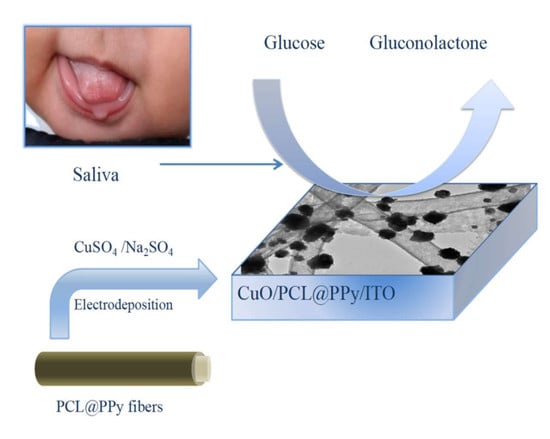
Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Instrument and Equipment
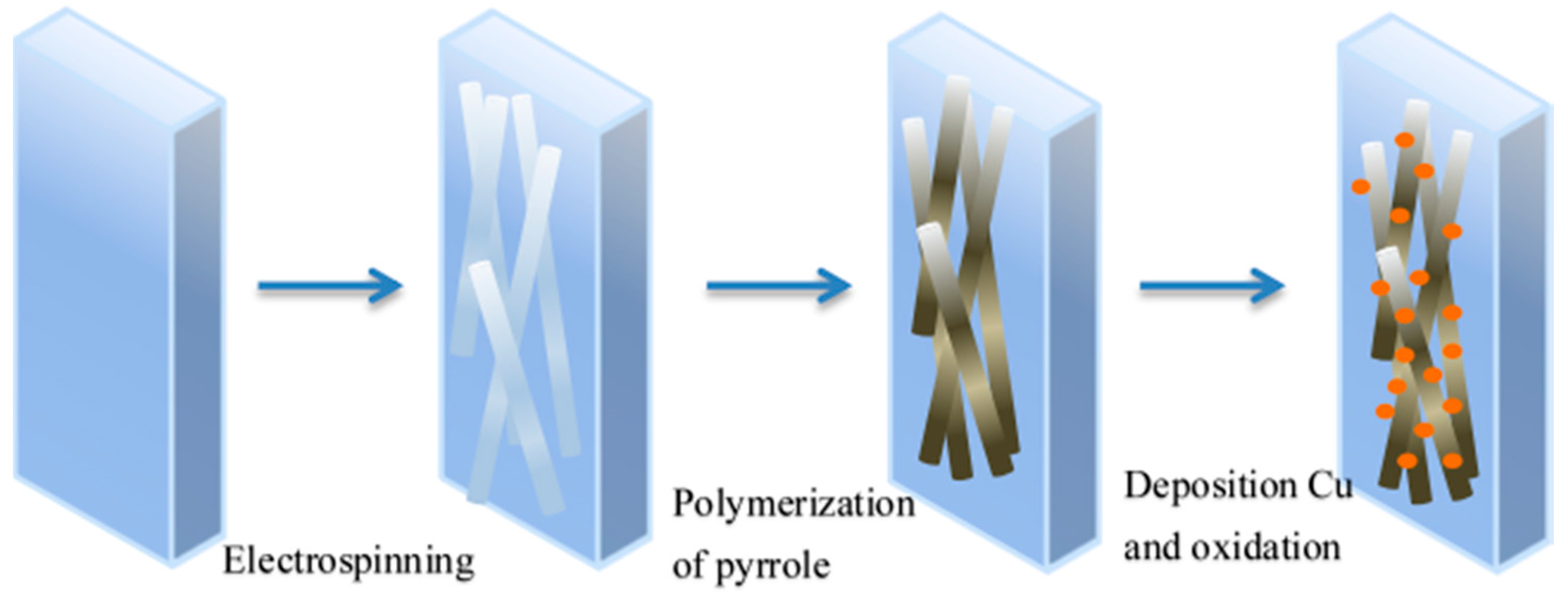
2.3. Preparation of Electrospinning PCL Nanofibers
2.4. Preparation of CuO/PCL@PPy/ITO
3. Results and Discussion
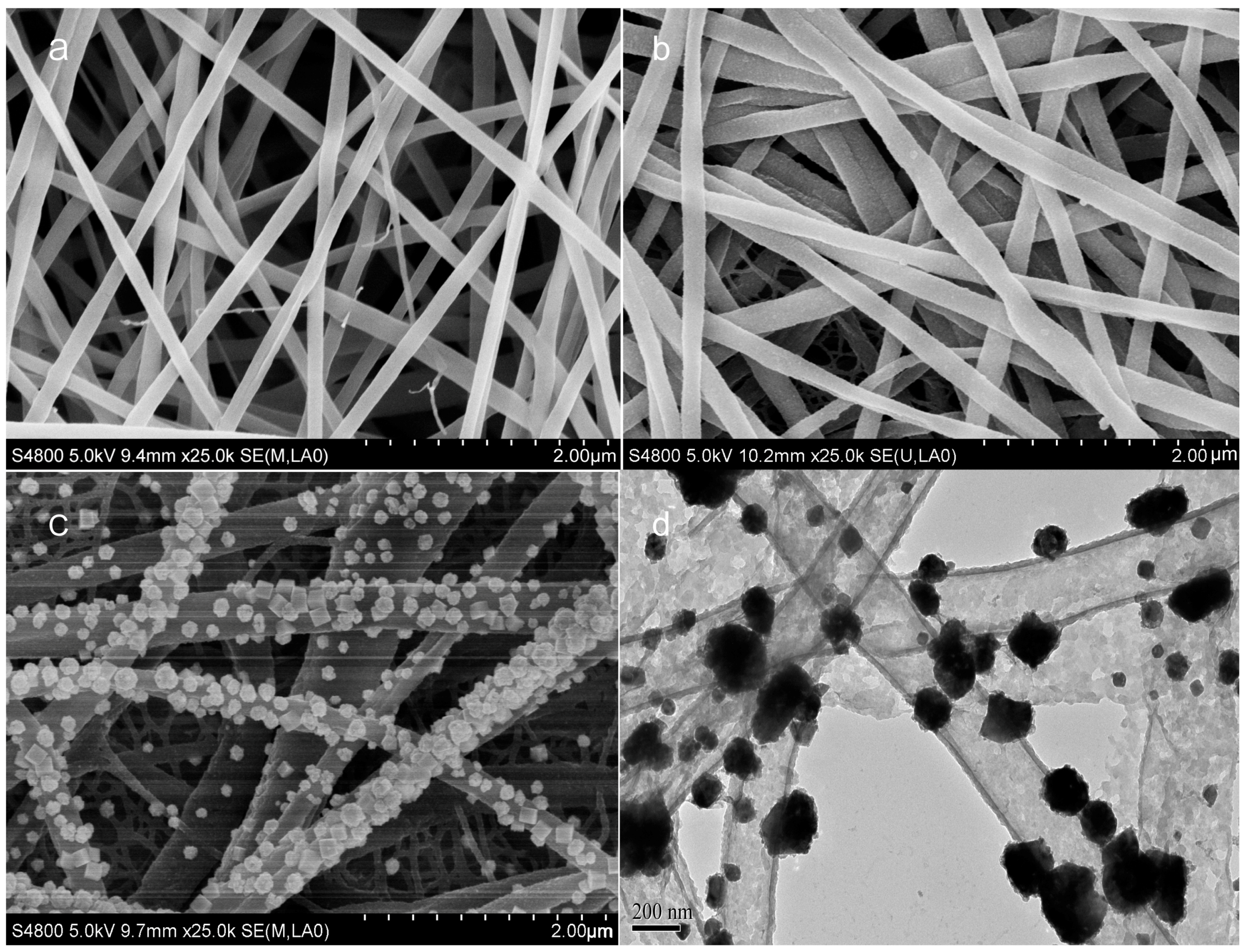
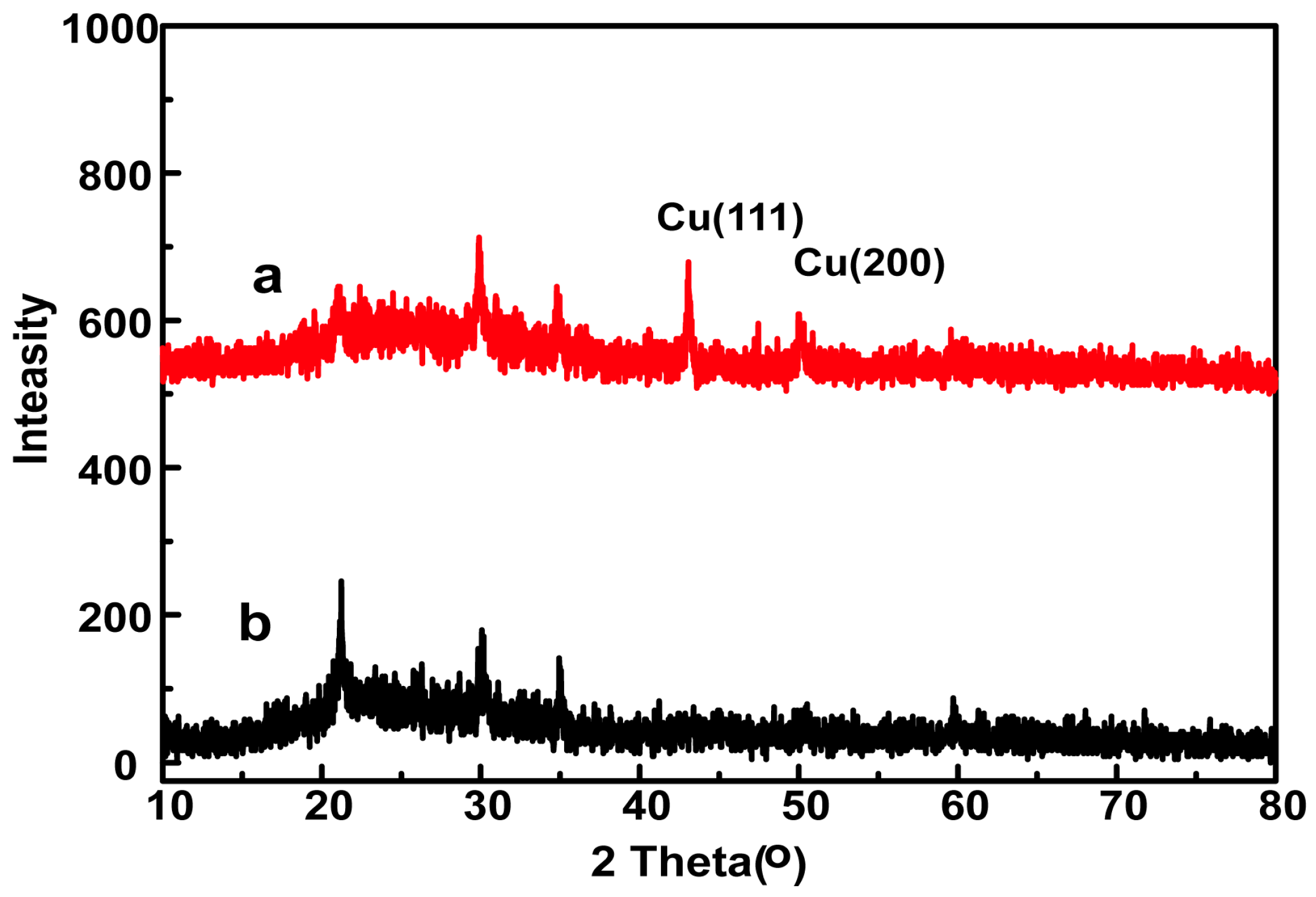
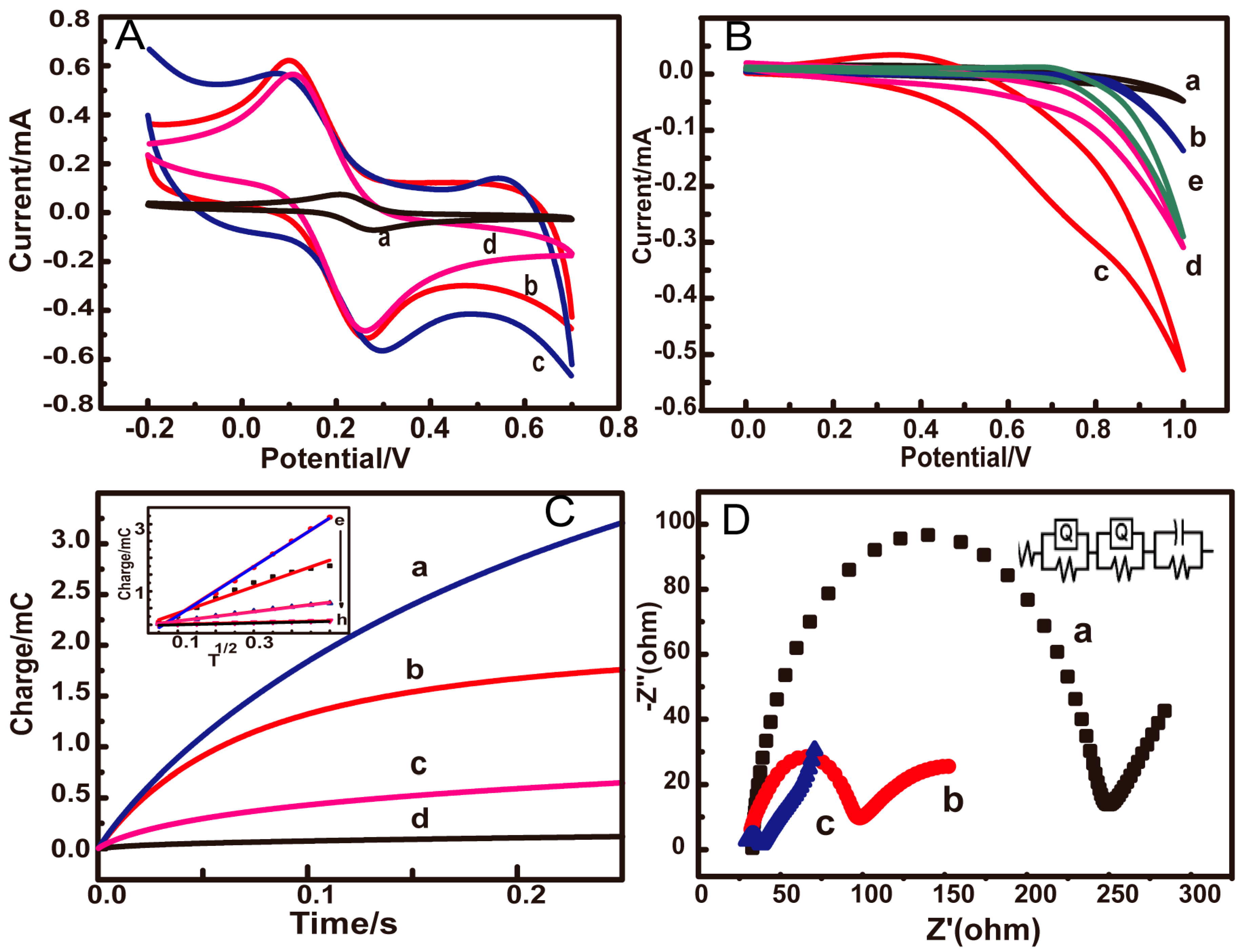
3.1. Characterization of as-Prepared Electrodes
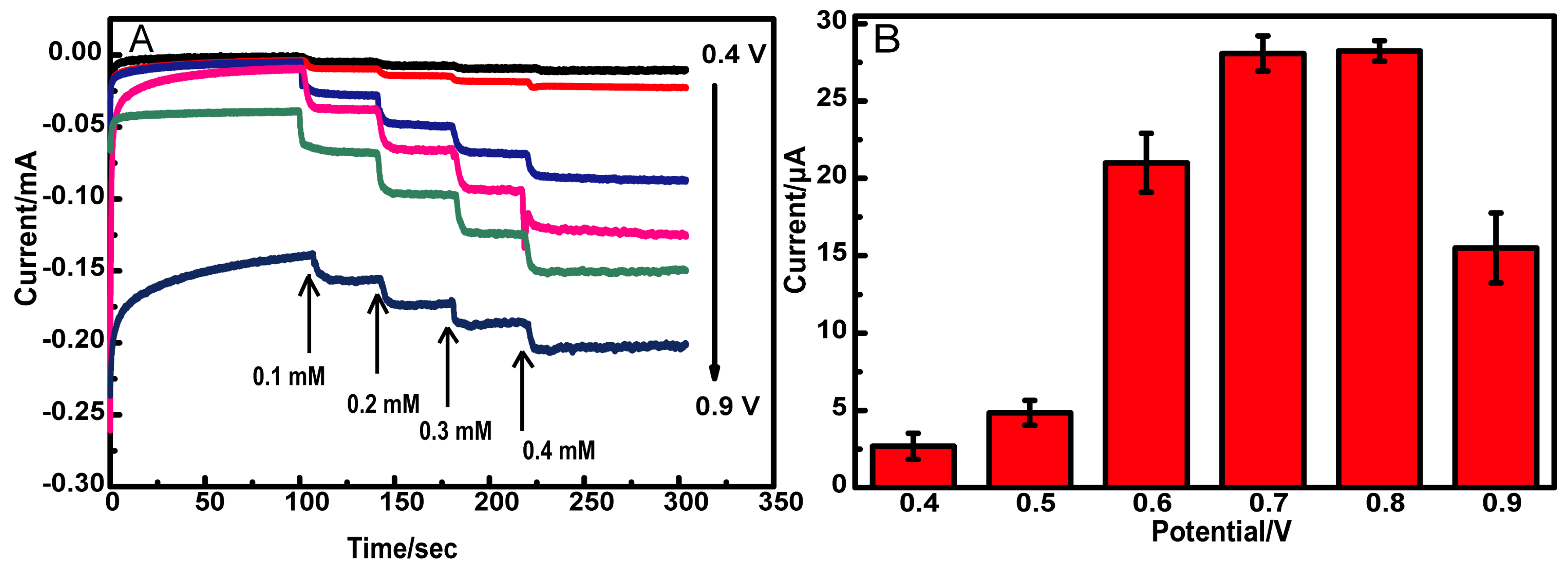
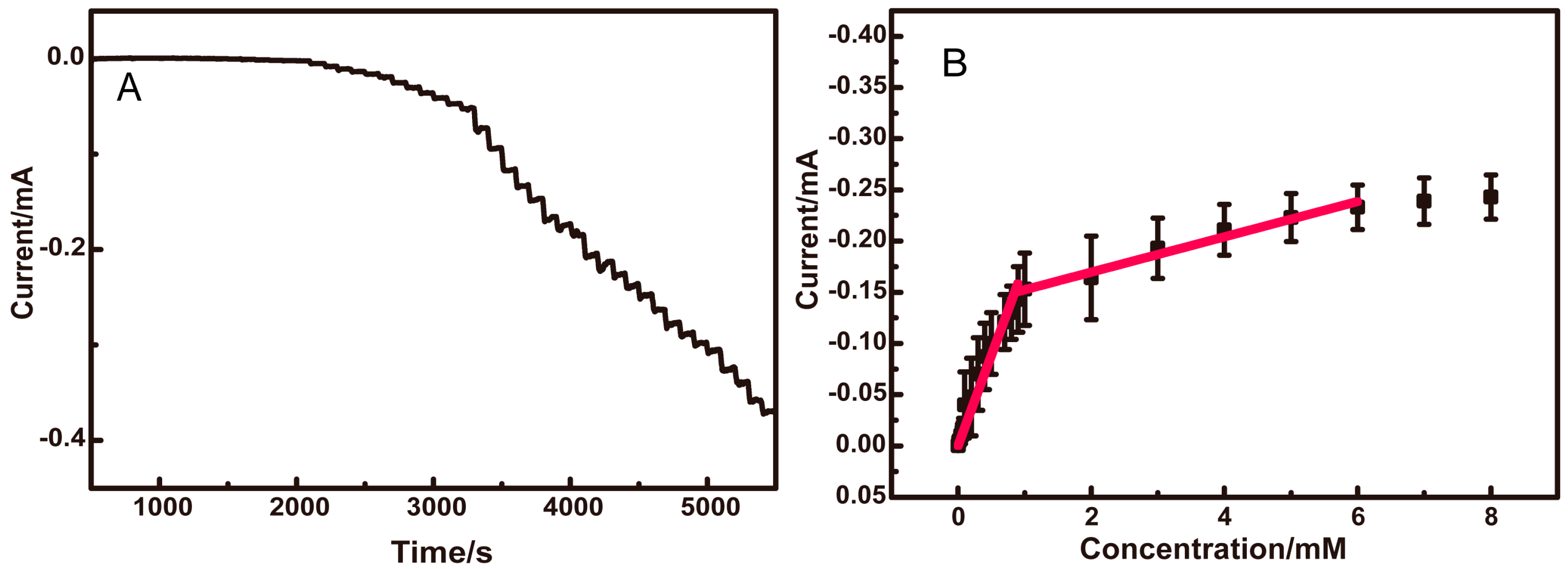
3.2. Electrochemical Response of Glucose
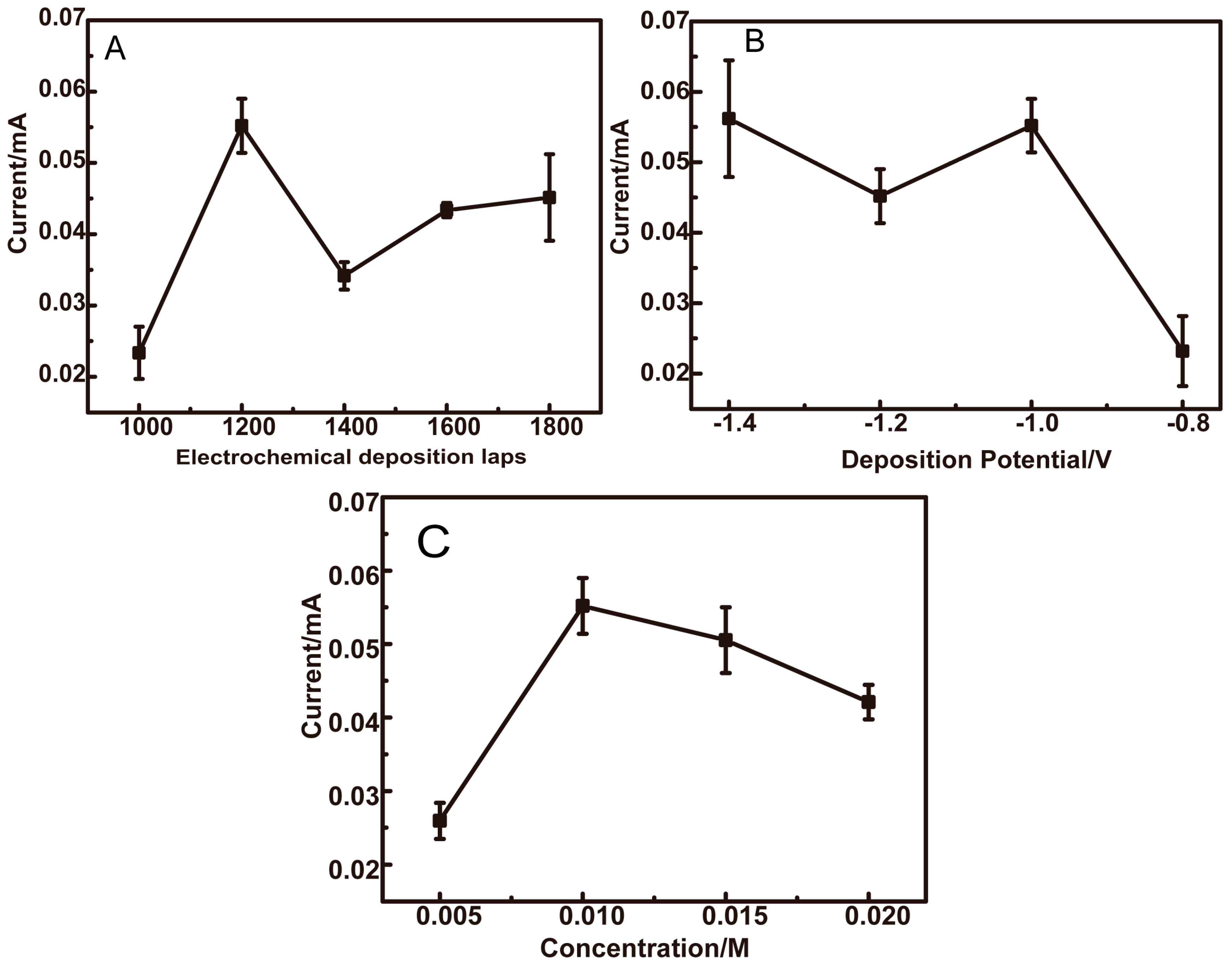
3.3. Optimization of Preparation Conditions for CuO/PCL@PPy/ITO Electrodes
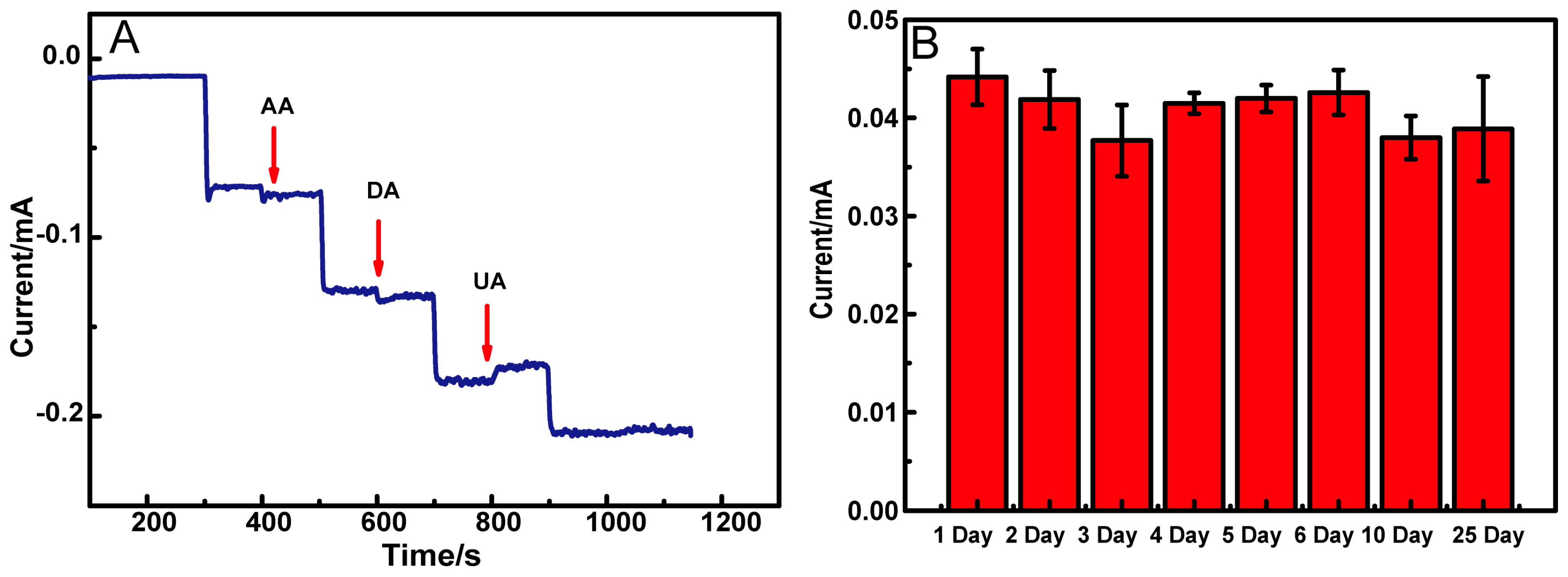
3.4. Selectivity and Stability Studies
3.5. Detection of Glucose in Real Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, J.D.; Turner, A.P. Home blood glucose biosensors: A commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lei, H.; Feng, L. A facile channel for D-glucose detection in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.W.; Kundys, M.; Jelen, P.S.; Jonsson-Niedziolka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, B.; Choi, C.S.; Lee, K.; Kim, S. Recent trends in the development of diagnostic tools for diabetes mellitus using patient saliva. TrAC Trends Anal. Chem. 2017, 89, 60–67. [Google Scholar] [CrossRef]
