Biofunctionalized Lysophosphatidic Acid/Silk Fibroin Film for Cornea Endothelial Cell Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SF Solution
2.2. Fabrication of LPA/SF
2.3. Characterizations
2.4. Isolation and Culture of Rabbit CEnCs (rCEnCs)
2.5. Morphology Analysis
2.6. Initial Attachment
2.7. Cell Viability
2.8. mRNAs Expression
2.9. Immunohistological Analysis
3. Results
3.1. Characterization of SF and LPA/SF
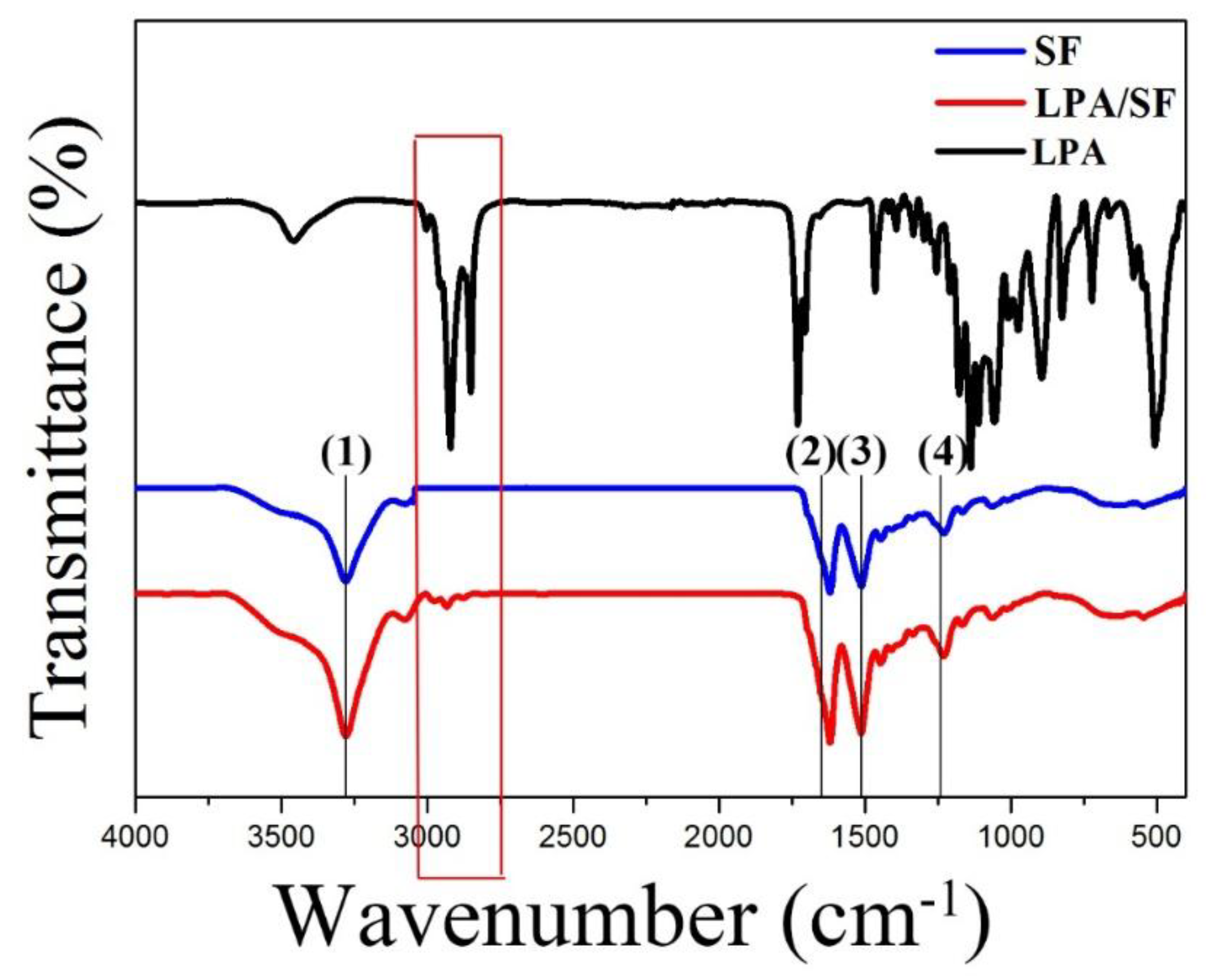
3.1.1. ATR-FTIR Spectroscopy
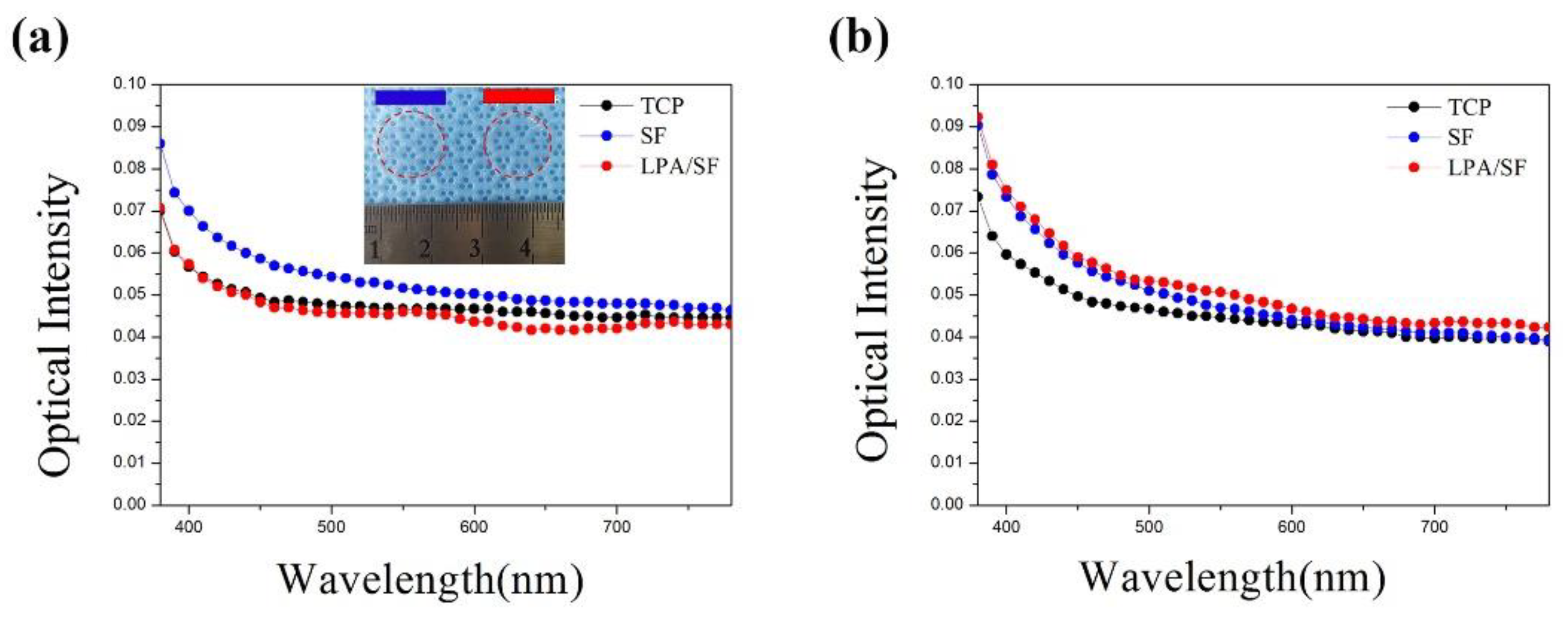
3.1.2. Transparency
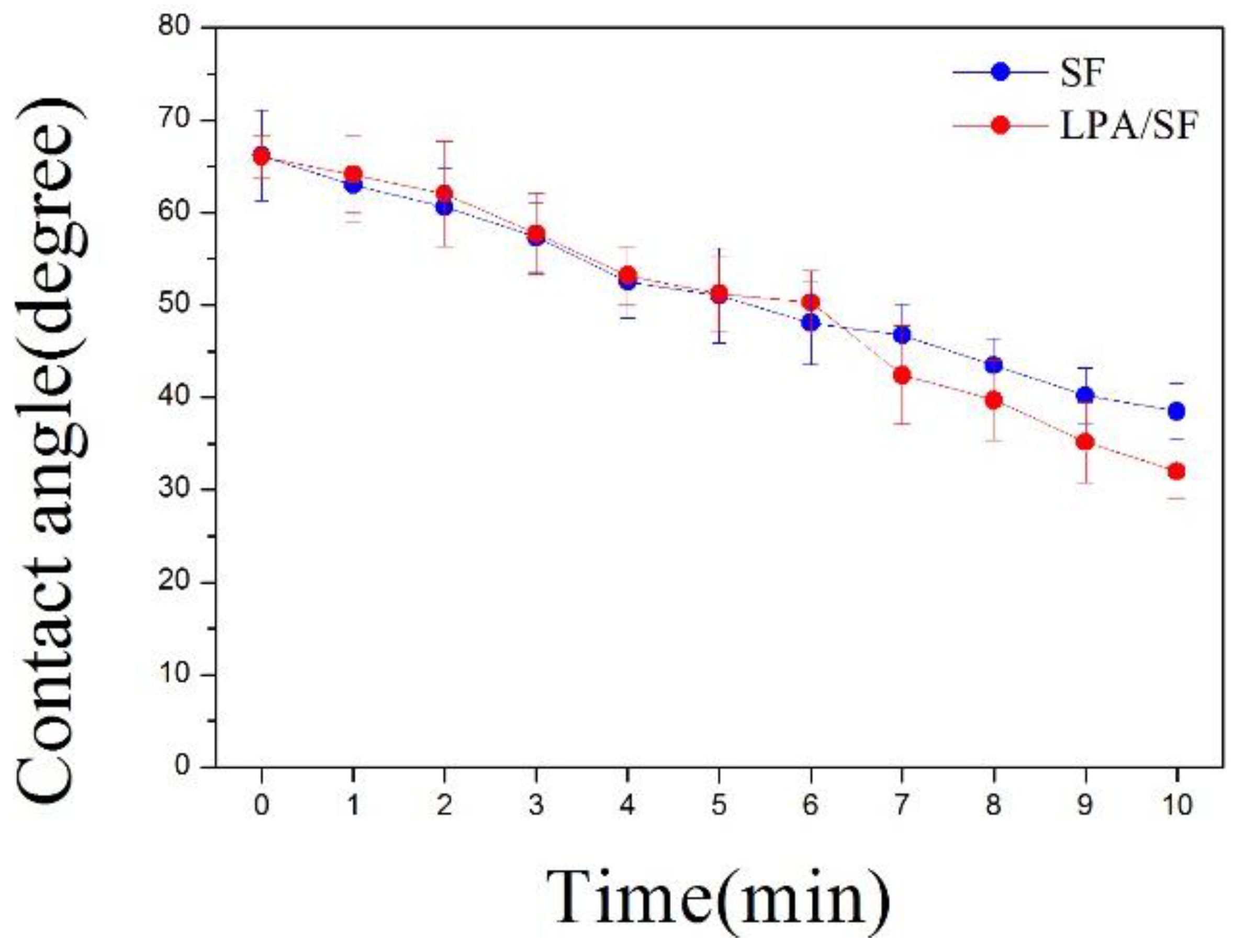
3.1.3. Hydrophilicity
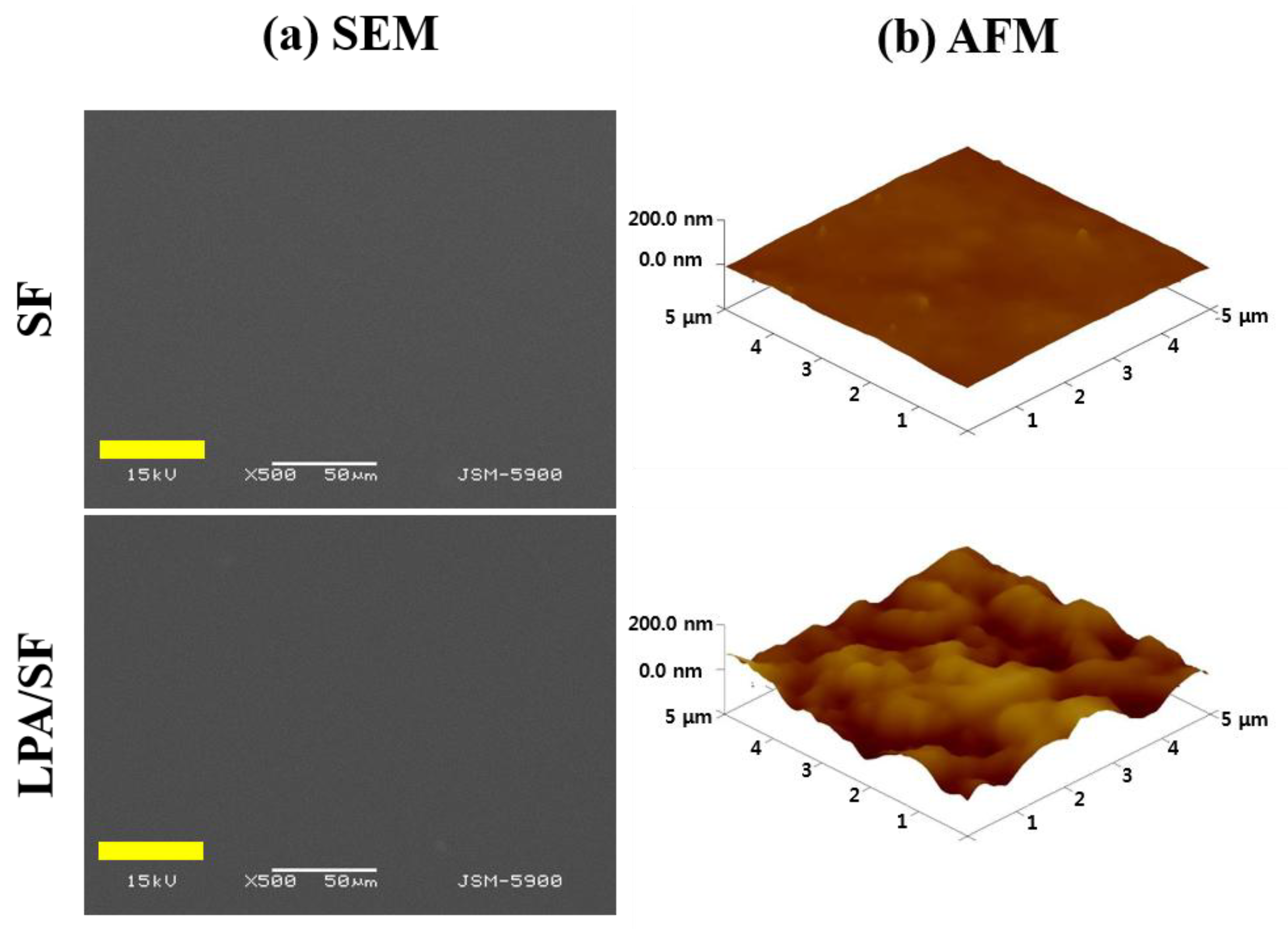
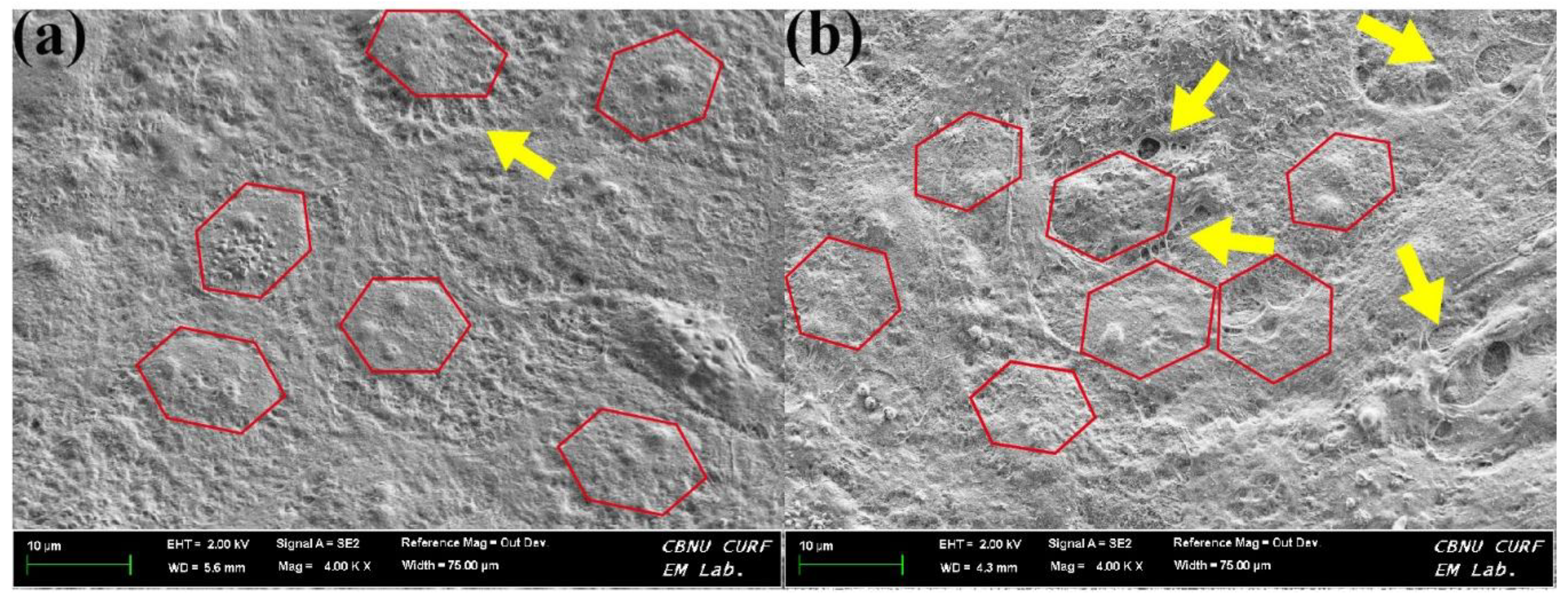
3.1.4. Surface Morphology and Roughness
3.2. In Vitro Study
3.2.1. Monolayer Formation
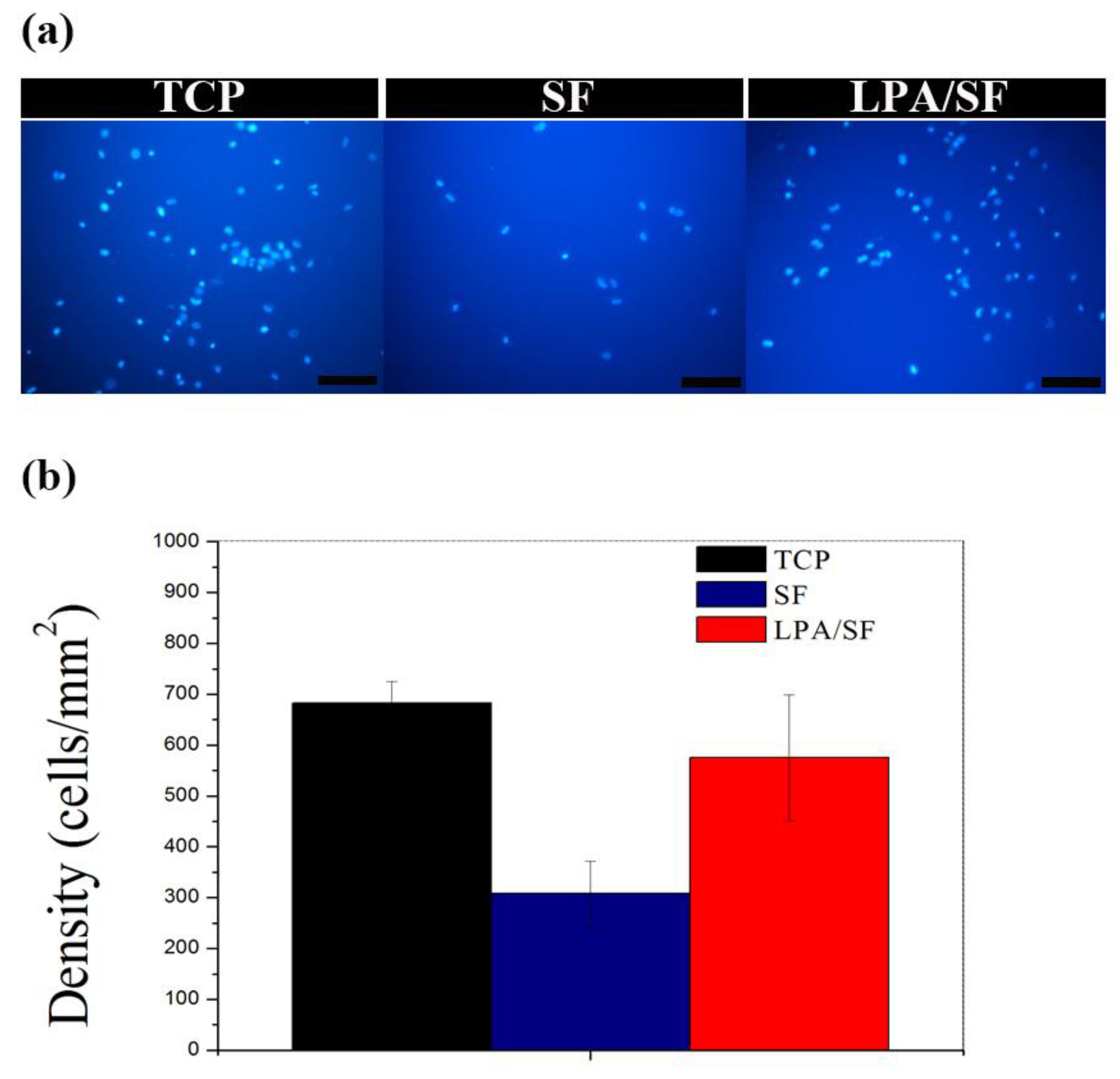
3.2.2. Initial Attachment
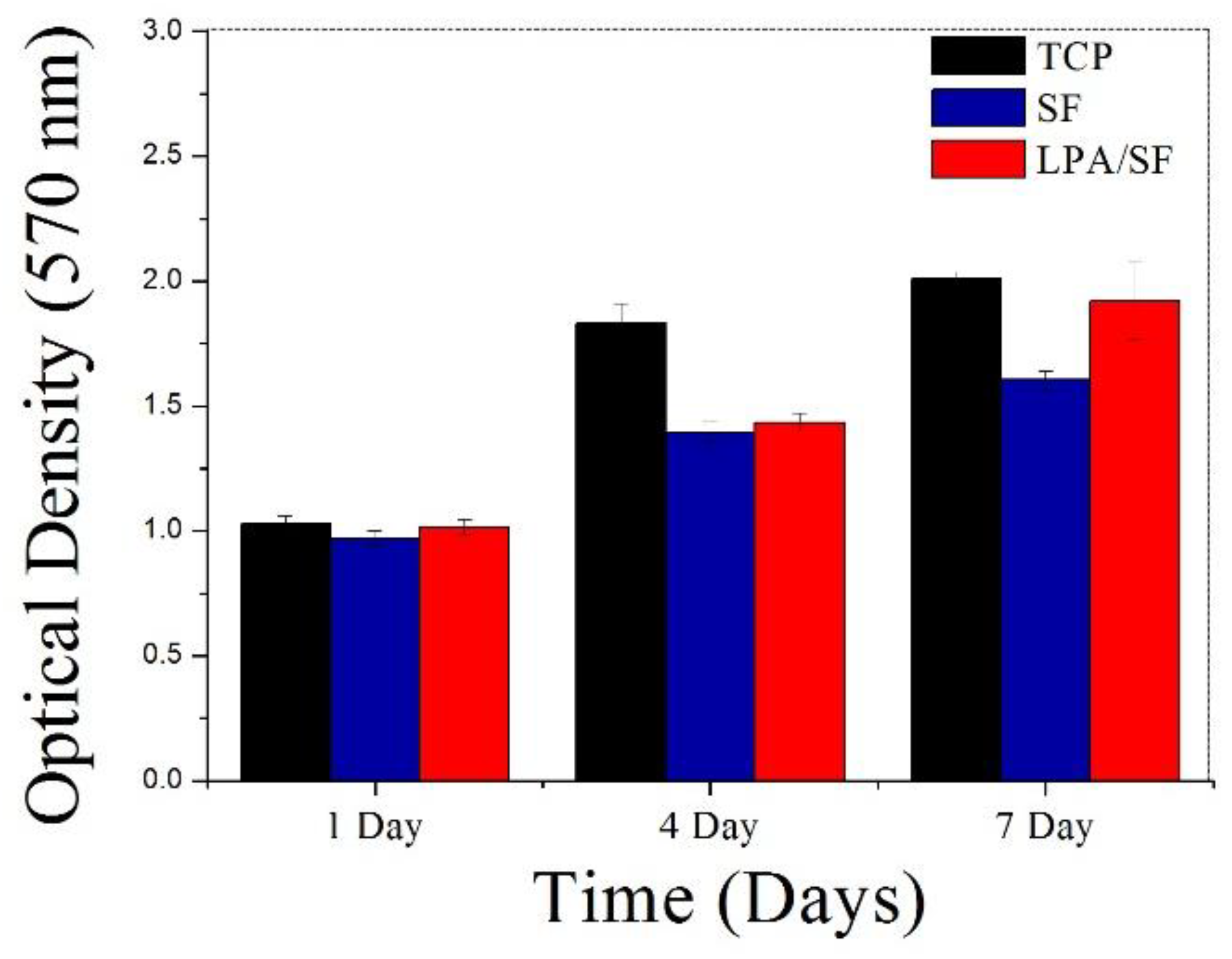
3.2.3. Cell Proliferation
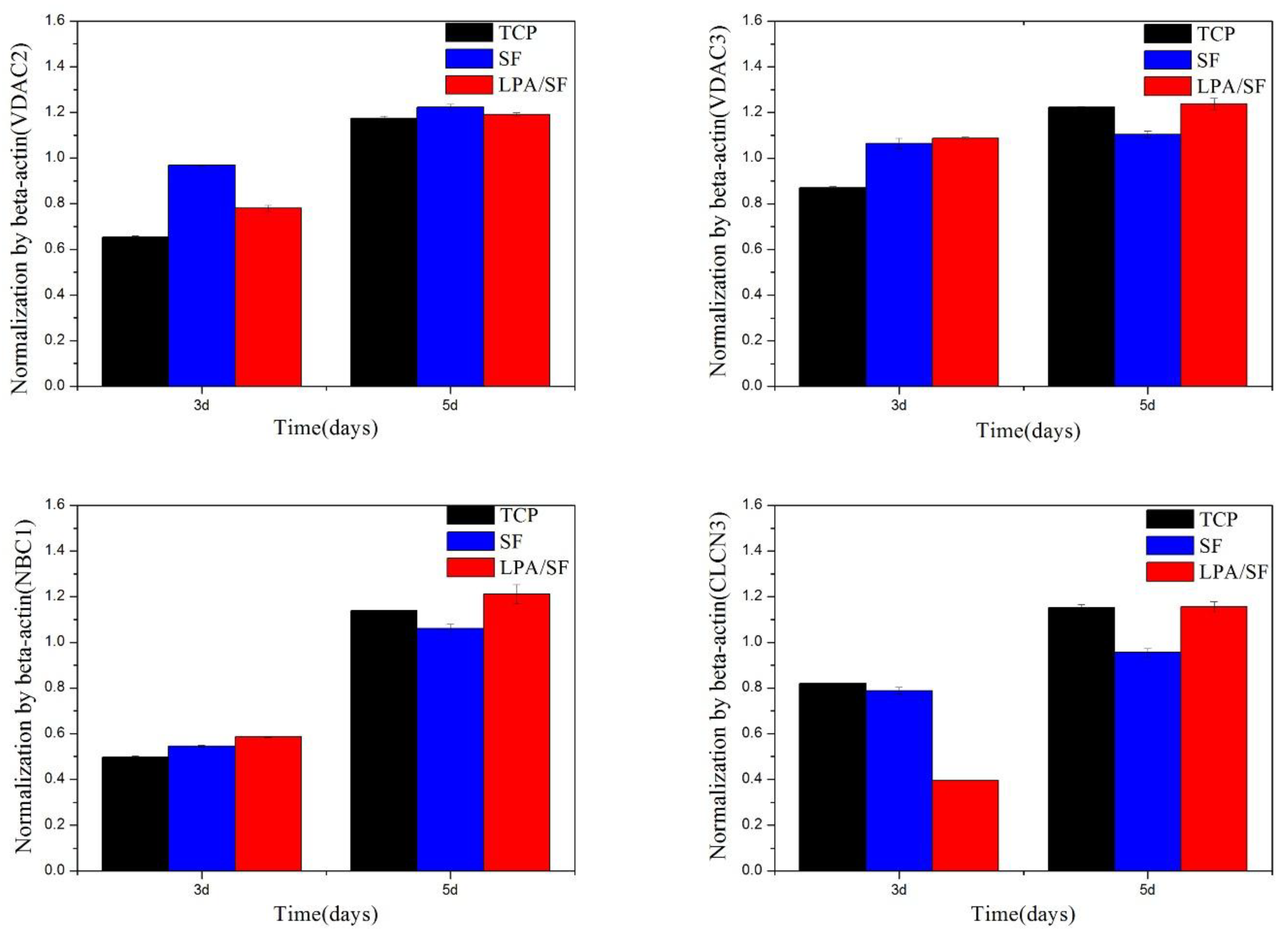
3.2.4. Specific mRNAs Expression
3.2.5. Immunohistological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Ghezzi, C.E.; Gomes, R.; Pollard, R.E.; Funderburgh, J.L.; Kaplan, D.L. In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 2017, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Sim, B.R.; Kim, J.I.; Khang, G. Functionalized silk fibroin film scaffold using β-Carotene for cornea endothelial cell regeneration. Colloids Surfaces B Biointerfaces 2018, 164, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Tripathy, N.; Cho, S.A.; Joo, C.K.; Lee, D.; Khang, G. Bioengineered neo-corneal endothelium using collagen type-I coated silk fibroin