Effect of SiO2 Nanoparticles on the Performance of PVdF-HFP/Ionic Liquid Separator for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Preparation of the PSiCIL Separators
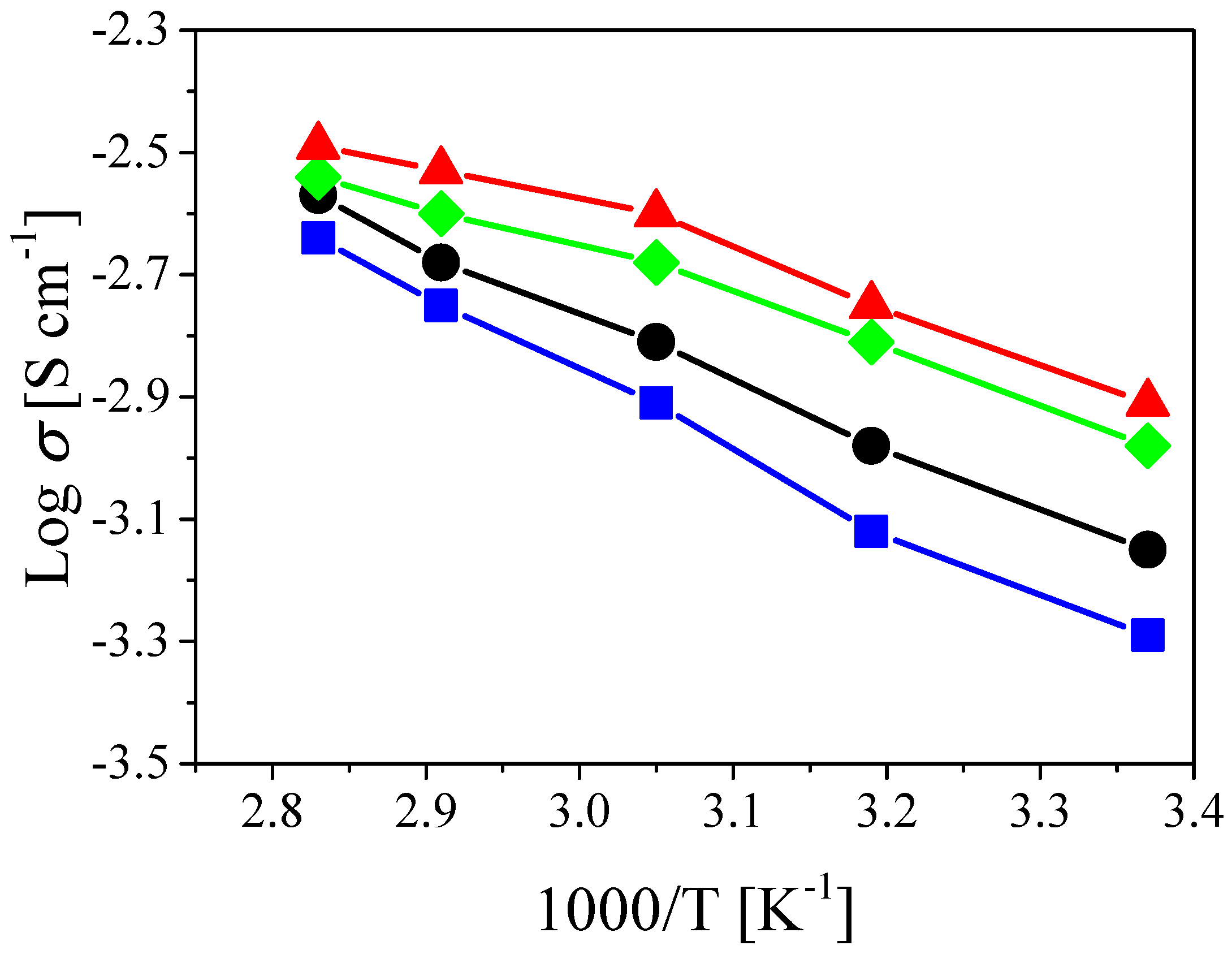
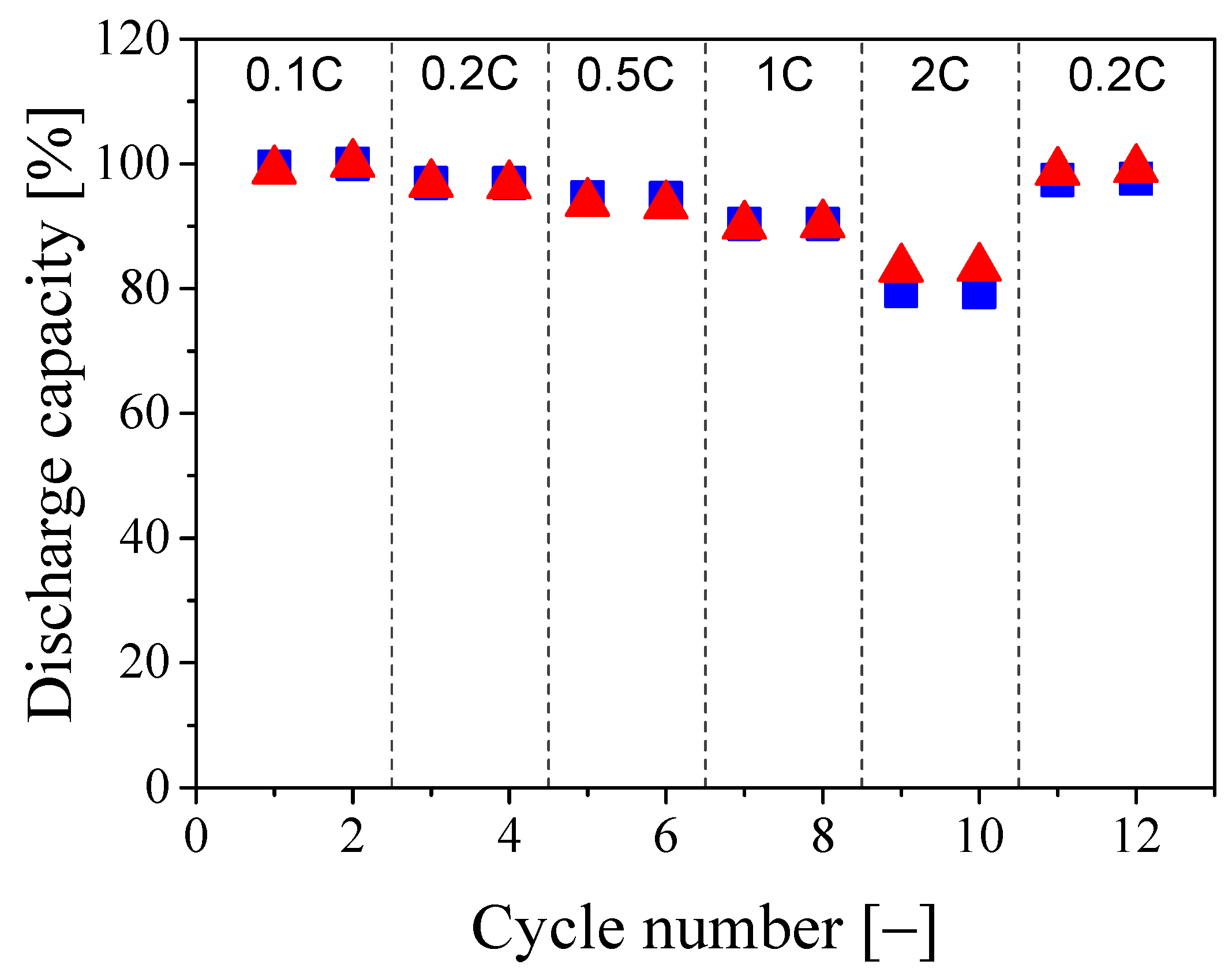
3.2. Electrochemical Properties of the PSiCIL Separators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y.K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation-approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: state of the Art and Perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef] [PubMed]
- Hammami, A.; Raymond, N.; Armand, M. Lithium-ion batteries: Runaway risk of forming toxic compounds. Nature 2003, 424, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Moganty, S.S.; Schaefer, J.L.; Archer, L.A. Ionic liquid-nanoparticle hybrid electrolytes. J. Mater. Chem. 2012, 22, 4066–4072. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Grugeon, S.; Laruelle, S.; Boyanov, S.; Lecocq, A.; Bertrand, J.P.; Marlair, G. In-depth safety-focused analysis of solvents used in electrolytes for large scale lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 9145–9155. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Tsao, C.H.; Hsu, C.H.; Chen, S.T.; Hsu, H.M. A new strategy for preparing oligomeric ionic liquid gel polymer electrolytes for high-performance and nonflammable lithium ion batteries. J. Membr. Sci. 2016, 499, 462–469. [Google Scholar] [CrossRef]