- Malik, S.; Khadgawat, R.; Anand, S.; Gupta, S. Non-invasive detection of fasting blood glucose level via electrochemical measurement of saliva. SpringerPlus 2016, 5, 701. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, H.; Abdel-Rehim, M. Saliva as an alternative specimen to plasma for drug bioanalysis: A review. TrAC Trends Anal. Chem. 2016, 83, 70–79. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, W.; Wang, M.L. Sensing of Salivary Glucose Using Nano-Structured Biosensors. Biosensors 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Jha, S.K. A paper strip based non-invasive glucose biosensor for salivary analysis. Biosens Bioelectron. 2015, 67, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sheng, Y.; Sun, Y.; Feng, J.; Wang, S.; Zhang, J.; Xu, J.; Jiang, D. A glucose oxidase-coupled DNAzyme sensor for glucose detection in tears and saliva. Biosens. Bioelectron. 2015, 70, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Fu, Y.; Zhang, W.; Ye, S.; Zhang, H.; Xie, F.; Gong, L.; Wei, Z.; Jin, H.; Chen, J. Highly sensitive detection of glucose: A quantitative approach employing nanorods assembled plasmonic substrate. Talanta 2017, 165, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, X.; Zhai, T.; Ling, Y.; Wang, H.; Tong, Y.; Li, Y. Free-standing nickel oxide nanoflake arrays: Synthesis and application for highly sensitive non-enzymatic glucose sensors. Nanoscale 2012, 4, 3123–3127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Su, L.; Zhang, Z.; Huo, D.; Hou, C.; Lei, Y. CuO nanowires based sensitive and selective non-enzymatic glucose detection. Sens. Actuators B Chem. 2014, 191, 86–93. [Google Scholar] [CrossRef]