film. Colloids Surfaces B Biointerfaces 2015, 136, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Yamagami, S.; Amano, S. Corneal endothelial regeneration and tissue engineering. Prog. Retin. Eye Res. 2013, 35, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aldave, A.J.; Dematteo, J.; Glasser, D.B.; Tu, E.Y.; Iliakis, B.; Nordlund, M.L.; Misko, J.; Verdier, D.D.; Yu, F. Report of the eye bank association of America medical advisory board subcommittee on fungal infection after corneal transplantation. Cornea 2013, 32, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.A.; Omenetto, F.G.; Kaplan, D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Myung, D.; Koh, W.; Bakri, A.; Zhang, F.; Marshall, A.; Ko, J.; Noolandi, J.; Carrasco, M.; Cochran, J.R.; Frank, C.W.; et al. Design and fabrication of an artificial cornea based on a photolithographically patterned hydrogel construct. Biomed. Microdevices 2007, 9, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Ilhan-Sarac, O.; Akpek, E.K. Current concepts and techniques in keratoprosthesis. Curr. Opin. Ophthalmol. 2005, 16, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Merrett, K.; Griffith, M.; Fagerholm, P.; Dravida, S.; Heyne, B.; Scaiano, J.C.; Watsky, M.A.; Shinozaki, N.; Lagali, N.; et al. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials 2008, 29, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Alaminos, M.; Sánchez-Quevedo, M.D.C.; Muñoz-Ávila, J.I.; Serrano, D.; Medialdea, S.; Carreras, I.; Campos, A. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lui, W.; Cui, L.; Wang, M.; Cao, Y. Tissue engineering of nearly transparent corneal stroma. Tissue Eng. 2005, 11, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Nandi, S.; Naskar, D.; Guha, R.; Chowdhury, S.; Pradhan, N.; Kundu, S.C.; Konar, A. Non-mulberry Silk Fibroin Biomaterial for Corneal Regeneration. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kasoju, N.; Bora, U. Silk fibroin in tissue engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.M.; Kim, D.K.; Park, H.J.; Ju, H.W.; Lee, O.J.; Kim, J.H.; Lee, J.M.; Lee, J.S.; Park, C.H. Fabrication and characterization of three-dimensional silk fibroin scaffolds using a mixture of salt/sucrose. Macromol. Res. 2014, 22, 1268–1274. [Google Scholar] [CrossRef]
- Kim, D.K.; Sim, B.R.; Khang, G. Nature-Derived Aloe Vera Gel Blended Silk Fibroin Film Scaffolds for Cornea Endothelial Cell Regeneration and Transplantation. ACS Appl. Mater. Interfaces 2016, 8, 15160–15168. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.I.; Hwang, T.I.; Sim, B.R.; Khang, G. Bioengineered Osteoinductive Broussonetia Kazinoki/ Silk Fibroin Composite Scaffolds for Bone Tissue Regeneration. ACS Appl. Mater. Interfaces 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; In Kim, J.; Sim, B.R.; Khang, G. Bioengineered porous composite curcumin/silk scaffolds for cartilage regeneration. Mater. Sci. Eng. C 2017, 78, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-S.; Song, J.E.; Tripathy, N.; Kim, H.; Moon, B.M.; Park, C.H.; Khang, G. Effect of pore sizes of silk scaffolds for cartilage tissue engineering. Macromol. Res. 2015, 23, 1091–1097. [Google Scholar] [CrossRef]
