Facile Synthesis of Ternary Alloy of CdSe1-xSx Quantum Dots with Tunable Absorption and Emission of Visible Light
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation Method
3. Characterization
4. Results and Discussion
4.1. Energy Dispersive X-ray Spectroscopy
4.2. X-ray Diffraction
4.3. Transmission Electron Microscopy
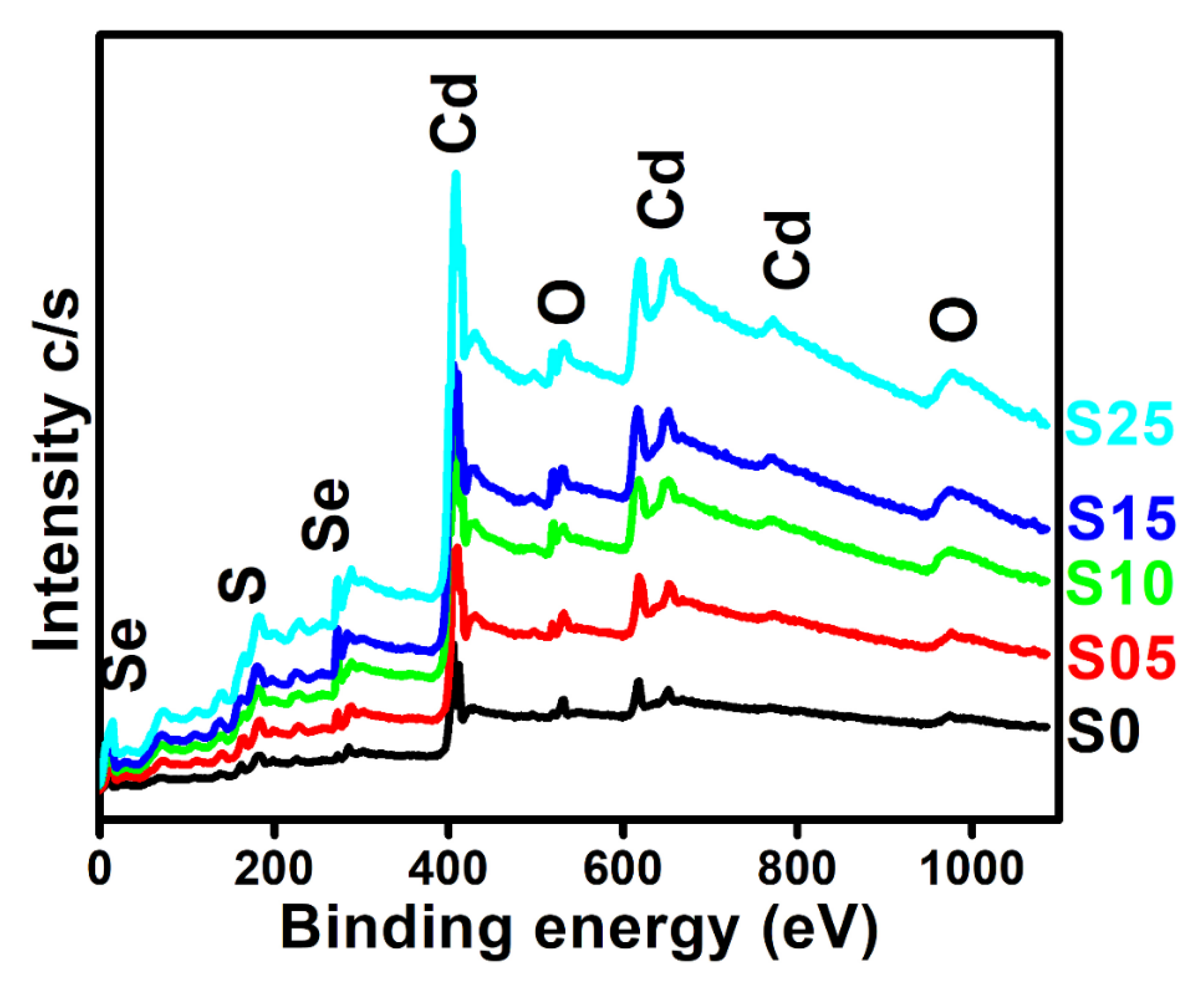
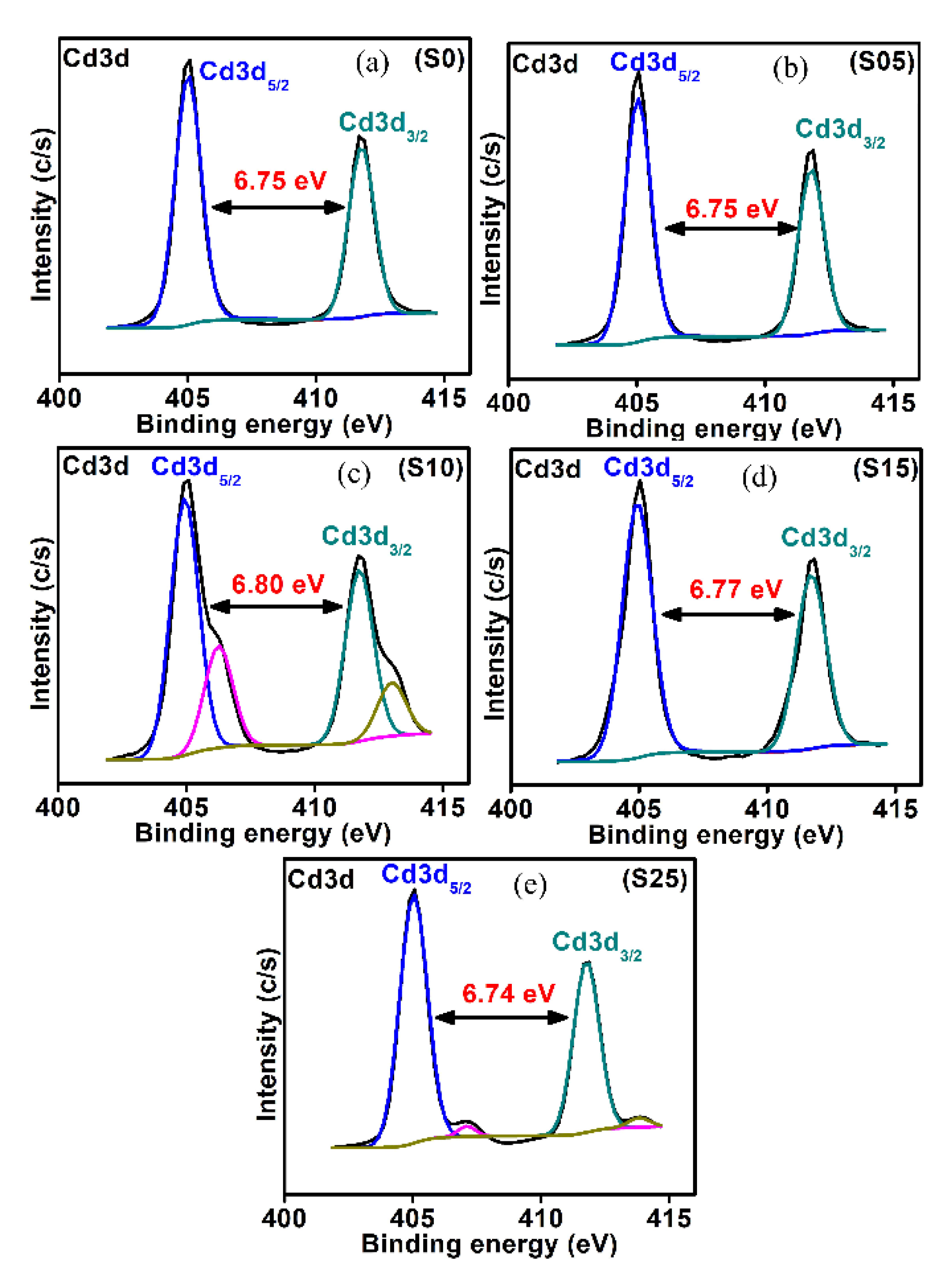
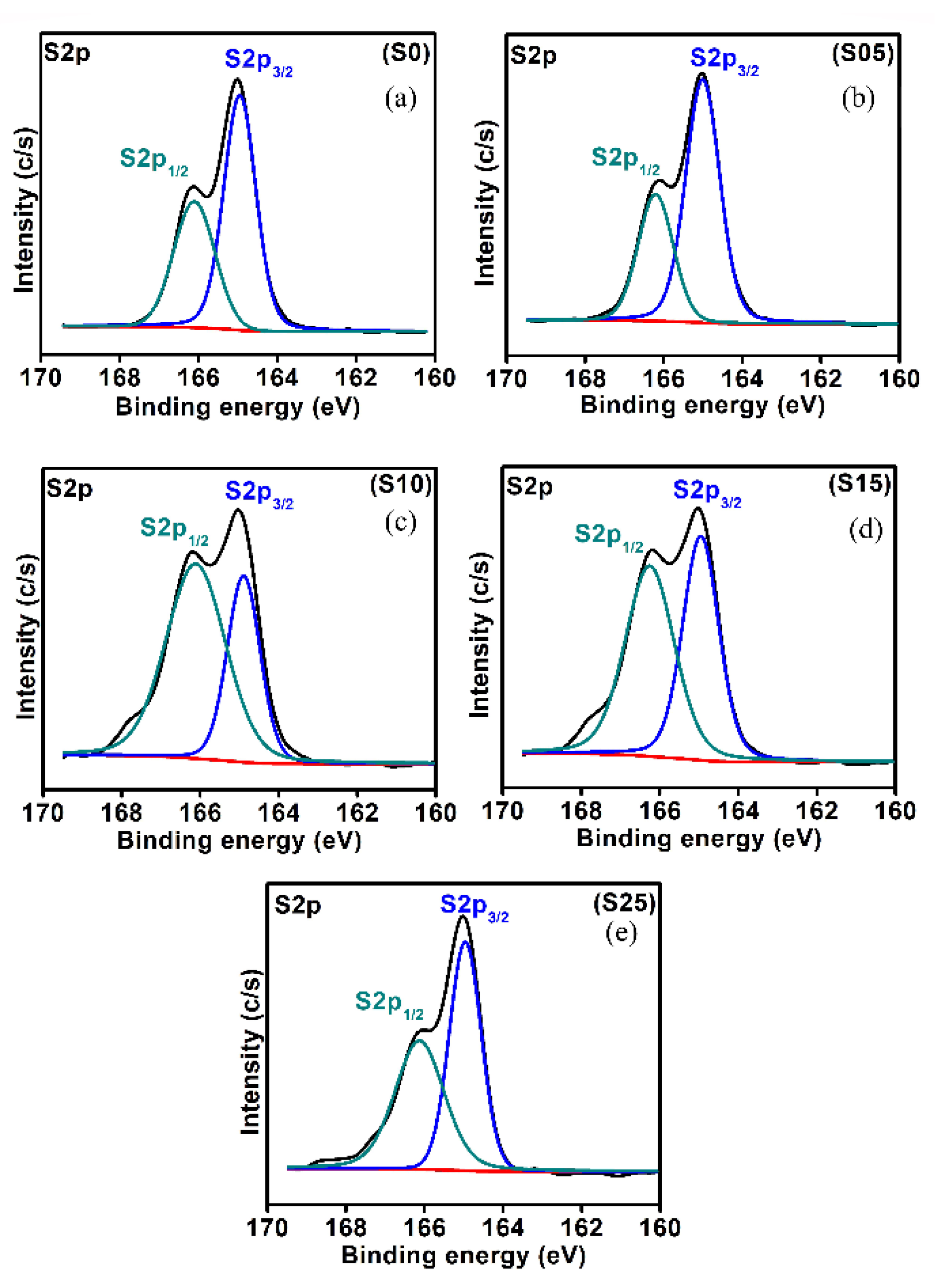
4.4. X-ray Photoelectron Spectroscopy (XPS)
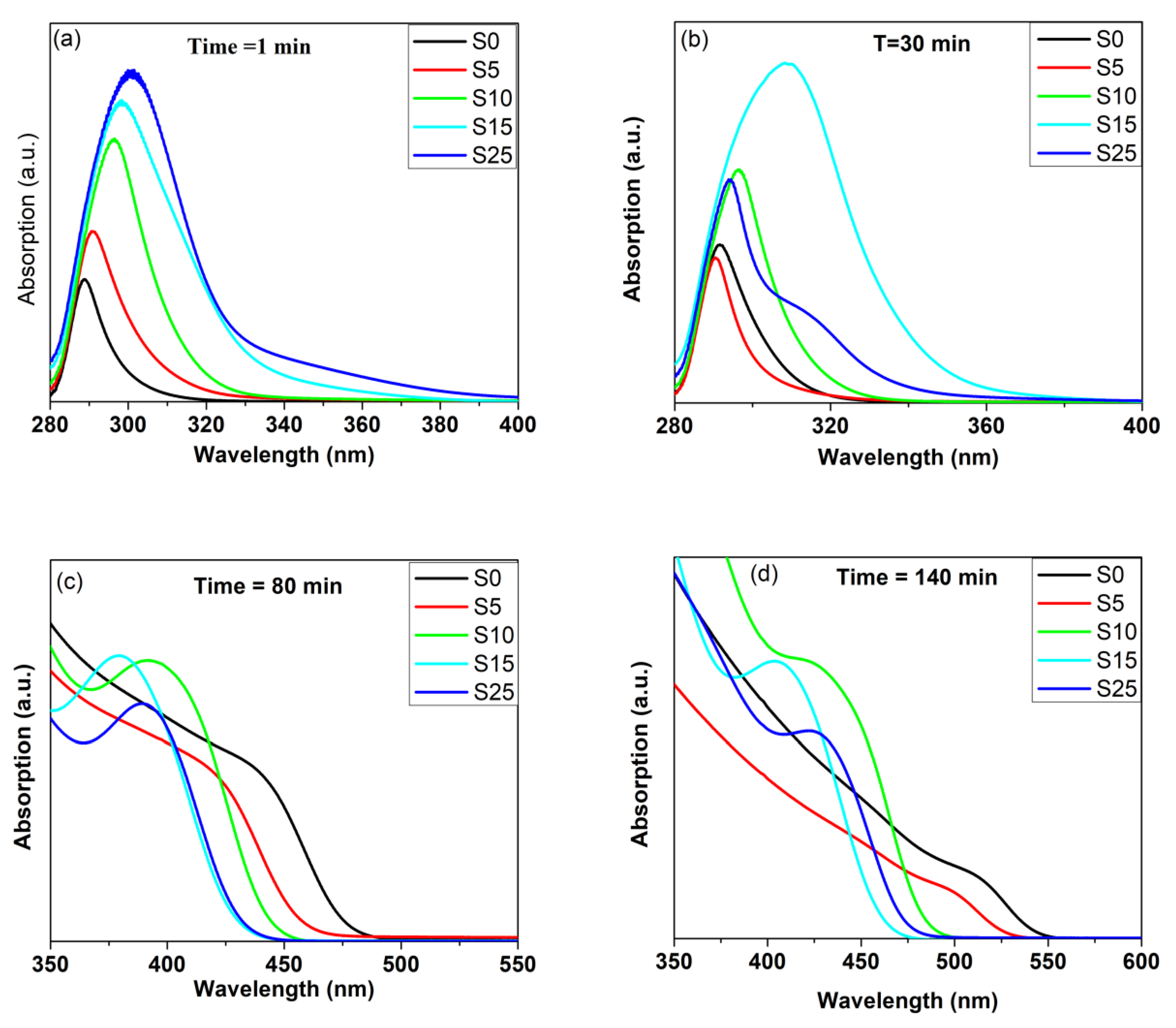
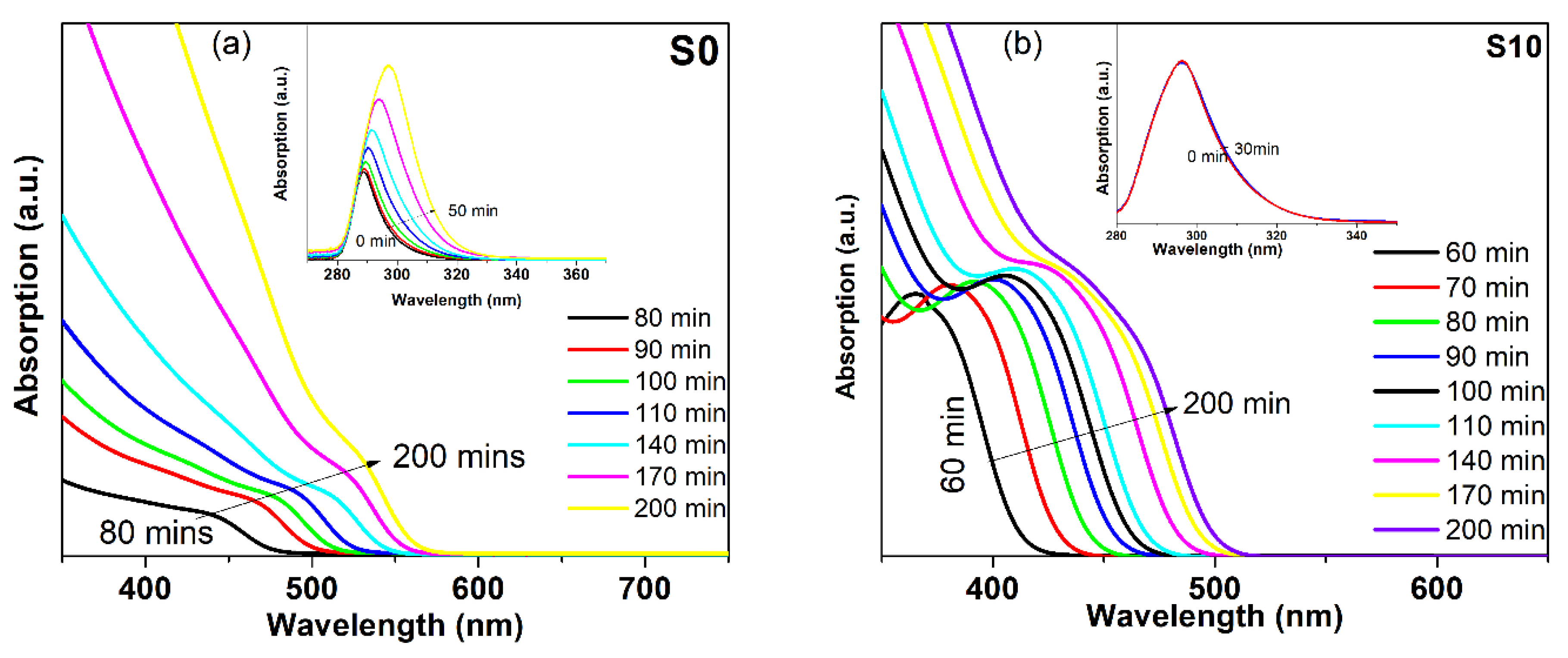
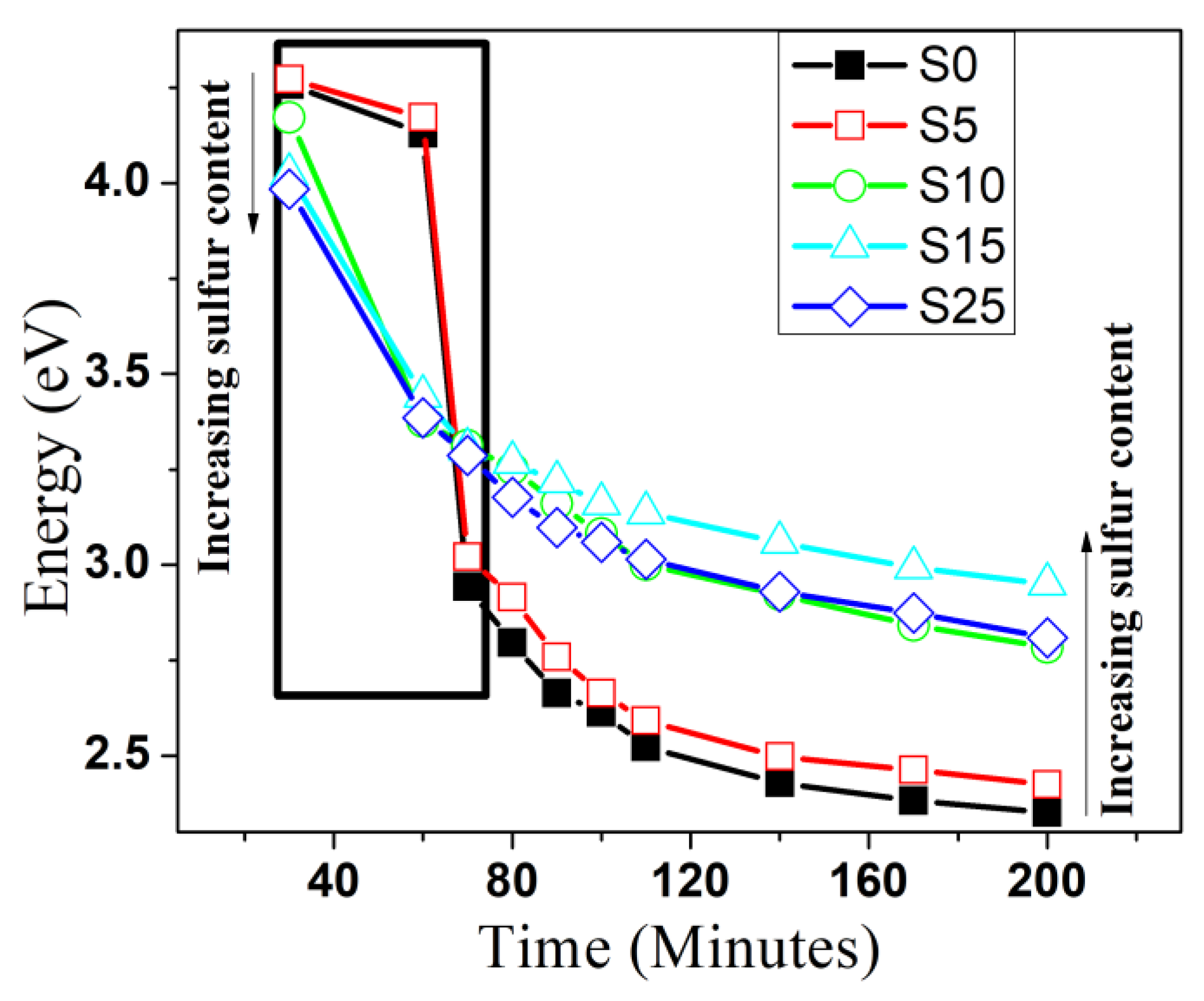
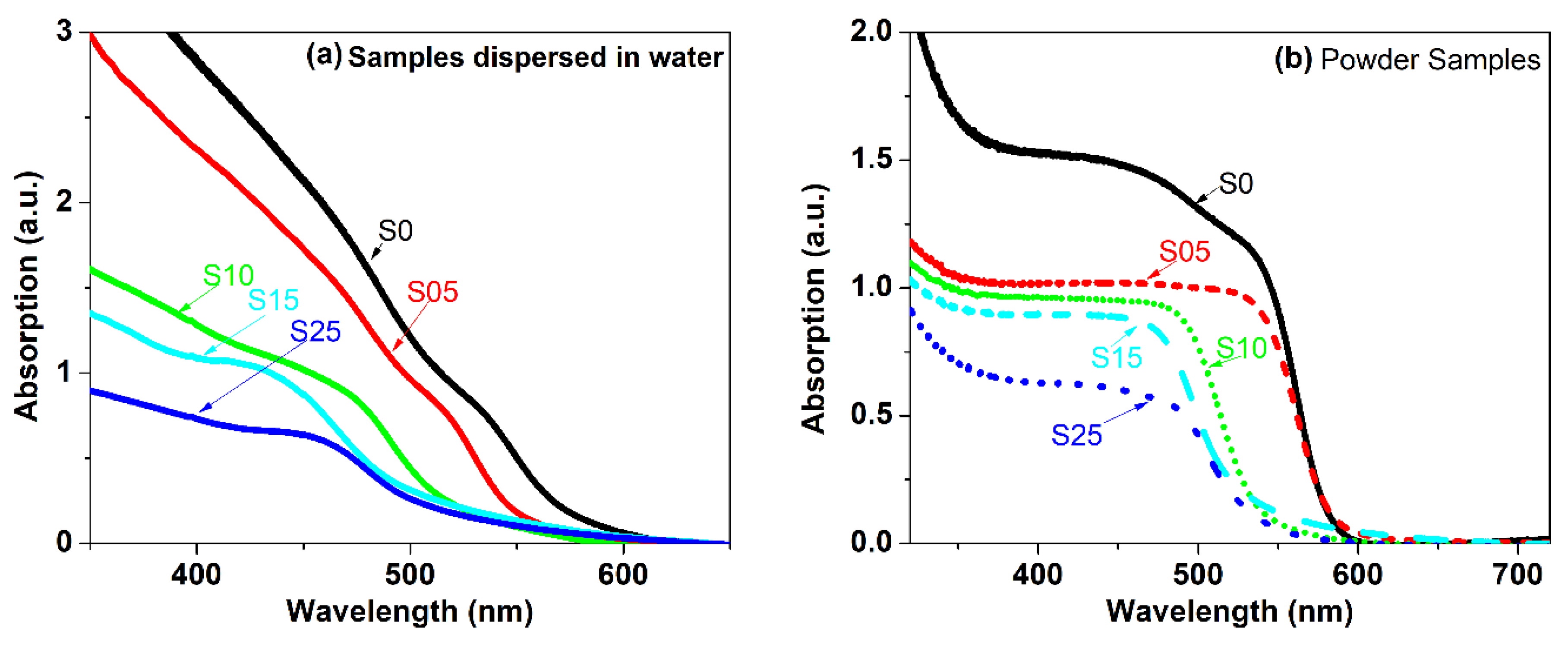
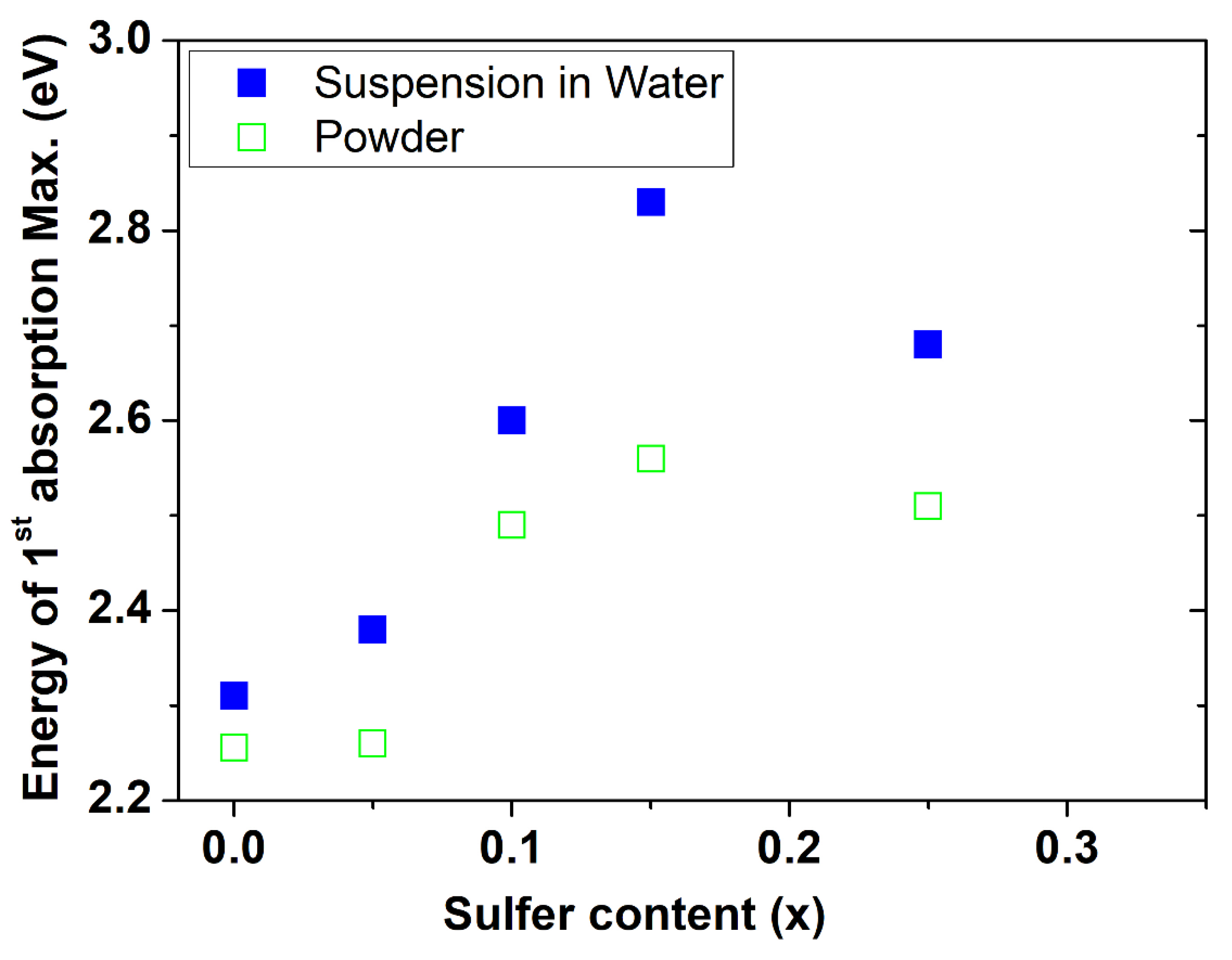
4.5. UV-Vis Absorption
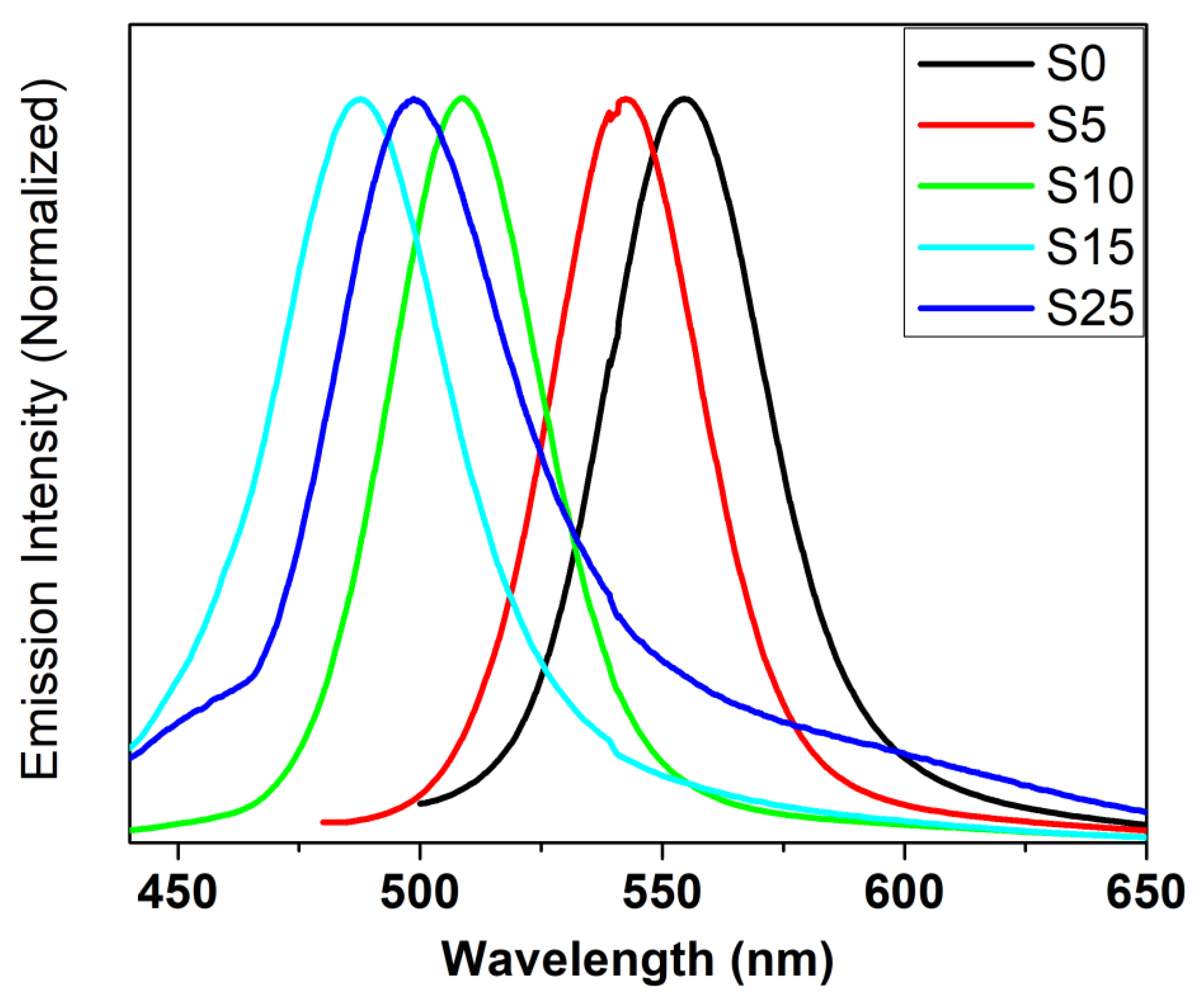
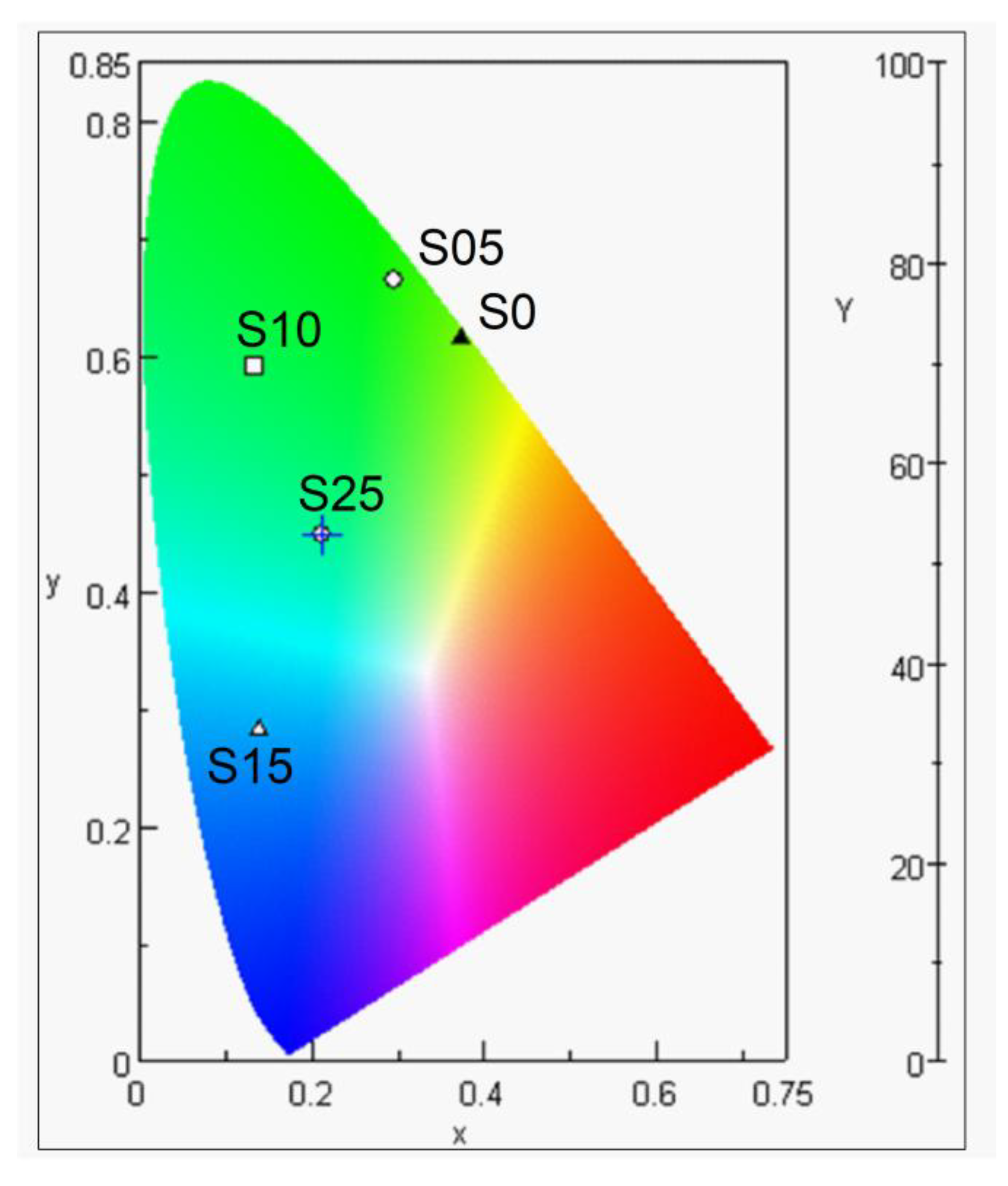
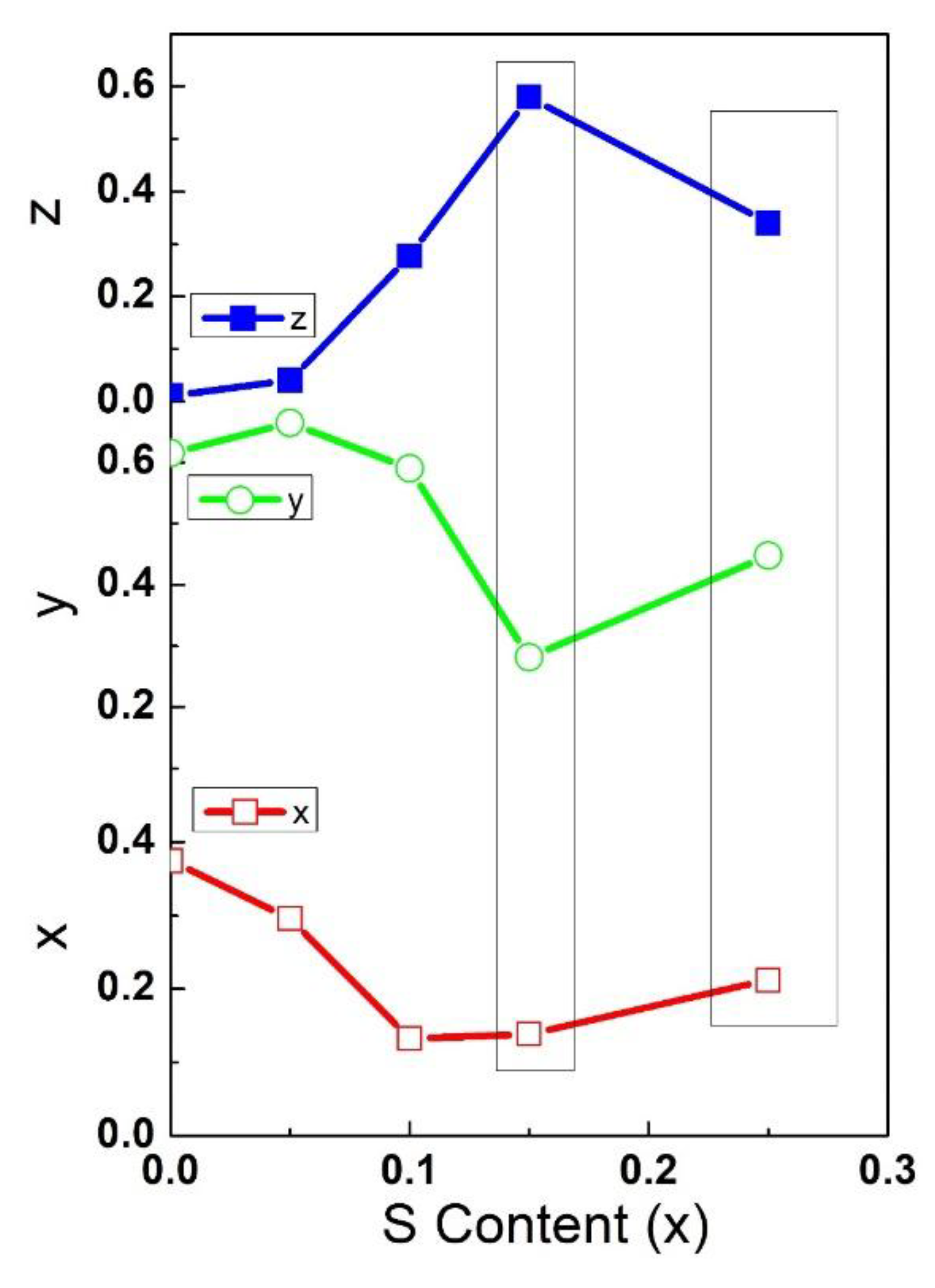
4.6. Luminescence
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rogach, A.L. Semiconductor Nanocrystal Quantum Dots Synthesis, Assembly, Spectroscopy and Applications; Springer: Wien, Austria; New York, NY, USA, 2008. [Google Scholar]
- Koole, R.; Groeneveld, E.; Vanmaekelbergh, D.; Meijerink, A.; Donegá, C.D. Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Zheng, H.M.; Wu, Y.; Alivisatos, A.P. Photovoltaic Devices Employing Ternary PbSxSe1-x Nanocrystals. Nano Lett. 2009, 9, 1699–1703. [Google Scholar]
- Eck, M.; Krueger, M. Correlation between CdSe QD Synthesis, Post-Synthetic Treatment, and BHJ Hybrid Solar Cell Performance. Nanomaterials 2016, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wu, B.; Guo, X.; Liu, X.; Rong, Z.; Liu, S.; Chen, Y.; Qin, D.; Xu, W.; Hou, L.; et al. Efficient CdTe Nanocrystal/TiO2 Hetero-Junction Solar Cells with Open Circuit Voltage Breaking 0.8 V by Incorporating a Thin Layer of CdS Nanocrystal. Nanomaterials 2018, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. CdS/graphene nanocomposite photocatalysts. Adv. Energy Mater. 2015, 5, 1500010. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liu, S.; Zhang, L. N-doped TiO2 Nanotubes as an Effective Additive to Improve the Catalytic Capability of Methanol Oxidation for Pt/Graphene Nanocomposites. Nanomaterials 2016, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Wageh, S.; Almazroai, L.S.; Alshahrie, A.; Al-Ghamdi, A.A. Enhanced Visible Light Photo-Catalytic Activity of ZnO and Ag-Doped ZnO (ZnO:Ag) Nanoparticles. J. Nanosci. Nanotechnol. 2018, 18, 7682–7690. [Google Scholar] [CrossRef]
- Prasad, S.; AlHesseny, H.S.; AlSalhi, M.S.; Devaraj, D.; Masilamai, V. A High Power, Frequency Tunable Colloidal Quantum Dot (CdSe/ZnS) Laser. Nanomaterials 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Vidor, F.F.; Meyers, T.; Hilleringmann, U. Inverter Circuits Using ZnO Nanoparticle Based Thin-Film Transistors for Flexible Electronic Applications. Nanomaterials 2016, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrsensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Zhang, Y.; Lu, M.; Ji, C.; Sun, C.; Chen, X.; Chen, H.; Colvin, V.L.; Yu, W.W. White Light-Emitting Diodes Based on AgInS2/ZnS Quantum Dots with Improved Bandwidth in Visible Light Communication. Nanomaterials 2016, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Wageh, S. Light Emitting Devices Based on CdSe Nanoparticles Capped with Mercaptoacetic Acid. IEEE J. Quantum Electron. 2014, 50, 741–748. [Google Scholar] [CrossRef]
- Rogach, A.L.; Harrison, M.T.; Kershaw, S.V.; Kornowski, A.; Burt, M.G.; Eychmuller, A.; Weller, H. Colloidally Prepared CdHgTe and HgTe Quantum Dots with Strong Near-Infrared Luminescence. Phys. Status Solidi B 2001, 224, 153–158. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Fan, Y.; Zhao, D. Synthesis of N-acetyl-L-cysteine-capped ZnCdSe quantum dots via hydrothermal method and their characterization. Sci. Technol. Adv. Mater. 2014, 15, 055001. [Google Scholar] [CrossRef] [PubMed]