- Ngo, Y.-L.T.; Hoa, L.T.; Chung, J.S.; Hur, S.H. Multi-dimensional Ag/NiO/reduced graphene oxide nanostructures for a highly sensitive non-enzymatic glucose sensor. J. Alloys Compd. 2017, 712, 742–751. [Google Scholar] [CrossRef]
- Su, Y.; Luo, B.; Zhang, J.Z. Controllable Cobalt Oxide/Au Hierarchically Nanostructured Electrode for Nonenzymatic Glucose Sensing. Anal. Chem. 2016, 88, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.; Lu, Y.; Sheng, K.; Liu, W.; Chen, C.; Li, Y.; Dong, B.; Song, H. Engineered IrO2@NiO Core-Shell Nanowires for Sensitive Non-enzymatic Detection of Trace Glucose in Saliva. Anal Chem. 2016, 88, 12346–12353. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Liu, J.; Chen, H.; Gao, Y.; Li, H. Copper/nickel nanoparticle decorated carbon nanotubes for nonenzymatic glucose biosensor. J. Solid State Electrochem. 2015, 19, 1511–1521. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Raj, M.A.; John, S.A. Nitrogen-Doped Graphene as a Robust Scaffold for the Homogeneous Deposition of Copper Nanostructures: A Nonenzymatic Disposable Glucose Sensor. ACS Sustain. Chem. Eng. 2017, 5, 1648–1658. [Google Scholar] [CrossRef]
