Curcumin–Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Preparation and Characterization of CD Micelles
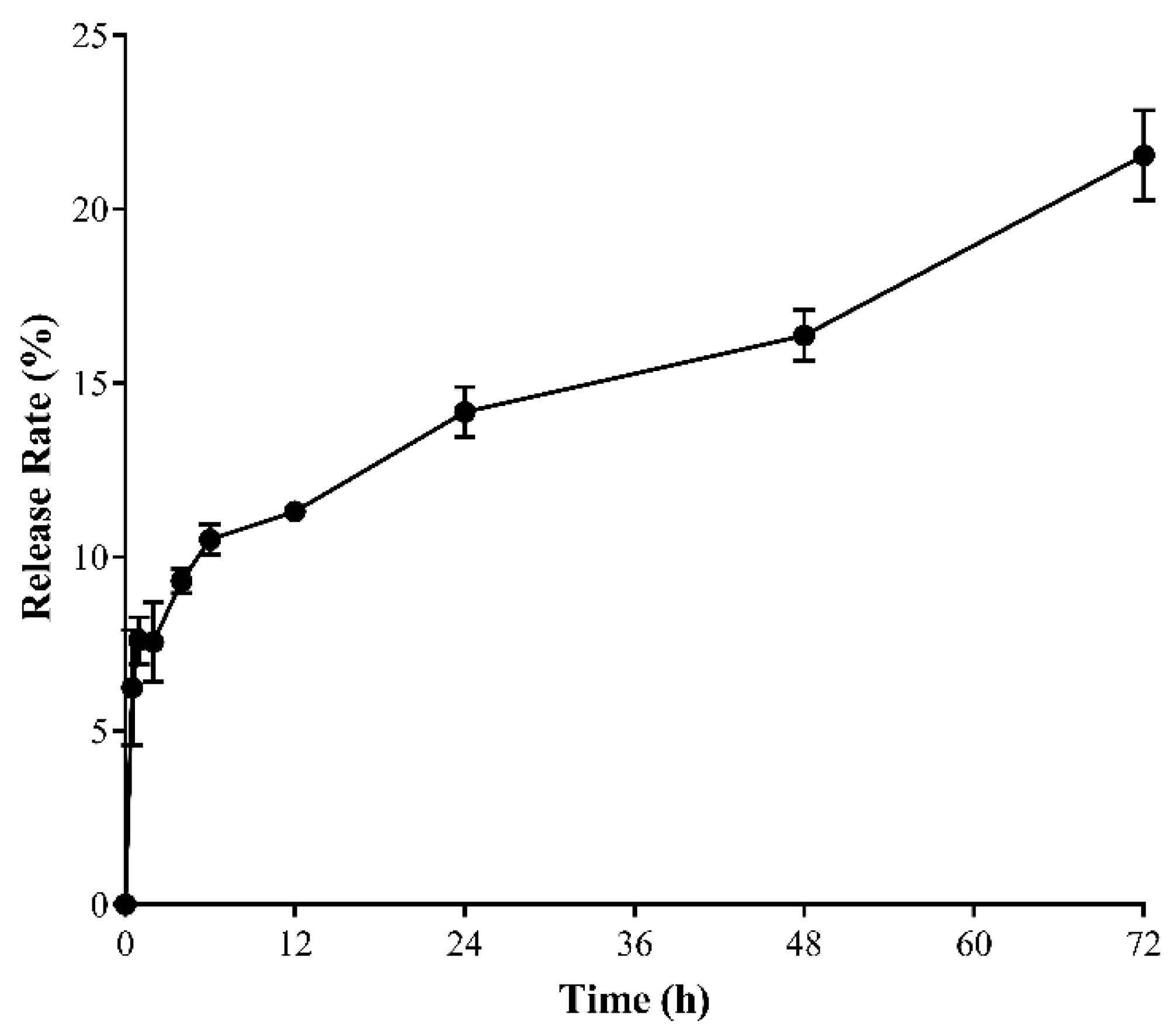
2.2. In Vitro SMA–CD Drug Release Studies
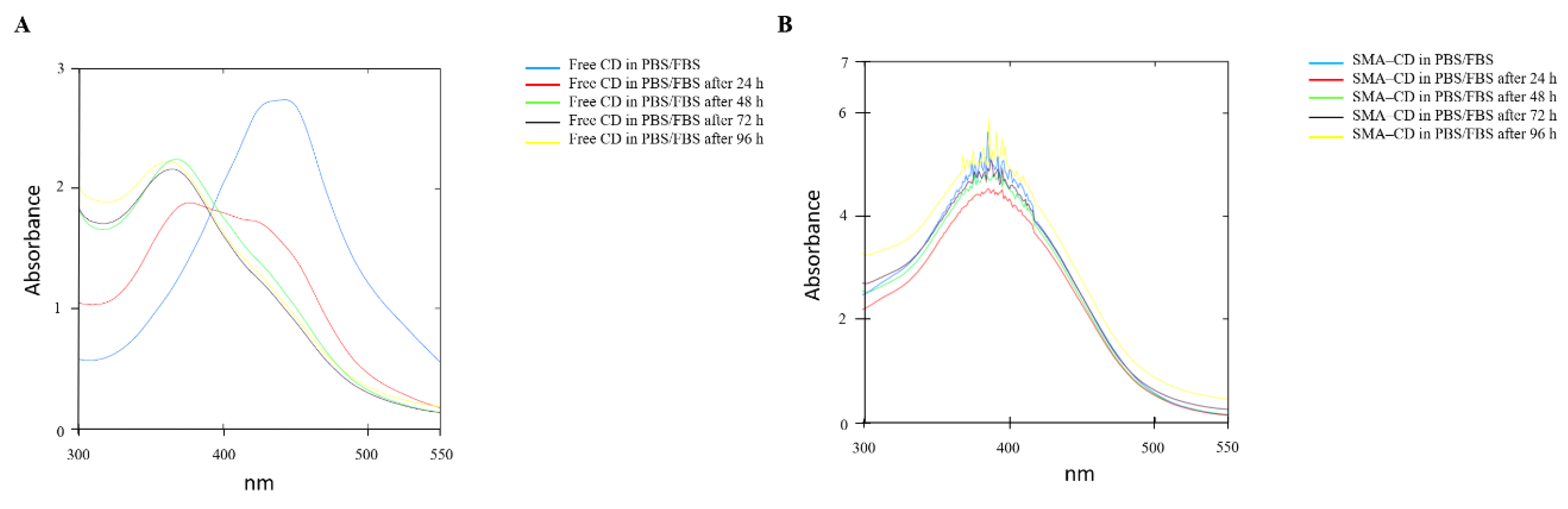
2.3. Drug Stability Studies of CD and SMA–-CD in Phosphate-Buffered Saline and PBS/Fetal Bovine Serum (FBS)
2.4. CD Interaction with Plasma Proteins
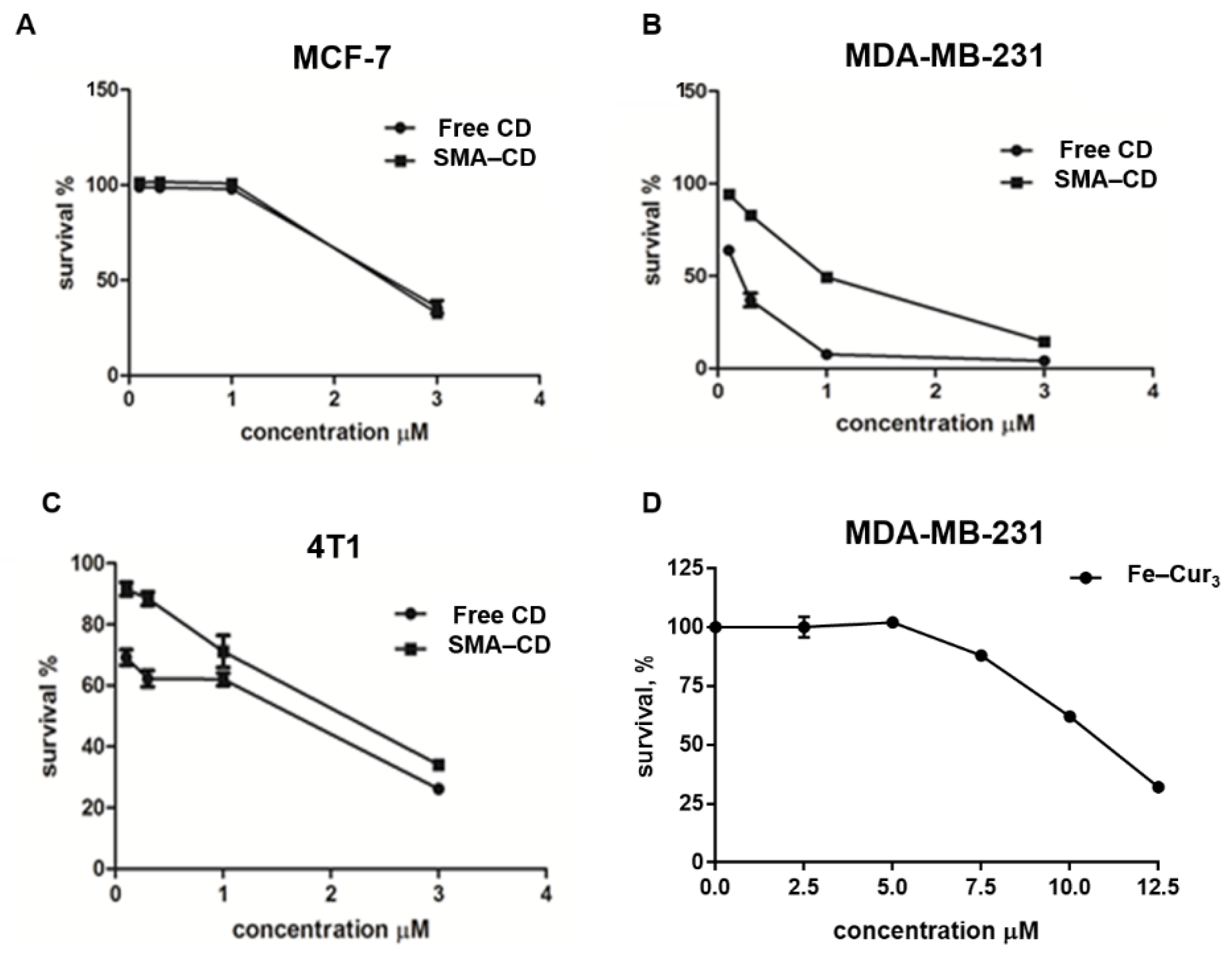
2.5. Cytotoxic Effect of Fe–Cur3, CD, and SMA–CD on Breast Cancer Cells
2.6. Maximum Tolerated Dose (MTD)
2.7. Biodistribution of CD and SMA–CD
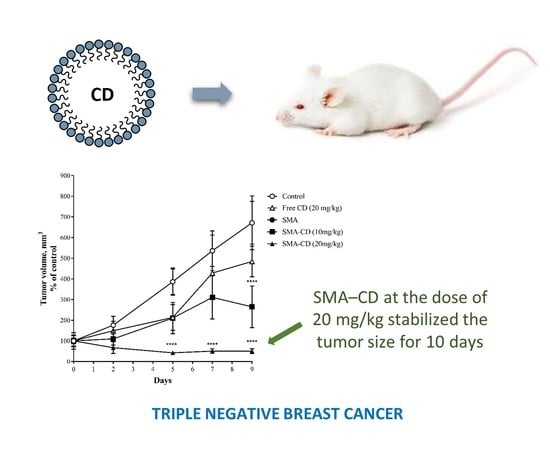
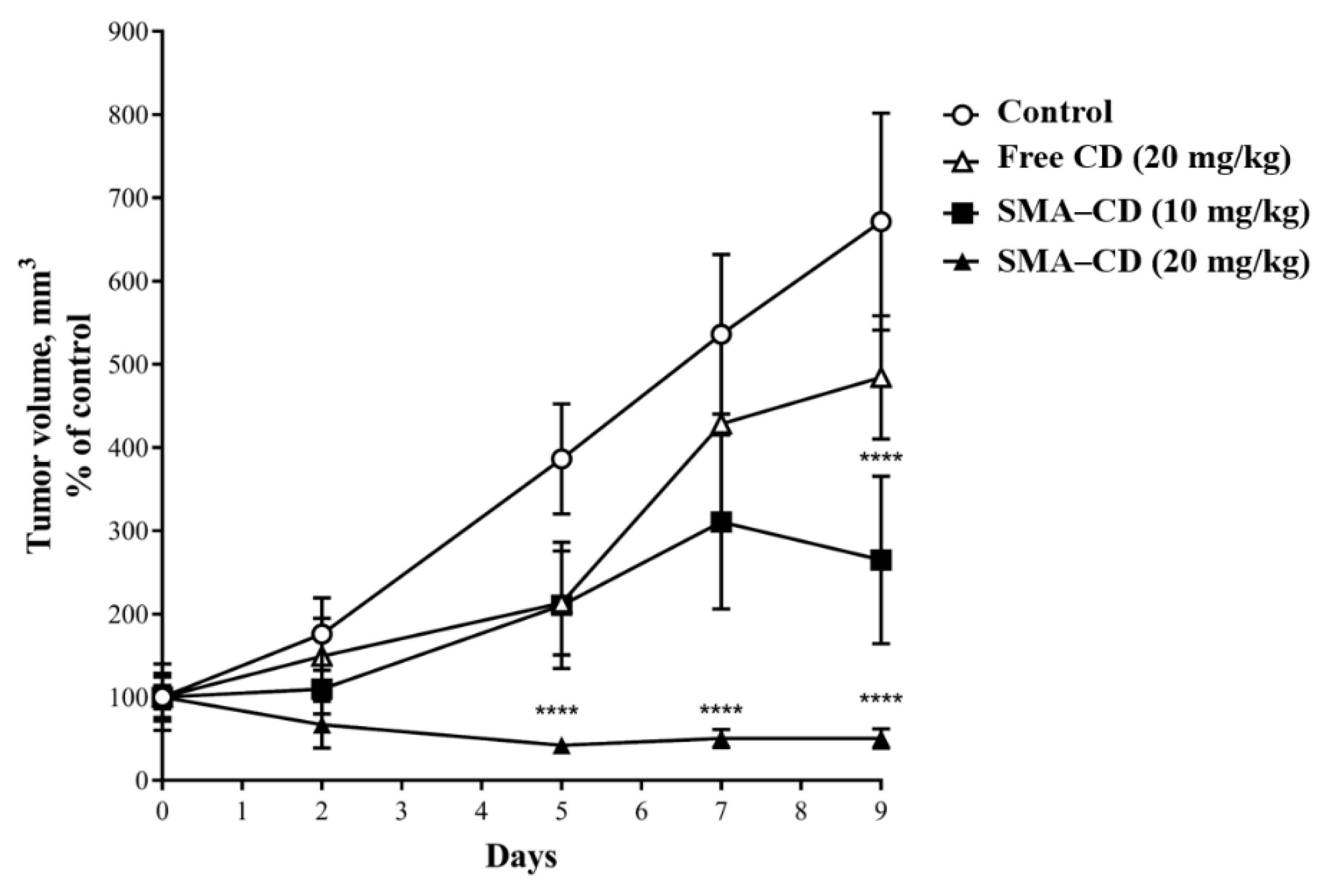
2.8. Effect of CD and SMA–CD on 4T1 Tumor Growth
3. Discussion
4. Materials and Methods
4.1. Synthesis of Fe–Cur3
4.2. Synthesis of CD
4.3. Preparation and Characterization of SMA–CD Micelles
4.3.1. Loading of the SMA–CD Micelles
4.3.2. Size, PDI, and Zeta Potential Determination of SMA–CD Micelles
4.3.3. Drug Release Studies of SMA–CD in Distilled Water
4.3.4. Drug Stability Studies of CD and SMA–CD in PBS or PBS/FBS
4.3.5. CD Interaction with Plasma Proteins
4.4. In Vitro Studies
4.4.1. Cell Culture
4.4.2. Cell Viability
4.5. In Vivo Studies
4.5.1. Maximum Tolerated Dose (MTD)
4.5.2. Biodistribution of CD and SMA–CD
4.5.3. Effect of CD and SMA–CD on 4T1 Tumor Growth