- Wageh, S.; Badr, M.H. Cd1-xZnxS Nanoparticles Stabilized by a Bifunctional Organic Molecule. Physica E 2008, 40, 2810–2813. [Google Scholar] [CrossRef]
- Al-Rasheedi, A.; Wageh, S.; Al-Zhrani, E.; Al-Ghamdi, A. Structural and optical properties of CdZnTe quantum dots capped with a bifunctional Molecule. J. Mater. Sci. Mater. Electron. 2017, 28, 9114–9125. [Google Scholar] [CrossRef]
- Ramírez-Herrera, D.E.; Rodríguez-Velázquez, E.; Alatorre-Meda, M.; Paraguay-Delgado, F.; Tirado-Guízar, A.; Pablo Taboada, P.; Pina-Luis, G. NIR-Emitting Alloyed CdTeSe QDs and Organic Dye Assemblies: A Nontoxic, Stable, and Efficient FRET System. Nanomaterials 2018, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Baslak, C.; Arslan, G.; Kus, M.Y. Removal of Rhodamine B from water by using CdTeSe quantum dot-cellulose membrane composites. RSC Adv. 2016, 6, 18549–18557. [Google Scholar] [CrossRef]
- Gurusinghe, N.P.; Hewa-Kasakarage, N.N.; Zamkov, M. Composition-tunable properties of CdSxTe1-x alloy nanocrystals. J. Phys. Chem. C 2008, 112, 12795–12800. [Google Scholar] [CrossRef]
- Wageh, S. Ternary ZnS:Te nanoparticles capped with 3-mercaptopropionic acid prepared in aqueous media. J. Mater. Sci. Mater. Electron. 2016, 27, 10877–10887. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jennings, J.R.; Mathews, N.; Wang, Q. Band engineered ternary solid solution CdSxSe1−x-sensitized mesoscopic TiO2 solar cells. Phys. Chem. Chem. Phys. 2012, 14, 7154–7161. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Myung, Y.; Choi, J.W.; Jang, D.M.; Lee, C.W.; Park, J.; Cha, E.H. Nb2O5 nanowire photoanode sensitized by a composition-tuned CdSxSe1−x shell. J. Mater. Chem. 2012, 22, 8413–8419. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, C.L.; Song, G.M.; Jin, X.; Xu, Z. In vivo skin penetration and metabolic path of quantum dots. Sci. China Life Sci. 2013, 56, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Elbaum, R.; Vega, S.; Hodes, G. Preparation and surface structure of nanocrystalline cadmium sulfide (Sulfoselenide) precipitated from dimethyl sulfoxide solutions. Chem. Mater. 2001, 13, 2272–2280. [Google Scholar] [CrossRef]
- Jang, F.; Jun, S.; Pu, L. High quality CdSeS nanocrystals synthesized by facile single injection process and their electroluminescence. Chem. Commun. 2003, 9, 2964–2965. [Google Scholar] [CrossRef]
- Qian, H.F.; Li, L.; Ren, J.C. One-step and rapid synthesis of high quality alloyed quantum dots (CdSe-CdS) in aqueous phase by microwave irradiation with controllable temperature. Mater. Res. Bull. 2005, 40, 1726–1736. [Google Scholar] [CrossRef]
- Swafford, L.A.; Weigand, L.A.; MJ Bowers, M.J.; McBride, J.R.; Rapaport, J.L.; Watt, T.L.; Dixit, S.K.; Feldman, L.C.; Rosenthal, S.J. Homogeneously alloyed CdSxSe1−x nanocrystals: Synthesis, characterization, and composition/size-dependent band gap. J. Am. Chem. Soc. 2006, 128, 12299–12306. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Y.; Vincent, M.; Kingston, D.; Descours, P.; Boivineau, T.; Zaman, M.B.; Wu, X.; Yu, K. Noninjection, one pot synthesis of photoluminescent colloidal homogeneously alloyed CdSeS quantumdots. J. Phys. Chem. C 2009, 113, 5193–5200. [Google Scholar] [CrossRef]
- Zou, Y.; Li, D.S.; Yang, D. Noninjection synthesis of CdS and alloyed CdSxSe1−x nanocrystals without nucleation initiators. Nanoscale Res. Lett. 2010, 5, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Unl¨u, C.; Tosun, G.U.; Sevim, S.; Özçelik, S. Developing a facile method for highly luminescent colloidal CdSxSe1−x ternary nanoalloys. J. Mater. Chem. C 2013, 1, 3026–3034. [Google Scholar] [CrossRef]