- Maaoui, H.; Singh, S.K.; Teodorescu, F.; Coffinier, Y.; Barras, A.; Chtourou, R.; Kurungot, S.; Szunerits, S.; Boukherroub, R. Copper oxide supported on three-dimensional ammonia-doped porous reduced graphene oxide prepared through electrophoretic deposition for non-enzymatic glucose sensing. Electrochim. Acta 2017, 224, 346–354. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Guo, M.; Zheng, Z.; Yu, T.; Wang, Y.; Rodriguez, E.G.; Lei, Y. Vertically Aligned CuO Nanowires Based Electrode for Amperometric Detection of Hydrogen Peroxide. Electroanalysis 2008, 20, 2153–2157. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, A.; Wang, G.; Wei, Y.; Wang, W.; Wu, H.; Fang, B. Fabrication of CuO nanowalls on Cu substrate for a high performance enzyme-free glucose sensor. CrystEngComm 2010, 12, 1120–1126. [Google Scholar] [CrossRef]
- Zhuang, Z.; Su, X.; Yuan, H.; Sun, Q.; Xiao, D.; Choi, M.M. An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 2008, 133, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Liu, G.; Wang, Z.; Wang, F.; Guo, X.; Wen, Y.; Yang, H. Reproducible preparation of a stable polypyrrole-coated-silver nanoparticles decorated polypyrrole-coated-polycaprolactone-nanofiber-based cloth electrode for electrochemical sensor application. Nanotechnology 2015, 26, 445704. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.G.; Yan, X.; Wang, X.X.; Zhao, H.; Guo, J.; Feng, J.Y.; Long, Y.Z. Chitosan nanostructures by in situ electrospinning for high-efficiency PM 2.5 capture. Nanoscale 2017, 9, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Barathi, V.A.; Lim, K.H.; Ramakrishna, S. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Zhang, H.; Nie, Y.; Nie, K.; Lv, X.; Sun, N.; Xie, X.; Ma, Y.; Sun, X. Sn nanoparticles@nitrogen-doped carbon nanofiber composites as high-performance anodes for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 6277–6283. [Google Scholar] [CrossRef]
- Yadav, K.; Nelson, C.T.; Hsu, S.L.; Hong, Z.; Clarkson, J.D.; Schleputz, C.M.; Damodaran, A.R.; Shafer, P.; Arenholz, E.; Dedon, L.R.; et al. Observation of polar vortices in oxide superlattices. Nature 2016, 530, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.; Kouwenhoven, L.P.; Petta, J.R.; Tarucha, S.; Vandersypen, L.M.K. Spins in few-electron quantum dots. Rev. Mod. Phys. 2007, 79, 1217–1265. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X.; Koh, Y.H.; Lei, B. Facilely fabricating PCL nanofibrous scaffolds with hierarchical pore structure for tissue engineering. Mater. Lett. 2014, 122, 62–65. [Google Scholar] [CrossRef]
- Wei, G.; Li, C.; Fu, Q.; Xu, Y.; Li, H. Preparation of PCL/silk fibroin/collagen electrospun fiber for urethral reconstruction. Int. Urol. Nephrol. 2015, 47, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, Y.; Li, L.; Xu, T.; Wu, Y.; Guo, X.; Wang, F.; Shen, H.; Wen, Y.; Yang, H. Stretched graphene tented by polycaprolactone and polypyrrole net–bracket for neurotransmitter detection. Appl. Surf. Sci. 2017, 396, 832–840. [Google Scholar] [CrossRef]