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noone, A.M.; Cronin, K.A.; Altekruse, S.F.; Howlader, N.; Lewis, D.R.; Petkov, V.I.; Penberthy, L. Cancer Incidence and Survival Trends by Subtype Using Data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer Epidemiol. Biomark. Prev. 2017, 26, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Almendro, V.; Fuster, G. Heterogeneity of breast cancer: Etiology and clinical relevance. Clin. Transl. Oncol. 2011, 13, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Livasy, C.A.; Perou, C.M.; Karaca, G.; Cowan, D.W.; Maia, D.; Jackson, S.; Tse, C.K.; Nyante, S.; Millikan, R.C. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum. Pathol. 2007, 38, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.; Van de Vijver, M. WHO Classification of Tumours of the Breast, 4th ed.; IARC: Lyon, France, 2012. [Google Scholar]
- Rakha, E.A.; Green, A.R. Molecular classification of breast cancer: What the pathologist needs to know. Pathology 2017, 49, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007, 13 Pt 1, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.; Ashraf, M.A.; Sarfraz, M. Natural cures for breast cancer treatment. Saudi Pharm. J. 2016, 24, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid. Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, V.; Rosengren, R.J.; Greish, K. Curcumin Analogues for the Treatment of Breast Cancer Revisited: The Nanotechnology Approach; Nova Science: Hauppauge, NY, USA, 2014; pp. 347–378. [Google Scholar]
- Tonnesen, H.H.; Karlsen, J.; van Henegouwen, G.B. Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z. Lebensm. Unters. Forsch. 1986, 183, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—Synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Mathur, A.; Bakhiet, M.; Taurin, S. Nanomedicine: Is it lost in translation? Ther. Deliv. 2018, 9, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Nehoff, H.; Parayath, N.N.; Taurin, S.; Greish, K. The influence of drug loading on caveolin-1 mediated intracellular internalization of doxorubicin nanomicelles in vitro. J. Nanomed. Nanotechnol. 2014, 5, 197. [Google Scholar] [CrossRef]
- Sahu, S.D.; Casciano, D.A. Handbook of Nanotoxicology, Nanomedicine and Stem Cell Use in Toxicology; Wiley: Hoboken, NJ, USA, 2014; Chapter 4; pp. 75–76. [Google Scholar]
- Lin, L.; Hutzen, B.; Ball, S.; Foust, E.; Sobo, M.; Deangelis, S.; Pandit, B.; Friedman, L.; Li, C.; Li, P.K. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci. 2009, 100, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Liang, G.; Xiao, J.; Huang, Z.F.; Zhou, H.P. Synthesis and biological evaluation of curcumin analogues without the beta-diketone moiety. FASEB J. 2008, 22, 720.13. [Google Scholar]
- Li, Y.; Hu, J.; Guan, F.; Song, L.; Fan, R.; Zhu, H.; Hu, X.; Shen, E.; Yang, B. Copper induces cellular senescence in human glioblastoma multiforme cells through downregulation of Bmi-1. Oncol. Rep. 2013, 29, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Dashtestani, F.; AlizadehZeinabad, H. Cytotoxic effect of albumin coated copper nanoparticle on human breast cancer cells of MDA-MB 231. PLoS ONE 2017, 12, e0188639. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Utage, B.; Mogle, P.; Kamble, R.; Hese, S.; Dawane, B.; Gacche, R. Evaluation of curcumin capped copper nanoparticles as possible inhibitors of human breast cancer cells and angiogenesis: A comparative study with native curcumin. AAPS PharmSciTech 2016, 17, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; El Brahmi, N.; Eloy, L.; Poupon, J.; Nicolas, V.; Steinmetz, A.; El Kazzouli, S.; Bousmina, M.M.; Blanchard-Desce, M.; Caminade, A.M.; et al. Anticancer copper(II) phosphorus dendrimers are potent proapoptotic Bax activators. Eur. J. Med. Chem. 2017, 132, 142–156. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zheng, K.; Yuan, C.; Chen, Z.; Huang, M. Be Active or Not: The Relative Contribution of Active and Passive Tumor Targeting of Nanomaterials. Nanotheranostics 2017, 1, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Taurin, S.; Nehoff, H.; Greish, K. Anticancer nanomedicine and tumor vascular permeability; Where is the missing link? J. Control. Release 2012, 164, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tsukigawa, K.; Liao, L.; Nakamura, H.; Fang, J.; Greish, K.; Otagiri, M.; Maeda, H. Synthesis and therapeutic effect of styrene–maleic acid copolymer-conjugated pirarubicin. Cancer Sci. 2015, 106, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Taurin, S.; Nehoff, H.; van Aswegen, T.; Greish, K. Tumor Vasculature, EPR Effect, and Anticancer Nanomedicine: Connecting the Dots. In Cancer Targeted Drug Delivery; Springer: New York, NY, USA, 2013; pp. 207–239. [Google Scholar]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical studies on binding of curcumin to bovine serum albumin. Photochem. Photobiol. 2003, 77, 597–603. [Google Scholar] [CrossRef]
- Barik, A.; Mishra, B.; Kunwar, A.; Kadam, R.M.; Shen, L.; Dutta, S.; Padhye, S.; Satpati, A.K.; Zhang, H.-Y.; Priyadarsini, K.I. Comparative study of copper (II)–curcumin complexes as superoxide dismutase mimics and free radical scavengers. Eur. J. Med. Chem. 2007, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Murugkar, A.; Gandhe, N.; Padhye, S. Enhanced antioxidant activities of metal conjugates of curcumin derivatives. Met. Based Drugs 2001, 8, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Sawa, T.; Fang, J.; Akaike, T.; Maeda, H. SMA-doxorubicin, a new polymeric micellar drug for effective targeting to solid tumours. J. Control. Release 2004, 97, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Fateel, M.; Abdelghany, S.; Rachel, N.; Alimoradi, H.; Bakhiet, M.; Alsaie, A. Sildenafil citrate improves the delivery and anticancer activity of doxorubicin formulations in a mouse model of breast cancer. J. Drug Target. 2018, 26, 610–615. [Google Scholar] [CrossRef] [PubMed]

| Micelle | Loading (wt/wt) | Size (nm) | PDI | Entrapment Efficiency (% EE) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| SMA–CD | 18% | 248 ± 68 | 0.274 | 80% | −0.11 ± 0.03 |
| Compound | IC50 (µM) 1 | ||
|---|---|---|---|
| MCF-7 | MDA-MB-231 | 4T1 | |
| Fe–Cur3 | >10 | >10 | >10 |
| CD | 3.37 ± 1.2 | 0.13 ± 0.07 | 0.89 ± 0.09 |
| SMA–CD | 3.37 ± 1.03 | 0.69 ± 0.13 | 1.94 ± 0.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greish, K.; Pittalà, V.; Taurin, S.; Taha, S.; Bahman, F.; Mathur, A.; Jasim, A.; Mohammed, F.; El-Deeb, I.M.; Fredericks, S.; et al. Curcumin–Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer. Nanomaterials 2018, 8, 884. https://doi.org/10.3390/nano8110884
Greish K, Pittalà V, Taurin S, Taha S, Bahman F, Mathur A, Jasim A, Mohammed F, El-Deeb IM, Fredericks S, et al. Curcumin–Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer. