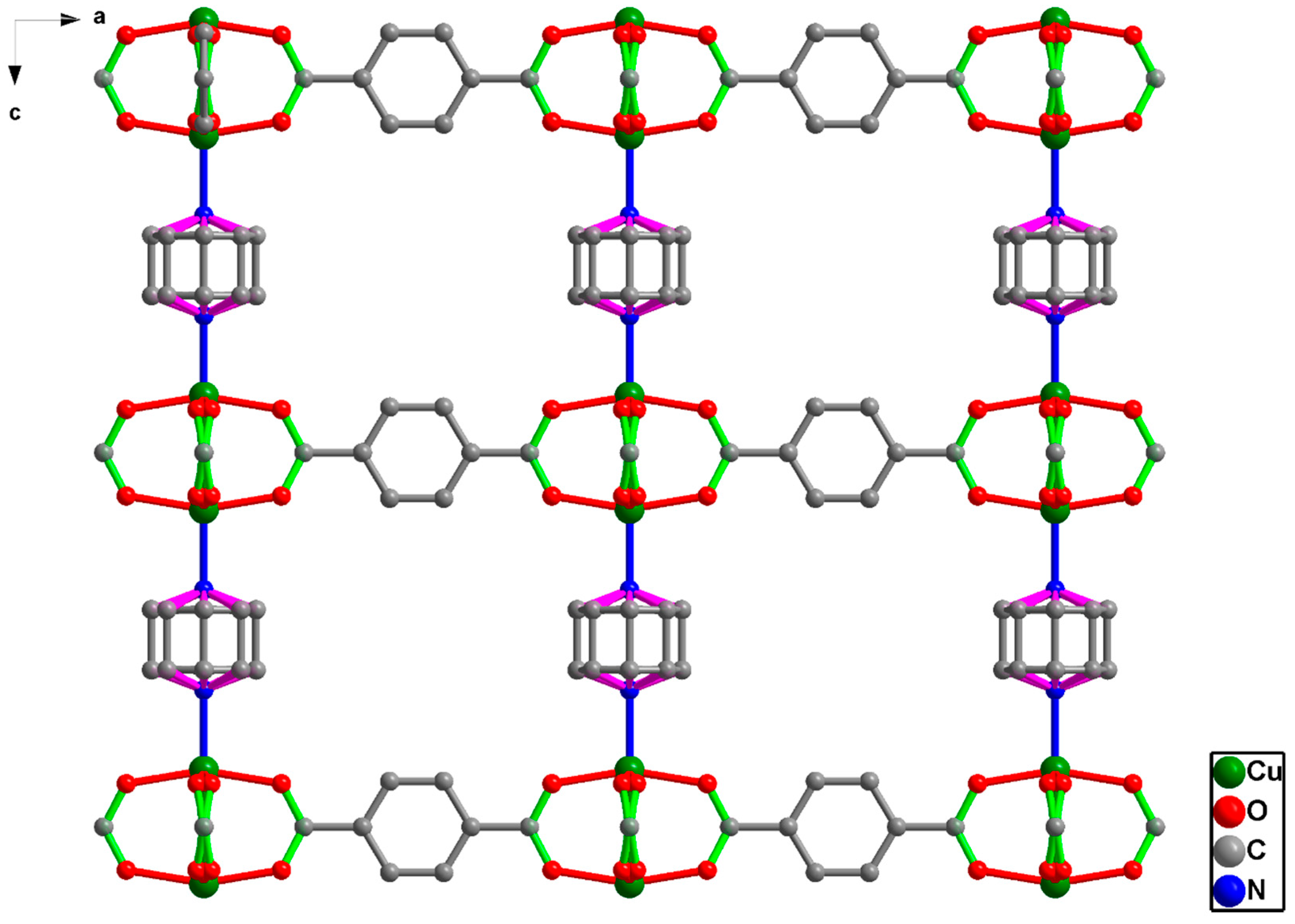
2.3. Preparation of [Cu2(BDC)2(DABCO)]
2.4. Preparation of CuNPs/NGO
2.5. Electrode Fabrication
3. Results and Discussion
3.1. Formation of [Cu2(BDC)2(DABCO)]
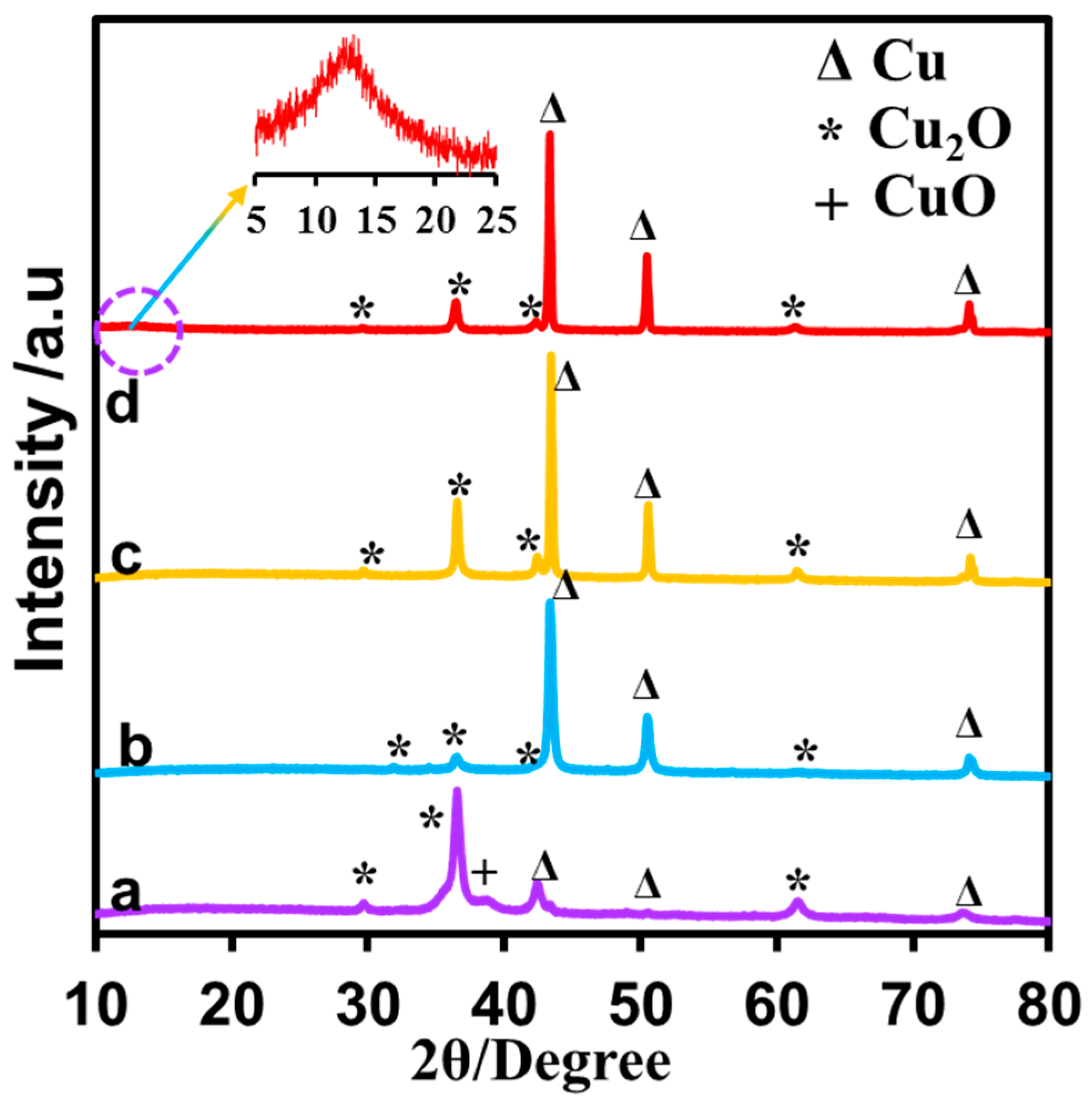
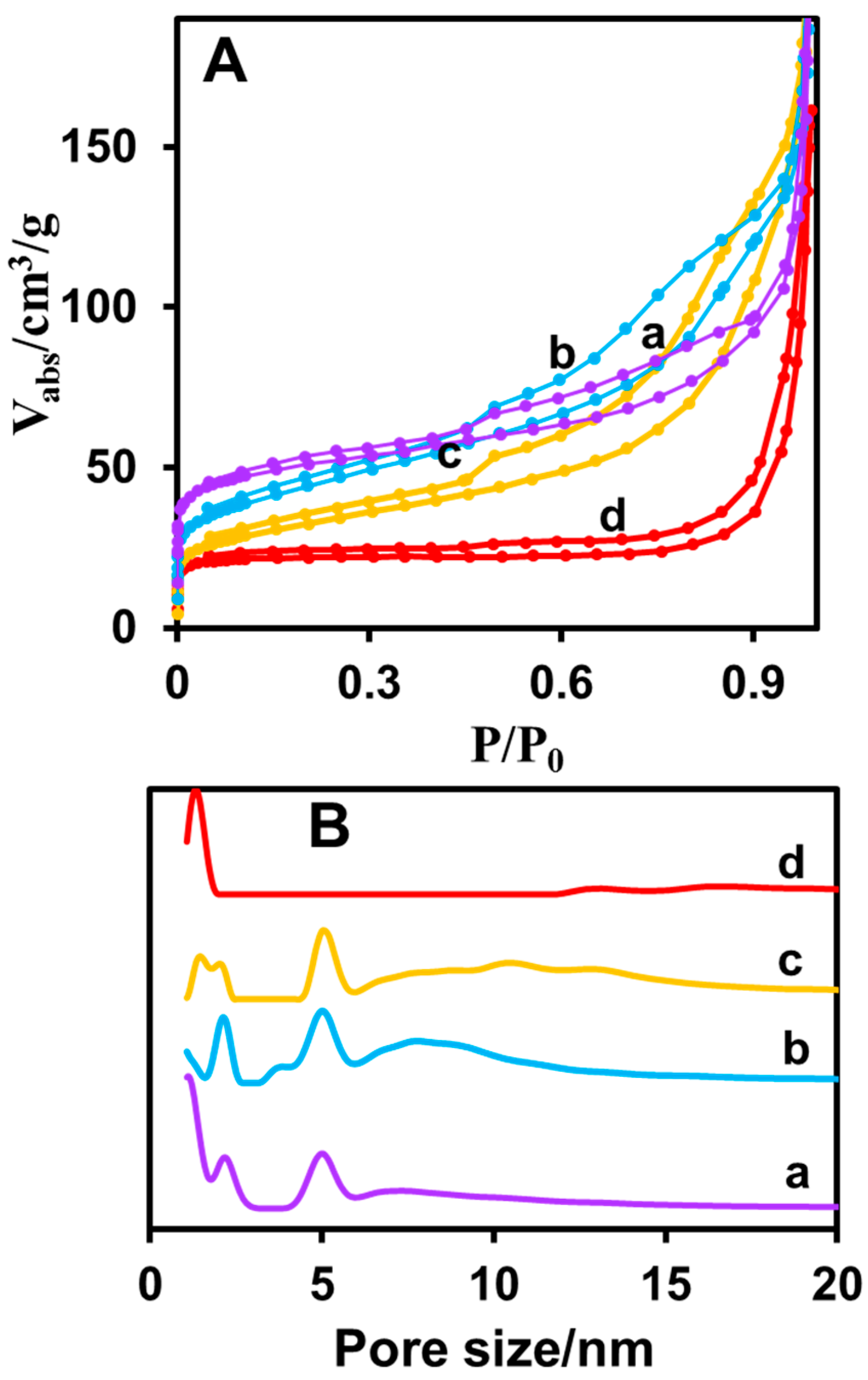
3.2. Characterization of Prepared CuNPs/NGO Nanocomposite
3.3. Electrochemical Behavior of CuNPs/NGO Nanocomposites
3.4. Amperometric i–t Determination
3.5. Selectivity Studies
3.6. Repeatability, Reproducibility and Stability
3.7. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Shin, H.S. Nanocages-augmented aligned polyaniline nanowires as unique platform for electrochemical non-enzymatic glucose biosensor. Appl. Catal. A Gen. 2016, 517, 21–29. [Google Scholar] [CrossRef]
- Jin, L.; Shang, L.; Guo, S.; Fang, Y.; Wen, D.; Wang, L.; Yin, J.; Dong, S. Biomolecule-stabilized Au nanoclusters as a fluorescence probe for sensitive detection of glucose. Biosens. Bioelectron. 2011, 26, 1965–1969. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Klein, P. Determination of sugars in honey by liquid chromatography. Saudi J. Biol. Sci. 2011, 18, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Devasenathipathy, R.; Chen, S.-M.; Wang, S.-F.; Devi, P.; Tai, Y. Electrodeposition of copper nanoparticles using pectin scaffold at graphene nanosheets for electrochemical sensing of glucose and hydrogen peroxide. Electrochim. Acta 2015, 176, 804–810. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Karuppiah, C.; Chen, S.-M.; Palanisamy, S.; Lou, B.-S.; Ali, M.A.; Al-Hemaid, F.M. A sensitive and selective enzyme-free amperometric glucose biosensor using a composite from multi-walled carbon nanotubes and cobalt phthalocyanine. RSC Adv. 2015, 5, 26762–26768. [Google Scholar] [CrossRef]
- Rana, S.; Mittal, S.K.; Kaur, N.; Banks, C.E. Disposable screen printed electrode modified with imine receptor having a wedge bridge for selective detection of Fe (ii) in aqueous medium. Sens. Actuators B Chem. 2017, 249, 467–477. [Google Scholar] [CrossRef]
- Renedo, O.D.; Alonso-Lomillo, M.; Martinez, M.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K.C.; Hart, J.P.; Cowell, D.C.; Arrigan, D.W. Voltammetric behavior and trace determination of cadmium at a calixarene modified screen-printed carbon electrode. Electroanalysis 2002, 14, 177–185. [Google Scholar] [CrossRef]
- Wang, G.; He, X.; Wang, L.; Gu, A.; Huang, Y.; Fang, B.; Geng, B.; Zhang, X. Non-enzymatic electrochemical sensing of glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- Mu, Y.; Jia, D.; He, Y.; Miao, Y.; Wu, H.-L. Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 2011, 26, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhang, P.; Chen, J.; Chen, D.; Quan, Y.; Gu, N.; Zhang, G.; Cui, R. Non-enzymatic sensing of glucose and hydrogen peroxide using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous copper, carbon black and nafion. Microchim. Acta 2016, 183, 1359–1365. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Chen, L.; Jia, J. Synthesis of copper nanorods for non-enzymatic amperometric sensing of glucose. Microchim. Acta 2016, 183, 2369–2375. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, W.; Sun, Y.; Sun, C. Enzyme-free glucose sensor based on a three-dimensional gold film electrode. Sens. Actuators B Chem. 2008, 134, 471–476. [Google Scholar] [CrossRef]
- Rong, L.-Q.; Yang, C.; Qian, Q.-Y.; Xia, X.-H. Study of the nonenzymatic glucose sensor based on highly dispersed Pt nanoparticles supported on carbon nanotubes. Talanta 2007, 72, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Han, M.; Du, Y.; Bao, J.; Dai, Z. Facile synthesis of porous tubular palladium nanostructures and their application in a nonenzymatic glucose sensor. Chem. Commun. 2010, 46, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Hameed, R.A. Amperometric glucose sensor based on nickel nanoparticles/carbon vulcan XC-72R. Biosens. Bioelectron. 2013, 47, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, Y.; Tian, H.; Hu, J. A novel non-enzymatic glucose sensor based on cobalt nanoparticles implantation-modified indium tin oxide electrode. Electroanalysis 2014, 26, 2693–2700. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.-D.; Ye, J.-S. Nonenzymatic electrochemical glucose sensor based on MnO2/MWNT nanocomposite. Electrochem. Commun. 2008, 10, 1268–1271. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.; Zhang, H.; Jiang, J.; Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-X.; Cao, W.-M.; Li, Y.; Liu, G.; Wen, Y.; Yang, H.-F.; Yang, S.-P. In situ growth of copper nanoparticles on multiwalled carbon nanotubes and their application as non-enzymatic glucose sensor materials. Electrochim. Acta 2010, 55, 3734–3740. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Q.; Wang, K.; Qian, J.; Dong, X.; Yang, Z.; Du, X.; Qiu, B. Enhanced non-enzymatic glucose sensing based on copper nanoparticles decorated nitrogen-doped graphene. Biosens. Bioelectron. 2014, 54, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Tang, Y.; Chou, L.-Y.; Sneed, B.T.; Brodsky, C.N.; Zhao, Z.; Tsung, C.-K. Yolk–shell nanocrystal@ZIF-8 nanostructures for gas-phase heterogeneous catalysis with selectivity control. J. Am. Chem. Soc. 2012, 134, 14345–14348. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, H.-C. Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, L.; Zhang, C.; Fu, J.; Jiang, J. Mil-53 (Fe): A metal–organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chem. A Eur. J. 2013, 19, 15105–15108. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regi, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhang, J.; Zhang, R.; Shi, H.; Wang, N.; Li, J.; Ma, F.; Zhang, D. Cu-based metal–organic framework as a novel sensing platform for the enhanced electro-oxidation of nitrite. Sens. Actuators B Chem. 2016, 222, 632–637. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, X.; Liu, T.; Zhao, H.; Lan, M. Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, F.; Zeng, B. One-step synthesis of a copper-based metal–organic framework–graphene nanocomposite with enhanced electrocatalytic activity. RSC Adv. 2015, 5, 22060–22065. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Luhana, C.; Wang, H.; Li, M.; Guo, L. Facile synthesis of a Cu-based MOF confined in macroporous carbon hybrid material with enhanced electrocatalytic ability. Chem. Commun. 2013, 49, 6885–6887. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, X.; Min, X.; Cheng, D.; Zhou, J.; Li, Y.; Xie, Z.; Liu, P.; Cai, W.; Zhang, C. Determination of catechol and hydroquinone with high sensitivity using MOF-graphene composites modified electrode. J. Electroanal. Chem. 2017, 789, 114–122. [Google Scholar] [CrossRef]
- Hosseini, H.; Ahmar, H.; Dehghani, A.; Bagheri, A.; Tadjarodi, A.; Fakhari, A.R. A novel electrochemical sensor based on metal-organic framework for electro-catalytic oxidation of l-cysteine. Biosens. Bioelectron. 2013, 42, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, M.; Liu, L.; Yang, X.; Xu, X. Electrochemical investigation of a new Cu-MOF and its electrocatalytic activity towards H2O2 oxidation in alkaline solution. Electrochem. Commun. 2013, 33, 131–134. [Google Scholar] [CrossRef]
- Arul, P.; John, S.A. Electrodeposition of CuO from -Cu-MOF on glassy carbon electrode: A non-enzymatic sensor for glucose. J. Electroanal. Chem. 2017, 799, 61–69. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Zhou, K.; Kabir, N.A.; Chen, Y.; Ke, X.; Van Tendeloo, G.; Verpoort, F. Tuning metal sites of DABCO MOF for gas purification at ambient conditions. Microporous Mesoporous Mater. 2015, 201, 277–285. [Google Scholar] [CrossRef]
- Yin, H.; Cui, Z.; Wang, L.; Nie, Q. In situ reduction of the Cu/Cu2O carbon spheres composite for enzymaticless glucose sensors. Sens. Actuators B Chem. 2016, 222, 1018–1023. [Google Scholar] [CrossRef]
- Zu, Y.; Tang, J.; Zhu, W.; Zhang, M.; Liu, G.; Liu, Y.; Zhang, W.; Jia, M. Graphite oxide-supported CaO catalysts for transesterification of soybean oil with methanol. Bioresour. Technol. 2011, 102, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.T.; Li, B.H.; Pei, T.; Lin, C.H.; Lee, S. Raman investigation on carbonization process of metal–organic frameworks. J. Raman Spectrosc. 2016, 47, 1271–1275. [Google Scholar] [CrossRef]
- Liu, X.; Long, L.; Yang, W.; Chen, L.; Jia, J. Facilely electrodeposited coral-like copper micro-/nano-structure arrays with excellent performance in glucose sensing. Sens. Actuators B Chem. 2018, 266, 853–860. [Google Scholar] [CrossRef]
- Balamurugan, J.; Thanh, T.D.; Karthikeyan, G.; Kim, N.H.; Lee, J.H. A novel hierarchical 3D N-Co-CNT@NG nanocomposite electrode for non-enzymatic glucose and hydrogen peroxide sensing applications. Biosens. Bioelectron. 2017, 89, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, J.; Tang, P.; Morante, J.R.; Arbiol, J.; Xu, C.; Li, Q.; Fransaer, J. Ultrasensitive binder-free glucose sensors based on the pyrolysis of in situ grown Cu-MOF. Sens. Actuators B Chem. 2018, 254, 272–281. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Chen, J.; Pang, H. Copper metal–organic framework nanocrystal for plane effect nonenzymatic electro-catalytic activity of glucose. Nanoscale 2014, 6, 10989–10994. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ivandini, T.A.; Makide, Y.; Fujishima, A.; Einaga, Y. Selective detection method derived from a controlled diffusion process at metal-modified diamond electrodes. Anal. Chem. 2006, 78, 7857–7860. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Liu, Y.; Li, X.; Zhao, J.; Ren, Z.; Pang, H. Nitrogen-doped carbon–copper nanohybrids as electrocatalysts in H2O2 and glucose sensing. ChemElectroChem 2014, 1, 799–807. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Jia, J.; Wang, J. Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sens. Actuators B Chem. 2012, 171, 580–587. [Google Scholar] [CrossRef]
- Sattayasamitsathit, S.; Thavarungkul, P.; Thammakhet, C.; Limbut, W.; Numnuam, A.; Buranachai, C.; Kanatharana, P. Fabrication of nanoporous copper film for electrochemical detection of glucose. Electroanalysis 2009, 21, 2371–2377. [Google Scholar] [CrossRef]
- Male, K.B.; Hrapovic, S.; Liu, Y.; Wang, D.; Luong, J.H. Electrochemical detection of carbohydrates using copper nanoparticles and carbon nanotubes. Anal. Chim. Acta 2004, 516, 35–41. [Google Scholar] [CrossRef]
- SoYoon, S.; Ramadoss, A.; Saravanakumar, B.; Kim, S.J. Novel Cu/CuO/ZnO hybrid hierarchical nanostructures for non-enzymatic glucose sensor application. J. Electroanal. Chem. 2014, 717, 90–95. [Google Scholar] [CrossRef]
- Liu, D.; Luo, Q.; Zhou, F. Nonenzymatic glucose sensor based on gold–copper alloy nanoparticles on defect sites of carbon nanotubes by spontaneous reduction. Synth. Met. 2010, 160, 1745–1748. [Google Scholar] [CrossRef]




| Sample | Cu wt % | N wt % | C wt % | H wt % | O wt % |
|---|---|---|---|---|---|
| CuNPs/NGO(600) | 71.63 | 2.10 | 22.84 | 1.72 | 1.71 |
| CuNPs/NGO(700) | 71.68 | 1.96 | 22.73 | 1.79 | 1.84 |
| CuNPs/NGO(800) | 71.34 | 1.94 | 23.82 | 1.83 | 1.07 |
| CuNPs/NGO(900) | 77.43 | 1.44 | 17.21 | 1.19 | 2.73 |
| Sample | Surface Area/m2 g−1 | Total Pore Volume (cc/g) P/Po ~ 0.99 | Pore Size (nm) | |
|---|---|---|---|---|
| BET | Langmuir | |||
| CuNPs/NGO(600) | 185 | 193 | 0.31 | 2, 5 |
| CuNPs/NGO(700) | 144 | 151 | 0.34 | 2, 3–5 |
| CuNPs/NGO(800) | 108 | 113 | 0.32 | 1–2, 5 |