- Saeed, K.; Park, S.-Y.; Lee, H.-J.; Baek, J.-B.; Huh, W.-S. Preparation of electrospun nanofibers of carbon nanotube/polycaprolactone nanocomposite. Polymer 2006, 47, 8019–8025. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Cao, Y.; Li, H. Electrochemical sensor for bisphenol A determination based on MWCNT/melamine complex modified GCE. Sens. Actuators B Chem. 2012, 171–172, 726–733. [Google Scholar] [CrossRef]
- Kang, X.; Mai, Z.; Zou, X.; Cai, P.; Mo, J. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 2007, 363, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, E.; Zhang, X. Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution. Electrochim. Acta 2014, 130, 253–260. [Google Scholar] [CrossRef]
- Periasamy, P.; Roy, P.; Wu, W.-P.; Huang, Y.-H.; Chang, H.-T. Glucose Oxidase and Horseradish Peroxidase Like Activities of Cuprous Oxide/Polypyrrole Composites. Electrochim. Acta 2016, 215, 253–260. [Google Scholar] [CrossRef]
- Li, X.; Yao, J.; Liu, F.; He, H.; Zhou, M.; Mao, N.; Xiao, P.; Zhang, Y. Nickel/Copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sens. Actuators B Chem. 2013, 181, 501–508. [Google Scholar] [CrossRef]
- Meng, F.; Shi, W.; Sun, Y.; Zhu, X.; Wu, G.; Ruan, C.; Liu, X.; Ge, D. Nonenzymatic biosensor based on Cu(x)O nanoparticles deposited on polypyrrole nanowires for improving detection range. Biosens. Bioelectron. 2013, 42, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nia, P.M.; Meng, W.P.; Lorestani, F.; Mahmoudian, M.R.; Alias, Y. Electrodeposition of copper oxide/polypyrrole/reduced graphene oxide as a nonenzymatic glucose biosensor. Sens. Actuators B Chem. 2015, 209, 100–108. [Google Scholar]