- Charles, Y.; Cao, Y.C.; Wang, J. One-Pot Synthesis of High-Quality Zinc-Blende CdS Nanocrystals. J. Am. Chem. Soc. 2004, 126, 14336–14337. [Google Scholar]
- Concina, I.; Natile, M.M.; Braga, A.; Vomiero, A.; Morandi, V.; Ortolani, L.; Ferroni, M.; Sberveglieri, G. One pot synthesis of bi-linker stabilised CdSe quantum dots. J. Phys. Conf. Ser. 2010, 245, 012067. [Google Scholar] [CrossRef]
- Khanna, P.K.; Srinivasa Rao, S.; Patil, K.R.; Singh, N.; Mehta, B.R. One-pot synthesis of oleic acid-capped cadmium chalcogenides (CdE: E = Se, Te) nano-crystals. J. Nanopart. Res. 2010, 12, 101–109. [Google Scholar] [CrossRef]
- Ouyang, J.; Kuijper, J.; Brot, S.; Kingston, D.; Wu, X.; Leek, D.M.; Hu, M.Z.; Ripmeester, J.A.; Yu, K. Photoluminescent Colloidal CdS Nanocrystals with High Quality via Noninjection One-Pot Synthesis in 1-Octadecene. J. Phys. Chem. C 2009, 113, 7579–7593. [Google Scholar] [CrossRef]
- Yu, K.; Ouyang, J.; Zaman, M.B.; Johnston, D.; Yan, F.J.; Li, G.; Ratcliffe, C.I.; Leek, D.M.; Wu, X.; Stupak, J.; et al. Single-Sized CdSe Nanocrystals with Bandgap Photoemission via a Noninjection One-Pot Approach. J. Phys. Chem. C 2009, 113, 3390–3401. [Google Scholar] [CrossRef]
- Jia, J.; Tian, J.; Mi, W.; Tian, W.; Liu, X.; Dai, J.; Wang, X. Growth kinetics of CdSe nanocrystals synthesized in liquid paraffin via one-pot method. J. Nanopart. Res. 2013, 15, 1724. [Google Scholar] [CrossRef]
- Chen, X.; Lou, Y.; Burda, C. Spectroscopic investigation of II.VI core-shell nanoparticles: CdSe/CdS. Int. J. Nanotechnol. 2004, 1, 105–118. [Google Scholar] [CrossRef]
- Mekis, I.; Talapin, D.V.; Kornowski, A.; Haase, M.; Weller, H. One-Pot Synthesis of Highly Luminescent CdSe/CdS Core-Shell Nanocrystals via Organometallic and “Greener” Chemical Approaches. J. Phys. Chem. B 2003, 107, 7454–7462. [Google Scholar] [CrossRef]
- Niu, Y.; Pu, C.; Lai, R.; Meng, R.; Lin, W.; Qin, H.; Peng, X. One-pot/three-step synthesis of zinc-blende CdSe/CdS core/shell nanocrystals with thick shells. Nano Res. 2017, 10, 1149–1162. [Google Scholar] [CrossRef]