| CuNPs/NGO(900) | 86 | 90 | 0.25 | 1.2 |
| Modified Electrode | a LR (μM) | b LOD (μM) | Sensitivity | Ref. |
|---|---|---|---|---|
| 3D N-Co-CNT@NG) | 25–10,830 | 0.1 | 9.05 µA mM−1 cm−2 | [40] |
| Cu@porous carbon | 1–6000 | 0.6 | 10,100 | [41] |
| [Cu3(c btc)2] nanocube | 1–2250 | 1 | 549 µA mM−1 cm−2 | [42] |
| Cu-d BDD | 1–50 | 10 | 2.3 µA mM−1 cm−2 | [43] |
| N-doped Carbon-Cu nanohybrids | 5–2100 | 0.7 | 223.6 µA mM−1 cm−2 | [44] |
| Graphene oxide and NiO nanofibers | 2–600 | 0.77 | 1100 | [45] |
| Cu nanoporous | 10–500 | 40 | 220 µA mM−1 cm−2 | [46] |
| Cu NPs/SWCNTs | 0.5–500 | 0.3 | 0.256 µA mM−1 cm−2 | [47] |
| Cu/CuO/ZnO | 100–1000 | 18 | 408 µA mM−1 cm−2 | [48] |
| AuCu/CNTs | 80–9260 | 4 | 22 µA mM−1 cm−2 | [49] |
| CuNPs/NGO | 1–1803 | 0.44 | 2500 µA mM−1 cm−2 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivasankar, K.; Rani, K.K.; Wang, S.-F.; Devasenathipathy, R.; Lin, C.-H. Copper Nanoparticle and Nitrogen Doped Graphite Oxide Based Biosensor for the Sensitive Determination of Glucose. Nanomaterials 2018, 8, 429. https://doi.org/10.3390/nano8060429
Sivasankar K, Rani KK, Wang S-F, Devasenathipathy R, Lin C-H. Copper Nanoparticle and Nitrogen Doped Graphite Oxide Based Biosensor for the Sensitive Determination of Glucose. Nanomaterials. 2018; 8(6):429. https://doi.org/10.3390/nano8060429
Chicago/Turabian StyleSivasankar, Kulandaivel, Karuppasamy Kohila Rani, Sea-Fue Wang, Rajkumar Devasenathipathy, and Chia-Her Lin. 2018. "Copper Nanoparticle and Nitrogen Doped Graphite Oxide Based Biosensor for the Sensitive Determination of Glucose" Nanomaterials 8, no. 6: 429. https://doi.org/10.3390/nano8060429
APA StyleSivasankar, K., Rani, K. K., Wang, S.-F., Devasenathipathy, R., & Lin, C.-H. (2018). Copper Nanoparticle and Nitrogen Doped Graphite Oxide Based Biosensor for the Sensitive Determination of Glucose. Nanomaterials, 8(6), 429. https://doi.org/10.3390/nano8060429