- Wang, T.; Su, W.; Fu, Y.; Hu, J. Controllably annealed CuO-nanoparticle modified ITO electrodes: Characterisation and electrochemical studies. Appl. Surf. Sci. 2016, 390, 795–803. [Google Scholar] [CrossRef]
- Ghanbari, K.; Babaei, Z. Fabrication and characterization of non-enzymatic glucose sensor based on ternary NiO/CuO/polyaniline nanocomposite. Anal. Biochem. 2016, 498, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Long, M.; Tan, L.; Zhang, Y.; Ouyang, J.; Liu, P.; Tang, A. Helical TiO2 Nanotube Arrays Modified by Cu-Cu2O with Ultrahigh Sensitivity for the Nonenzymatic Electro-oxidation of Glucose. ACS Appl. Mater. Interfaces 2015, 7, 12719–12730. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.D.; Han, Y.; Wang, Y.; Xu, J.; Song, Y.Y. One-Step to Prepare Self-Organized Nanoporous NiO/TiO2 Layers and Its Use in Non-Enzymatic Glucose Sensing. Sci. Rep. 2013, 3, 3323. [Google Scholar] [CrossRef] [PubMed]

| Samples | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Saliva | 0 | 38.87 | 0 | 6.05% |
| 50 | 91.47 | 105.1% | 3.26% | |
| 150 | 197.5 | 105.6% | 5.78% | |
| 250 | 279.8 | 96.36% | 5.80% |
| Modified Electrodes | Detection Limit (μM) | Detection Potential (V) | Linear Range | Reference |
|---|---|---|---|---|
| Au/ammonia-doped-prGO/CuO | 0.25 μM | +0.50 | (0.00025–6 mM) | [18] |
| CuO/rGO | 0.1 μM | +0.4 V | 0.0004–3.3 mM | [37] |
| Cu2O/Ppy LT paper electrodes | NA | 0 | 1–40 mM | [38] |
| Ni-Cu/TiO2 NTs | 5 μM | +0.55 V | 0.01–3.2 mM | [39] |
| CuxO/PPy/Au | 6.2 μM | +0.6 V | 0–8 mM | [40] |
| CuxO/Ppy/rGO/GCE | 0.03 μM | +0.2 V | 0.1–100 mM | [41] |
| CuO/ITO | 0.7 μM | +0.59 V | 0–4.4 mM | [42] |
| NiO/CuO/PANI | 2.0 μM | +0.6 V | 0.02–2.500 mM | [43] |
| Cu−Cu2O/TiO2/Ti electrode | 8.6 μM | +0.65 V | 0.1–2.5 mM | [44] |
| NiO/TiO2 | 1.0 μM | +0.47 V | 0.005–12.1 mM | [45] |
| CuO/PCL@PPy/ITO | 2 μM | +0.7 V | 0.002–6 mM | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Jin, W.; Wang, Z.; Cheng, H.; Huang, X.; Guo, X.; Ying, Y.; Wu, Y.; Wang, F.; Wen, Y.; et al. Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials 2018, 8, 133. https://doi.org/10.3390/nano8030133
Xu T, Jin W, Wang Z, Cheng H, Huang X, Guo X, Ying Y, Wu Y, Wang F, Wen Y, et al. Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials. 2018; 8(3):133. https://doi.org/10.3390/nano8030133
Chicago/Turabian StyleXu, Ting, Wen Jin, Zhenzhen Wang, Haiyan Cheng, Xinhua Huang, Xiaoyu Guo, Ye Ying, Yiping Wu, Feng Wang, Ying Wen, and et al. 2018. "Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva" Nanomaterials 8, no. 3: 133. https://doi.org/10.3390/nano8030133
APA StyleXu, T., Jin, W., Wang, Z., Cheng, H., Huang, X., Guo, X., Ying, Y., Wu, Y., Wang, F., Wen, Y., & Yang, H. (2018). Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials, 8(3), 133. https://doi.org/10.3390/nano8030133