- Nguyen, T.-L.; Michael, M.; Mulvaney, P. Synthesis of Highly Crystalline CdSe@ZnO Nanocrystals via Monolayer-by-Monolayer Epitaxial Shell Deposition. Chem. Mater. 2014, 26, 4274–4279. [Google Scholar] [CrossRef]
- Lim, J.; Jun, S.; Jang, E.; Baik, H.; Kim, H.; Cho, J. Preparation of Highly Luminescent Nanocrystals and Their Application to Light-Emitting Diodes. Adv. Mater. 2007, 19, 1927–1932. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, P.; Pan, K.; He, T.; He, X.; Zhou, C.; He, Y. One-pot aqueous-phase synthesis of ultra-small CdSe/CdS/CdZnS core–shell–shell quantum dots with high-luminescent efficiency and good stability. J. Nanopart. Res. 2013, 15, 1680. [Google Scholar] [CrossRef]
- AbouZeid, K.M.; Mohamed, M.B.; El-Shall, M.S. Self-organization of Au–CdSe hybrid nanoflowers at different length scales via bi-functional diamine linkers. J. Nanopart. Res. 2016, 18, 8. [Google Scholar] [CrossRef]
- Kong, L.; Chu, X.; Wang, C.; Yang, X.; Zhou, L. Non-injection and one-pot approach to CdSe: Eu3+ hybrid nanocrystals with tunable photoluminescence from green to red. J. Nanopart. Res. 2017, 19, 20. [Google Scholar] [CrossRef]
- Verma, S.K.; Verma, R.; Li, N.; Xiong, D.; Tian, S.; Xiang, W.; Zhang, Z.; Xie, Y.; Zhao, X. Fabrication and band engineering of Cu-doped CdSe0.6Te0.4-alloyed quantum dots for solar cells. Sol. Energy Mater. Sol. Cells 2016, 157, 161–170. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Zitoun, D.; Gedanken, A. One-Pot Synthesis and Characterization of Mn2+-Doped Wurtzite CdSe Nanocrystals Encapsulated with Carbon. J. Phys. Chem. C 2008, 112, 7624–7630. [Google Scholar] [CrossRef]
- Wang, R.; Calvignanello, O.; Ratcliffe, C.I.; Wu, X.; Leek, D.M.; Zaman, M.B.; Kingston, D.; Ripmeester, J.A.; Yu, K. Homogeneously-Alloyed CdTeSe Single-Sized Nanocrystals with Bandgap Photoluminescence. J. Phys. Chem. C 2009, 113, 3402–3408. [Google Scholar] [CrossRef]
- Rogach, A.L.; Katsikas, L.; Kornowski, A.; Su, D.; Eychmüller, A.; Weller, H. Synthesis and characterization of thiol-stabilized CdTe nanocrystals. Ber. Bunsenges. Phys. Chem. 1996, 100, 1772–1778. [Google Scholar] [CrossRef]
- Wageh, S. Raman and Photoluminescence Study of CdSe Nanoparticles Capped with a Bifunctional Molecule. Physica E 2007, 39, 8–14. [Google Scholar] [CrossRef]
- John, F.; William, F.; Peter, E.; Kenneth, D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics, Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Jilani, A.; Iqbal, J.; Rafique, S.; Abdel-wahab, M.S.; Jamil, Y.; Al-Ghamdi, A.A. Morphological, optical and X-ray photoelectron chemical state shift investigations of ZnO thin films. Optik 2016, 127, 6358–6365. [Google Scholar] [CrossRef]
- Katari JE, B.; Colvin, V.L.; Alivisatos, A.P. X-ray Photoelectron Spectroscopy of CdSe Nanocrystals with Applications to Studies of the Nanocrystal Surface. J. Phys. Chem. 1994, 98, 4109–4117. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Ghamdi1, A.A.; Yakuphanoglu, F. Band edge emission of ZnS nanoparticles prepared by excess of thiourea as a source of sulfur. J. Sol.-Gel. Sci. Technol. 2013, 66, 443–451. [Google Scholar] [CrossRef]
- Bruss, L.E. Zero-dimensional “excitons” in semiconductor clusters. IEEE J. Quantum Electron. 1986, 22, 1909–1914. [Google Scholar] [CrossRef]
- Takagahara, T. Effects of dielectric confinement and electron-hole exchange interaction on excitonic states in semiconductor quantum dots. Phys. Rev. B 1993, 47, 4569. [Google Scholar] [CrossRef]
- Laheld UE, H.; Einevoll, G.T. Excitons in CdSe quantum dots. Phys. Rev. B 1997, 55, 5184–5204. [Google Scholar] [CrossRef]
- Wei, S.-H.; Zhang, S.B.; Zunger, A. First-principles calculation of band offsets, optical bowings, and defects in CdS, CdSe, CdTe, and their alloys. J. Appl. Phys. 2000, 87, 1304–1311. [Google Scholar] [CrossRef]
- Mane, R.S.; Lokhande, C.D. Studies on chemically deposited cadmium sulphoselenide (CdSSe) films. Thin Solid Films 1997, 304, 56–60. [Google Scholar] [CrossRef]
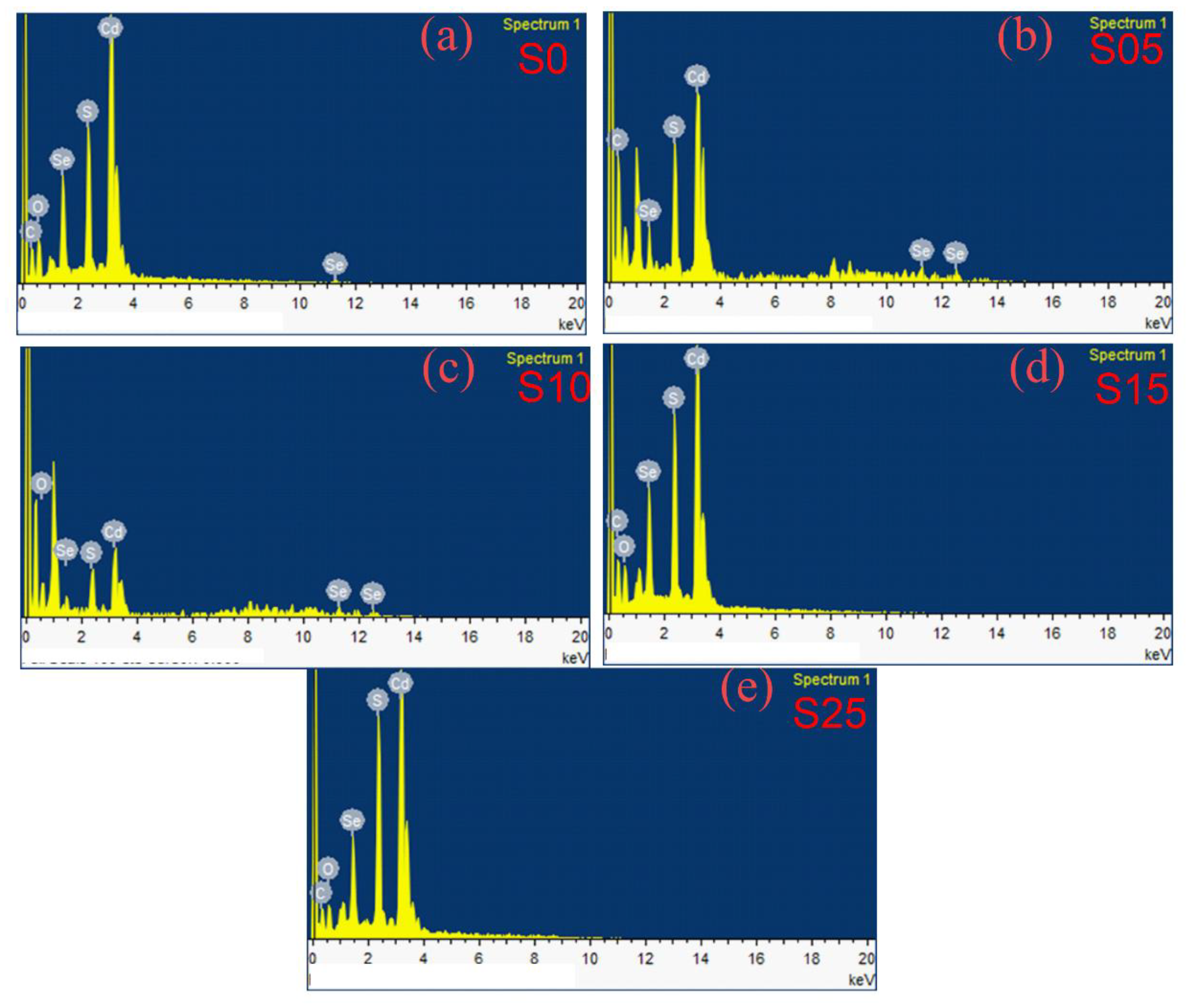
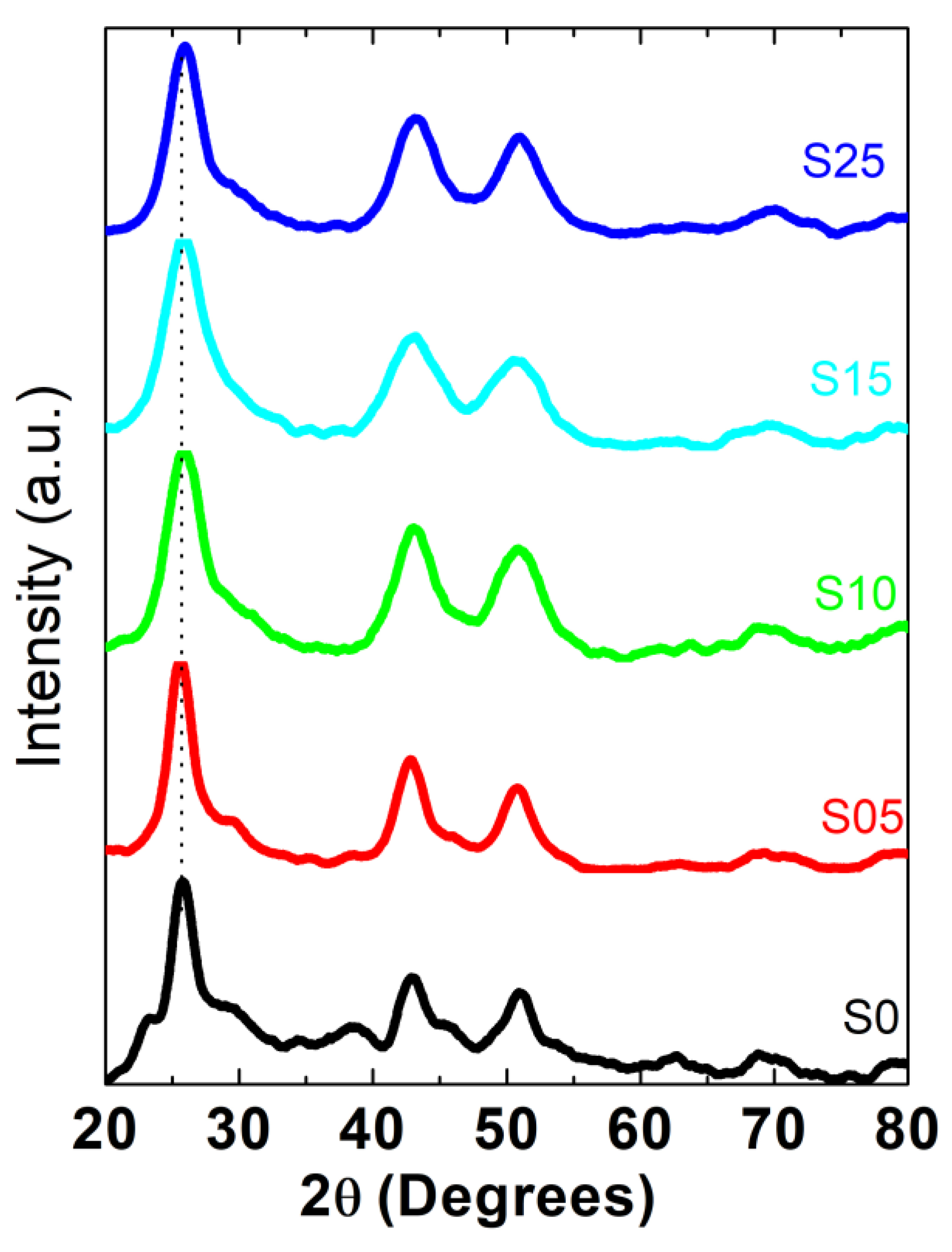
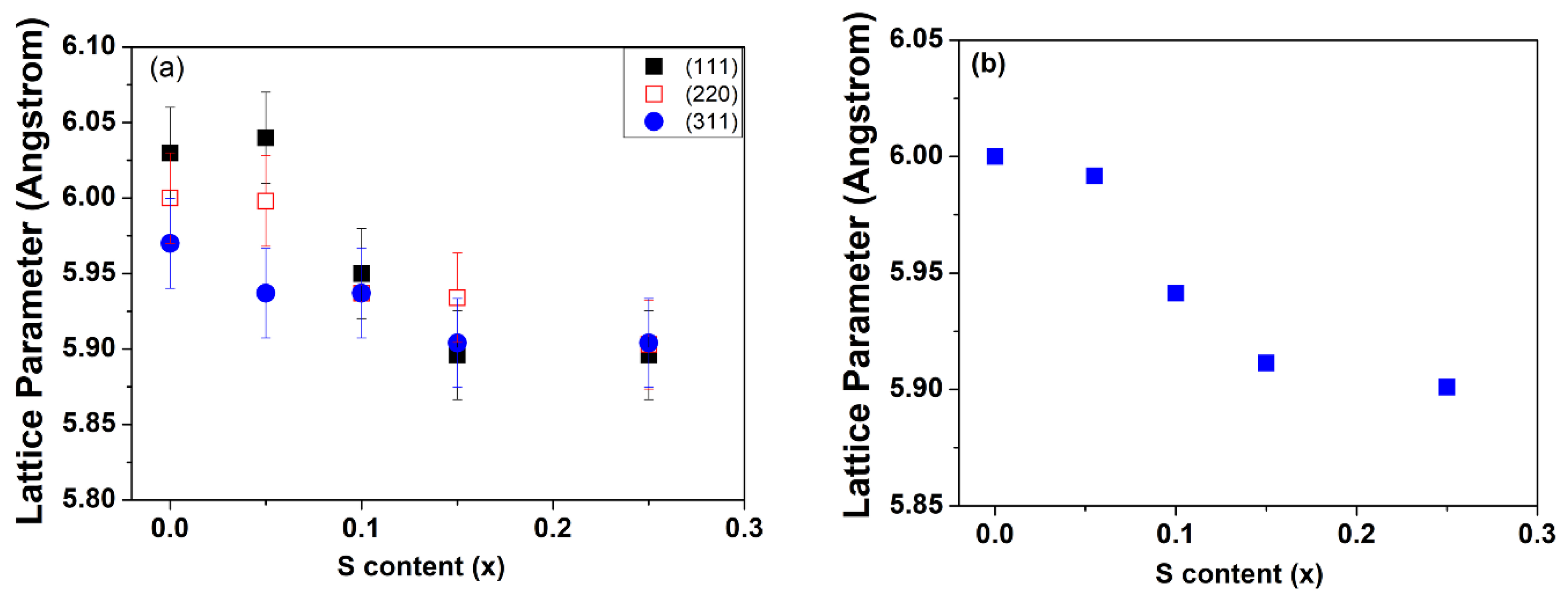
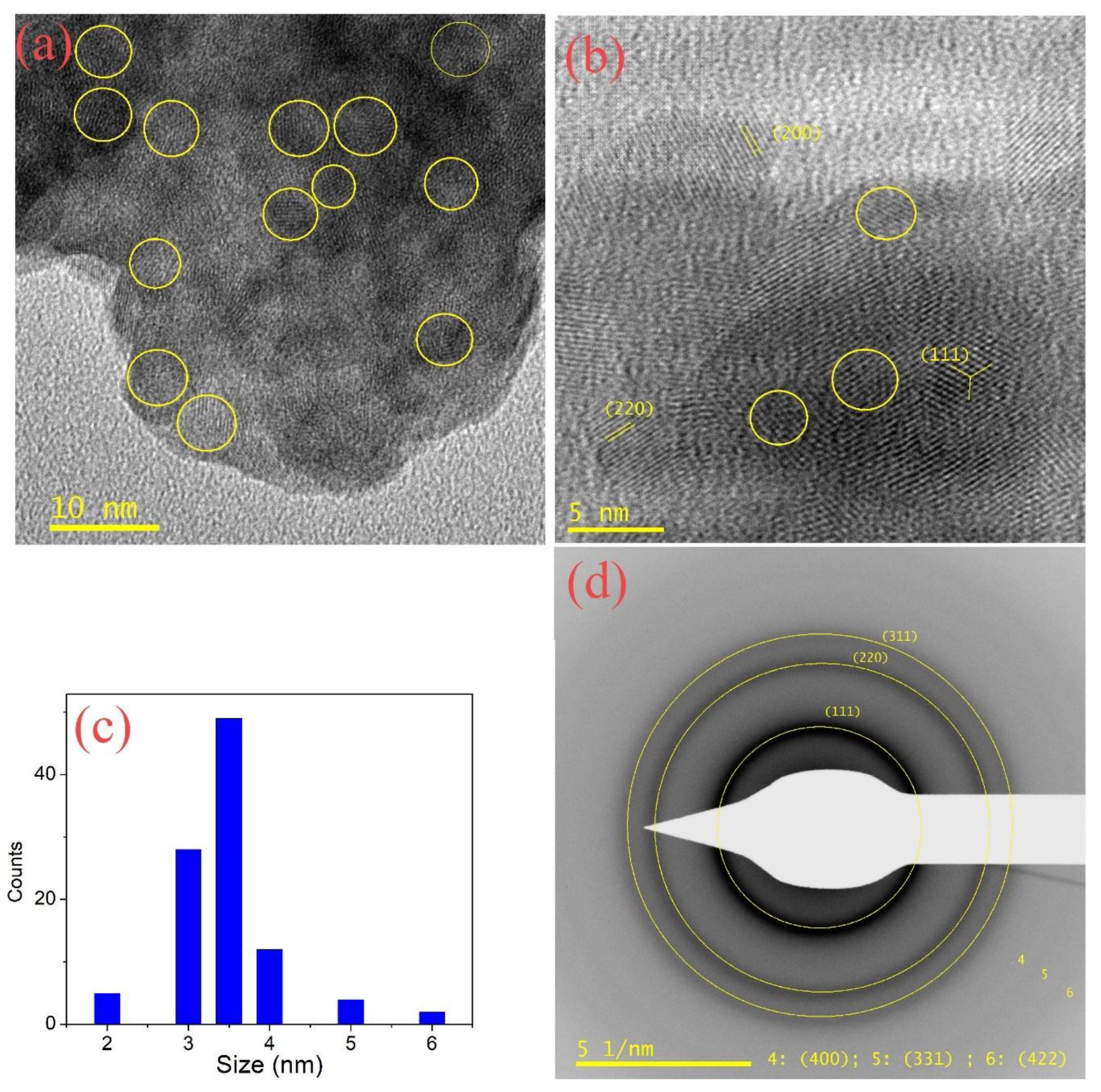
| Sample | S/Se + S | S/Se + S | Band Gap | Diameter (nm) | Diameter (nm) | Bowing Parameter |
|---|---|---|---|---|---|---|
| Precursors | EDX | (eV) | X-ray | UV-Vis | ||
| S0 | 0 | 0.139 | 2.26 | 3.75 | 3.65 | 0 |
| S05 | 0.05 | 0.183 | 2.26 | 3.80 | 3.80 | 0.2 |
| S10 | 0.1 | 0.190 | 2.49 | 2.44 | 2.40 | 1 |
| S15 | 0.15 | 0.196 | 2.56 | 2.10 | 2.13 | 1.3 |
| S25 | 0.25 | 0.211 | 2.51 | 2.37 | 2.37 | 1.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wageh, S.; Al-Ghamdi, A.; Jilani, A.; Iqbal, J. Facile Synthesis of Ternary Alloy of CdSe1-xSx Quantum Dots with Tunable Absorption and Emission of Visible Light. Nanomaterials 2018, 8, 979. https://doi.org/10.3390/nano8120979
Wageh S, Al-Ghamdi A, Jilani A, Iqbal J. Facile Synthesis of Ternary Alloy of CdSe1-xSx Quantum Dots with Tunable Absorption and Emission of Visible Light. Nanomaterials. 2018; 8(12):979. https://doi.org/10.3390/nano8120979
Chicago/Turabian StyleWageh, S., Ahmed Al-Ghamdi, Asim Jilani, and Javed Iqbal. 2018. "Facile Synthesis of Ternary Alloy of CdSe1-xSx Quantum Dots with Tunable Absorption and Emission of Visible Light" Nanomaterials 8, no. 12: 979. https://doi.org/10.3390/nano8120979
APA StyleWageh, S., Al-Ghamdi, A., Jilani, A., & Iqbal, J. (2018). Facile Synthesis of Ternary Alloy of CdSe1-xSx Quantum Dots with Tunable Absorption and Emission of Visible Light. Nanomaterials, 8(12), 979. https://doi.org/10.3390/nano8120979