- Rollins, H.W.; Harrup, M.K.; Dufek, E.J.; Jamison, D.K.; Sazhin, S.V.; Gering, K.L.; Daubaras, D.L. Fluorinated phosphazene co-solvents for improved thermal and safety performance in lithium-ion battery electrolytes. J. Power Sources 2014, 263, 66–74. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Tachikawa, N.; Forsyth, M.; Pringle, J.M.; Howlett, P.C.; Elliott, G.D.; Davis, J.H.; Watanabe, M.; Simon, P.; Angell, C.A. Energy applications of ionic liquids. Energy Environ. Sci. 2014, 7, 232–250. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids-new “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, J.; Welton, T.; Armstrong, D.W. Characterizing ionic liquids on the basis of multiple solvation interactions. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Borgel, V.; Markevich, E.; Aurbach, D.; Semrau, G.; Schmidt, M. On the application of ionic liquids for rechargeable Li batteries: High voltage systems. J. Power Sources 2009, 189, 331–336. [Google Scholar] [CrossRef]
- Lux, S.F.; Schmuck, M.; Appetecchi, G.B.; Passerini, S.; Winter, M.; Balducci, A. Lithium insertion in graphite from ternary ionic liquid–lithium salt electrolytes: II. Evaluation of specific capacity and cycling efficiency and stability at room temperature. J. Power Sources 2009, 192, 606–611. [Google Scholar] [CrossRef]
- Schmitz, R.W.; Murmann, P.; Schmitz, R.; Müller, R.; Krämer, L.; Kasnatscheew, J.; Isken, P.; Niehoff, P.; Nowak, S.; Roeschenthaler, G.V. Investigations on novel electrolytes, solvents and SEI additives for use in lithium-ion batteries: systematic electrochemical characterization and detailed analysis by spectroscopic methods. Prog. Solid State Chem. 2014, 42, 65–84. [Google Scholar] [CrossRef]
- Di Leo, R.A.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Battery electrolytes based on saturated ring ionic liquids: Physical and electrochemical properties. Electrochim. Acta 2013, 109, 27–32. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; He, X.; Wang, J.; Liu, H.; Röser, S.; Rad, B.R.; Evertz, M.; Streipert, B.; Li, J.; Wagner, R. High voltage LiNi0.5Mn1.5O4/Li4Ti5O12 lithium ion cells at elevated temperatures: carbonate-versus ionic liquid-based electrolytes. ACS Appl. Mater. Interfaces 2016, 8, 25971–25978. [Google Scholar] [CrossRef] [PubMed]
- Nádherná, M.; Reiter, J.; Moškon, J.; Dominko, R. Lithium bis (fluorosulfonyl) imide-PYR14TFSI ionic liquid electrolyte compatible with graphite. J. Power Sources 2011, 196, 7700–7706. [Google Scholar] [CrossRef]
- Kalhoff, J.; Bresser, D.; Bolloli, M.; Alloin, F.; Sanchez, J.; Passerini, S. Enabling LiTFSI-based electrolytes for safer lithium-ion batteries by using linear fluorinated carbonates as (co)solvent. ChemSusChem 2014, 7, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, V.; Gnanaraj, J.; Madhavi, S.; Liu, H. Lithium-ion conducting electrolyte salts for lithium batteries. Chem. Eur. J. 2011, 17, 14326–14346. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.M.; Nahm, K. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Stolarska, M.; Niedzicki, L.; Borkowska, R.; Zalewska, A.; Wieczorek, W. Structure, transport properties and interfacial stability of PVdF/HFP electrolytes containing modified inorganic filler. Electrochim. Acta 2007, 53, 1512–1517. [Google Scholar] [CrossRef]
- Chung, S.; Wang, Y.; Persi, L.; Croce, F.; Greenbaum, S.; Scrosati, B.; Plichta, E. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J. Power Sources 2001, 97, 644–648. [Google Scholar] [CrossRef]
- Wachtler, M.; Ostrovskii, D.; Jacobsson, P.; Scrosati, B. A study on PVdF-based SiO2-containing composite gel-type polymer electrolytes for lithium batteries. Electrochim. Acta 2004, 50, 357–361. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Karthick, S.; Priya, A.S.; Ramalingam, S.; Subramania, A. Effect of nanoscale CeO2 on PVDF-HFP-based nanocomposite porous polymer electrolytes for Li-ion batteries. J. Solid State Electrochem. 2008, 12, 1135–1141. [Google Scholar] [CrossRef]
- Wu, C.G.; Lu, M.I.; Tsai, C.C.; Chuang, H.J. PVdF-HFP/metal oxide nanocomposites: the matrices for high-conducting, low-leakage porous polymer electrolytes. J. Power Sources 2006, 159, 295–300. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Kim, J.K.; Manuel, J.; Chauhan, G.S.; Ahn, J.H.; Nah, C. Ionic conductivity and electrochemical properties of nanocomposite polymer electrolytes based on electrospun poly (vinylidene fluoride-co-hexafluoropropylene) with nano-sized ceramic fillers. Electrochim. Acta 2008, 54, 228–234. [Google Scholar] [CrossRef]