- Sim, B.R.; Kim, H.M.; Kim, S.M.; Kim, D.K.; Song, J.E.; Park, C.-H.; Khang, G. Osteogenesis differentiation of rabbit bone marrow-mesenchymal stem cells in silk scaffold loaded with various ratios of hydroxyapatite. Polymer 2016, 40, 915–924. [Google Scholar] [CrossRef]
- Lee, D.H.; Tripathy, N.; Shin, J.H.; Song, J.E.; Cha, J.G.; Min, K.D.; Park, C.H.; Khang, G. Enhanced osteogenesis of β-tricalcium phosphate reinforced silk fibroin scaffold for bone tissue biofabrication. Int. J. Biol. Macromol. 2017, 95, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Suganya, S.; Venugopal, J.; Ramakrishna, S.; Lakshmi, B.S.; Dev, V.R. Aloe Vera/Silk Fibroin/Hydroxyapatite Incorporated Electrospun Nanofibrous Scaffold for Enhanced Osteogenesis. J. Biomater. Tissue Eng. 2014, 4, 9–19. [Google Scholar] [CrossRef]
- Meinel, L.; Betz, O.; Fajardo, R.; Hofmann, S.; Nazarian, A.; Cory, E.; Hilbe, M.; McCool, J.; Langer, R.; Vunjak-Novakovic, G.; et al. Silk based biomaterials to heal critical sized femur defects. Bone 2006, 39, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.K.; Lee, S.E.; Cha, S.R.; Kim, C.H.; Jeong, H.K.; Kim, S.Y.; Shin, J.H.; Song, J.E.; Park, C.H.; Khang, G. Osteogenic Differentiation of Bone Marrow Stem Cell in Silk Fibroin Scaffold. Int. J. Tissue Regen. 2015, 6, 56–63. [Google Scholar]
- Shen, W.; Chen, X.; Hu, Y.; Yin, Z.; Zhu, T.; Hu, J.; Chen, J.; Zheng, Z.; Zhang, W.; Ran, J.; et al. Long-term effects of knitted silk-collagen sponge scaffold on anterior cruciate ligament reconstruction and osteoarthritis prevention. Biomaterials 2014, 35, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, D.H.; Song, J.E.; Cho, S.J.; Park, C.H.; Khang, G. Effect of silk sponge concentrations on skin regeneration. Polymer 2017, 41, 1–6. [Google Scholar] [CrossRef]
- Hardy, J.G.; Scheibel, T.R. Composite materials based on silk proteins. Prog. Polym. Sci. 2010, 35, 1093–1115. [Google Scholar] [CrossRef]
- Kim, E.Y.; Tripathy, N.; Park, J.Y.; Lee, S.E.; Joo, C.K.; Khang, G. Silk fibroin film as an efficient carrier for corneal endothelial cells regeneration. Macromol. Res. 2015, 23, 189–195. [Google Scholar] [CrossRef]
- Lee, S.E.; Cha, S.R.; Jang, N.K.; Kim, S.Y.; Kim, E.Y.; Song, J.E.; Park, C.H.; Khang, G. Effect of degumming time of silk films on growth of corneal endothelial cells for tissue engineered endothelialized neo-corneas. Polymer 2016, 40, 181–187. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.R.; Kim, D.K.; Jeon, Y.S.; Jeon, H.Y.; Kook, Y.J. Graphene Oxide as an Additive on Silk Fibroin Film for Corneal Endothelial Cell Regeneration. Int. J. Tissue Regen. 2016, 7, 108–112. [Google Scholar]
- Sim, B.R.; Kim, D.K.; Lee, D.H.; Jeon, Y.S.; Tripathy, N. Effect of Quercetin / Silk Fibroin Film on Proliferation of Corneal Endothelial Cells. Int. J. Tissue Regen. 2016, 7, 1–5. [Google Scholar]
- Feng, J.; Mineda, K.; Wu, S.H.; Mashiko, T.; Doi, K.; Kuno, S.; Kinoshita, K.; Kanayama, K.; Asahi, R.; Sunaga, A.; et al. An injectable non-cross-linked hyaluronic-acid gel containing therapeutic spheroids of human adipose-derived stem cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Sim, B.R.; Jeon, H.Y.; Song, J.E.; Tripathy, N.; Lee, D.; Khang, G. Silk Fibroin Film Scaffold for Cornea Endothelial Regeneration and Transplantation. Int. J. Tissue Regen. 2016, 7, 72–76. [Google Scholar]
- Kim, S.M.; Kim, E.Y.; Kuk, H.; Kim, S.N.; Sim, B.R.; Kim, D.K.; Joo, C.; Khang, G. A Study for Corneal Endothelial Cell Growth Behavior on Silk Fibroin Substrata. Int. J. Tissue Regen. 2015, 6, 49–55. [Google Scholar]
- Noguchi, K.; Herr, D.; Mutoh, T.; Chun, J. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 2009, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Nakane, S.; Kishimoto, S.; Waku, K.; Yoshioka, Y.; Tokumura, A. Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J. Lipid Res. 2002, 43, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-P.; Yin, J.; Yu, F.-S.X. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest. Ophthalmol. Vis. Sci. 2007, 48, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G. Physiological responses to lysophosphatidic acid and related glycero-phospholipids. Prostaglandins Other Lipid Mediat. 2001, 64, 47–62. [Google Scholar] [CrossRef]
- Jalink, K.; Hordijk, P.L.; Moolenaar, W.H. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. BBA-Rev. Cancer 1994, 1198, 185–196. [Google Scholar] [CrossRef]
- Nishida, K. Tissue engineering of the cornea. Cornea 2003, 22, S28–S34. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Brugnano, J.; Sun, S.; Vase, A.; Orwin, E. The development of a tissue-engineered cornea: Biomaterials and culture methods. Pediatr. Res. 2008, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Wei, R.-H.; Zhao, S.-Z. Evaluation of corneal cell growth on tissue engineering materials as artificial cornea scaffolds. Int. J. Ophthalmol. 2013, 6, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, N.; Rodríguez-Barrientos, C.A.; Aznar-Cervantes, S.D.; Chacón, M.; Cenis, J.L.; Riestra, A.C.; Sánchez-Avila, R.M.; Persinal, M.; Brea-Pastor, A.; Fernández-Vega Cueto, L.; et al. Silk fibroin films for corneal endothelial regeneration: Transplant in a rabbit descemet membrane endothelial keratoplasty. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3357–3365. [Google Scholar] [CrossRef]
- Madden, P.W.; Lai, J.N.X.; George, K.A.; Giovenco, T.; Harkin, D.G.; Chirila, T.V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 2011, 32, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Melles, G.R.J.; Ong, T.S.; Ververs, B.; van der Wees, J. Descemet membrane endothelial keratoplasty (DMEK). Cornea 2006, 25, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Nakahara, Y.; Xuan, D.; Wu, D.; Zhao, F.-K.; Li, X.-Y.; Zhang, J.-S. Study on the optical property and biocompatibility of a tissue engineering cornea. Int. J. Ophthalmol. 2012, 5, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Prasad, T.; Raj, V.; Anil Kumar, P.R.; Sreenivasan, K.; Kumary, T.V. A cytocompatible poly(n-isopropylacrylamide-co-glycidylmethacrylate) coated surface as new substrate for corneal tissue engineering. J. Bioact. Compat. Polym. 2010, 25, 58–74. [Google Scholar] [CrossRef]
- Kong, B.; Mi, S. Electrospun scaffolds for corneal tissue engineering: A review. Materials (Basel) 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal Tissue Engineering: Recent Advances and Future Perspectives. Tissue Eng. Part B Rev. 2015, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef]
- Röder, A.; García-Gareta, E.; Theodoropoulos, C.; Ristovski, N.; Blackwood, K.; Woodruff, M. An Assessment of Cell Culture Plate Surface Chemistry for in Vitro Studies of Tissue Engineering Scaffolds. J. Funct. Biomater. 2015, 6, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 2004, 256–257, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wiener, M. Regeneration of the cornea. J. Am. Med. Assoc. 1909, LIII, 762–765. [Google Scholar] [CrossRef]
- Bernardo, A.A.; Bernardo, C.M.; Espiritu, D.J.; Arruda, J.A.L. The Sodium Bicarbonate Cotransporter: Structure, Function, and Regulation. Semin. Nephrol. 2006, 26, 352–360. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Jeon, H.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Biofunctionalized Lysophosphatidic Acid/Silk Fibroin Film for Cornea Endothelial Cell Regeneration. Nanomaterials 2018, 8, 290. https://doi.org/10.3390/nano8050290
Choi JH, Jeon H, Song JE, Oliveira JM, Reis RL, Khang G. Biofunctionalized Lysophosphatidic Acid/Silk Fibroin Film for Cornea Endothelial Cell Regeneration. Nanomaterials. 2018; 8(5):290. https://doi.org/10.3390/nano8050290
Chicago/Turabian StyleChoi, Joo Hee, Hayan Jeon, Jeong Eun Song, Joaquim Miguel Oliveira, Rui Luis Reis, and Gilson Khang. 2018. "Biofunctionalized Lysophosphatidic Acid/Silk Fibroin Film for Cornea Endothelial Cell Regeneration" Nanomaterials 8, no. 5: 290. https://doi.org/10.3390/nano8050290
APA StyleChoi, J. H., Jeon, H., Song, J. E., Oliveira, J. M., Reis, R. L., & Khang, G. (2018). Biofunctionalized Lysophosphatidic Acid/Silk Fibroin Film for Cornea Endothelial Cell Regeneration. Nanomaterials, 8(5), 290. https://doi.org/10.3390/nano8050290