- Raghavan, P.; Choi, J.W.; Ahn, J.H.; Cheruvally, G.; Chauhan, G.S.; Ahn, H.J.; Nah, C. Novel electrospun poly (vinylidene fluoride-co-hexafluoropropylene)-in situ SiO2 composite membrane-based polymer electrolyte for lithium batteries. J. Power Sources 2008, 184, 437–443. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456. [Google Scholar] [CrossRef]
- Jeong, H.S.; Lee, S.Y. Closely packed SiO2 nanoparticles/poly (vinylidene fluoride-hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2011, 196, 6716–6722. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: a Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Bishop, K.J.; Wilmer, C.E.; Soh, S.; Grzybowski, B.A. Nanoscale forces and their uses in self-assembly. Small 2009, 5, 1600–1630. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Deka, M. Nanofiber Reinforced Composite Polymer Electrolyte Membranes. In Nanofibers; INTECH: Rijeka, Croatia, 2010; pp. 13–38. [Google Scholar]
- Sheng, X.; Xie, D.; Zhang, X.; Zhong, L.; Wu, H.; Morbidelli, M. Uniform distribution of graphene oxide sheets into a poly-vinylidene fluoride nanoparticle matrix through shear-driven aggregation. Soft Matter 2016, 12, 5876–5882. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zaccone, A.; Tsoutsoura, A.; Lattuada, M.; Morbidelli, M. High shear-induced gelation of charge-stabilized colloids in a microchannel without adding electrolytes. Langmuir 2009, 25, 4715–4723. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lattuada, M.; Sandkühler, P.; Sefcik, J.; Morbidelli, M. Role of sedimentation and buoyancy on the kinetics of diffusion limited colloidal aggregation. Langmuir 2003, 19, 10710–10718. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S.; Kumar, T.P.; Kulandainathan, M.A.; Ravi, G.; Wilson, J. Nanofiller incorporated poly (vinylidene fluoride–hexafluoropropylene)(PVdF-HFP) composite electrolytes for lithium batteries. J. Power Sources 2006, 159, 1316–1321. [Google Scholar] [CrossRef]
- Caimi, S.; Wu, H.; Morbidelli, M. PVdF-HFP and Ionic Liquid-Based, Freestanding Thin Separator for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 5224–5232. [Google Scholar] [CrossRef]
- Caimi, S.; Cingolani, A.; Jaquet, B.; Siggel, M.; Lattuada, M.; Morbidelli, M. Tracking of fluorescently labeled polymer particles reveals surface effects during shear-controlled aggregation. Langmuir 2017, 33, 14038–14044. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, A.; Baur, D.; Caimi, S.; Storti, G.; Morbidelli, M. Preparation of Perfusive Chromatographic Materials via Shear-Induced Reactive Gelation. J. Chromatogr. A 2018, 1538, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Kumar, A. Ionic conduction in P(VDF-HFP)/PVDF-(PC+DEC)-LiClO4 polymer gel electrolytes. Electrochim. Acta 2004, 49, 2581–2589. [Google Scholar] [CrossRef]
- Meng, X.; Wu, H.; Storti, G.; Morbidelli, M. Effect of dispersed polymeric nanoparticles on the bulk polymerization of methyl methacrylate. Macromolecules 2016, 49, 7758–7766. [Google Scholar] [CrossRef]
- Li, Z.; Su, G.; Wang, X.; Gao, D. Micro-porous P(VDF-HFP)-based polymer electrolyte filled with Al2O3 nanoparticles. Solid State Ion. 2005, 176, 1903–1908. [Google Scholar] [CrossRef]
- Wieczorek, W.; Stevens, J.; Florjańczyk, Z. Composite polyether based solid electrolytes. The Lewis acid–base approach. Solid State Ion. 1996, 85, 67–72. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, J.; Hong, L. In situ preparation of poly (ethylene oxide)-SiO2 composite polymer electrolytes. J. Power Sources 2004, 129, 303–311. [Google Scholar] [CrossRef]
- Scrosati, B.; Croce, F.; Panero, S. Progress in lithium polymer battery R&D. J. Power Sources 2001, 100, 93–100. [Google Scholar]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar] [CrossRef]
- Ferrari, S.; Quartarone, E.; Mustarelli, P.; Magistris, A.; Fagnoni, M.; Protti, S.; Gerbaldi, C.; Spinella, A. Lithium ion conducting PVdF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis (trifluoromethanesulfonyl)-imide ionic liquid. J. Power Sources 2010, 195, 559–566. [Google Scholar] [CrossRef]
- Wu, F.; Chen, N.; Chen, R.; Zhu, Q.; Qian, J.; Li, L. “Liquid-in-Solid” and “Solid-in-Liquid” Electrolytes with High Rate Capacity and Long Cycling Life for Lithium-Ion Batteries. Chem. Mater. 2016, 28, 848–856. [Google Scholar] [CrossRef]
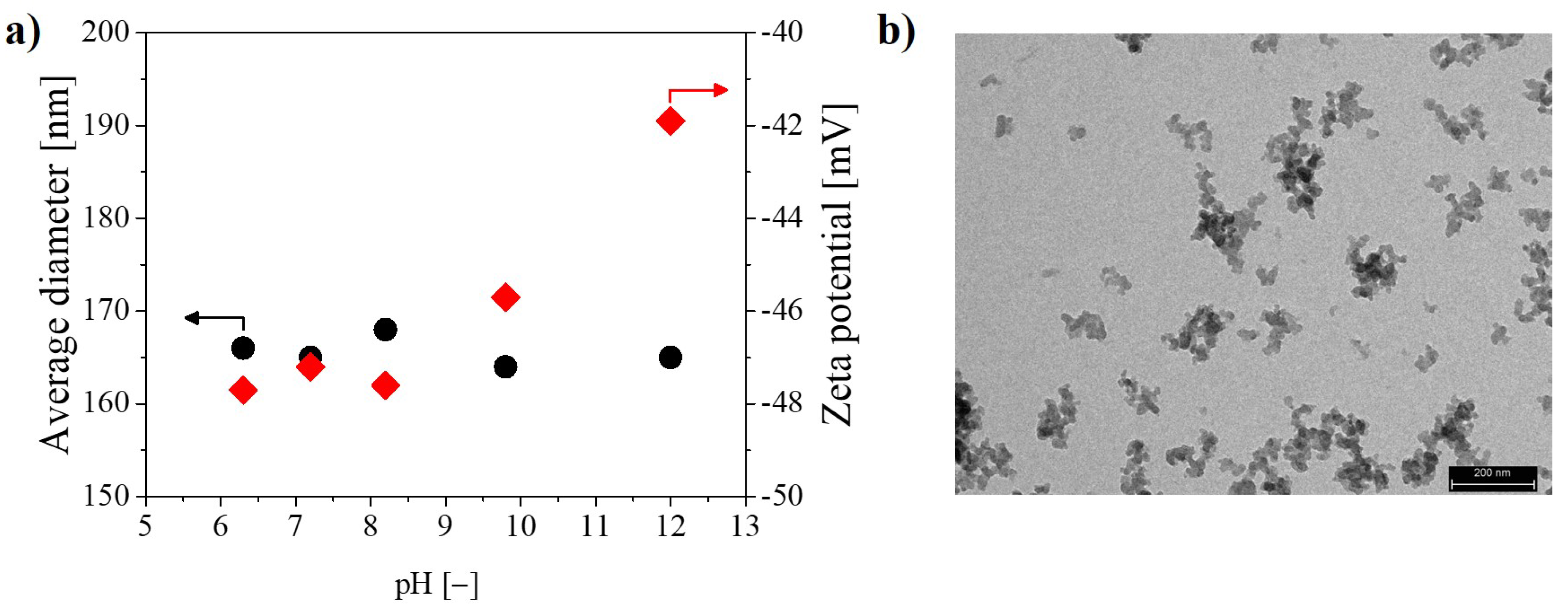
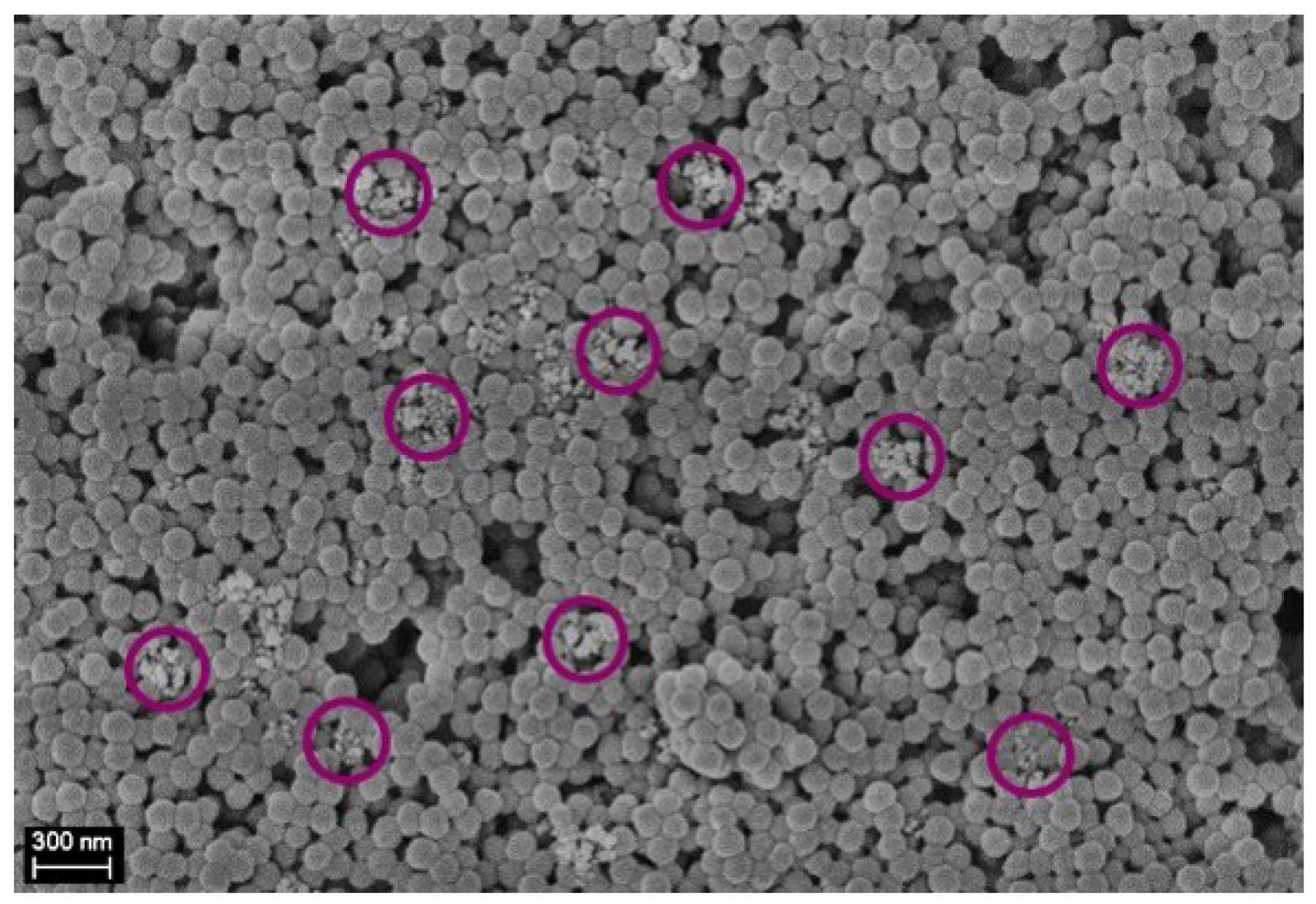

| Average Clusters Diameter [nm] | PDI [−] | Zeta Potential [mV] | |
|---|---|---|---|
| Tixosil 365 | 160 | 0.19 | −47.9 |
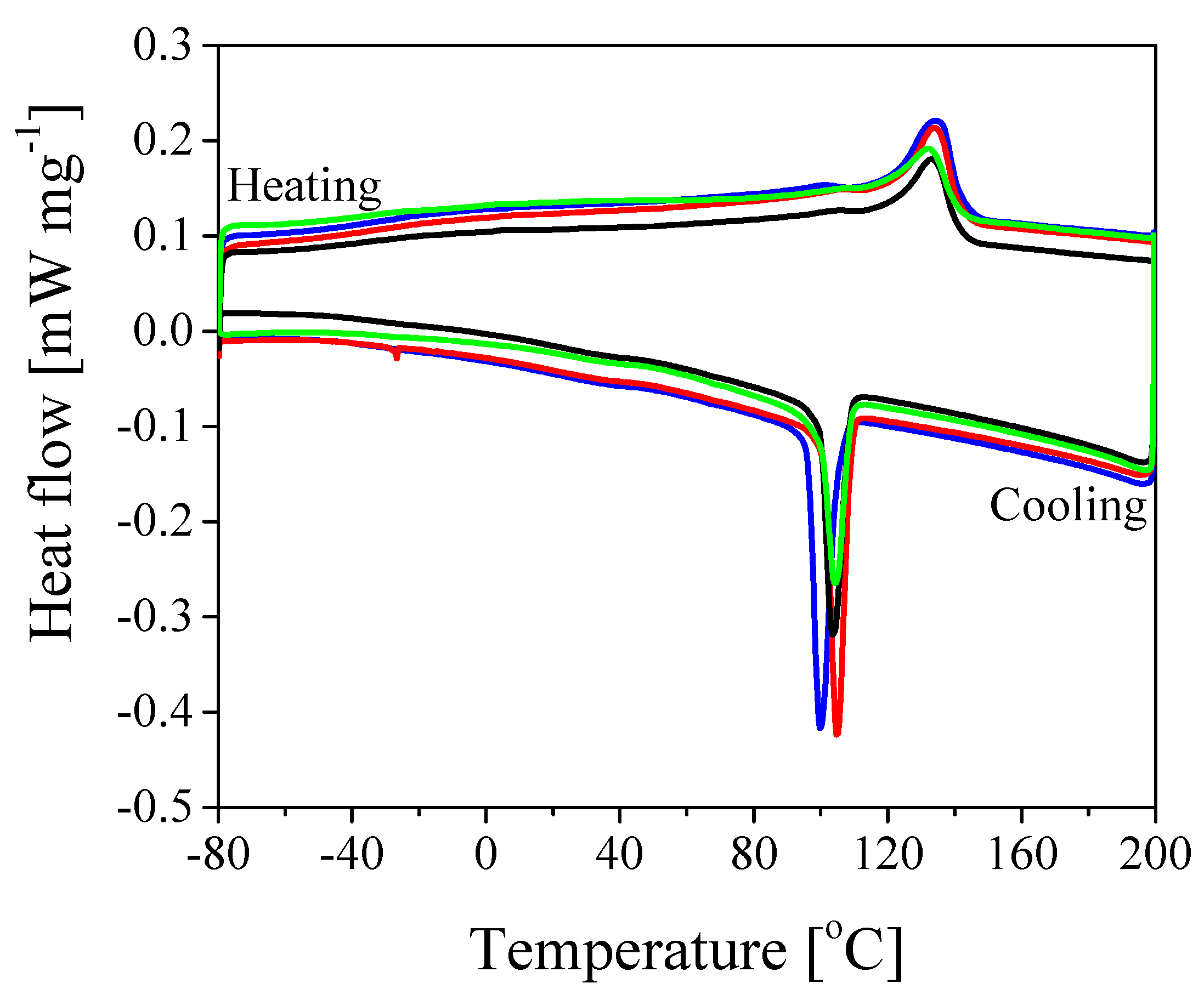
| T [C] | T [C] | ΔH [J g] | X [%] | |
|---|---|---|---|---|
| Pure | 133.6 | 97.6 | 22.19 | 21.2 |
| 10% SiO | 132.8 | 100 | 18.21 | 17.4 |
| 20% SiO | 130.5 | 99.7 | 16.92 | 16.2 |
| 30% SiO | 130.6 | 99.6 | 15.66 | 14.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caimi, S.; Klaue, A.; Wu, H.; Morbidelli, M. Effect of SiO2 Nanoparticles on the Performance of PVdF-HFP/Ionic Liquid Separator for Lithium-Ion Batteries. Nanomaterials 2018, 8, 926. https://doi.org/10.3390/nano8110926
Caimi S, Klaue A, Wu H, Morbidelli M. Effect of SiO2 Nanoparticles on the Performance of PVdF-HFP/Ionic Liquid Separator for Lithium-Ion Batteries. Nanomaterials. 2018; 8(11):926. https://doi.org/10.3390/nano8110926
Chicago/Turabian StyleCaimi, Stefano, Antoine Klaue, Hua Wu, and Massimo Morbidelli. 2018. "Effect of SiO2 Nanoparticles on the Performance of PVdF-HFP/Ionic Liquid Separator for Lithium-Ion Batteries" Nanomaterials 8, no. 11: 926. https://doi.org/10.3390/nano8110926
APA StyleCaimi, S., Klaue, A., Wu, H., & Morbidelli, M. (2018). Effect of SiO2 Nanoparticles on the Performance of PVdF-HFP/Ionic Liquid Separator for Lithium-Ion Batteries. Nanomaterials, 8(11), 926. https://doi.org/10.3390/nano8110926






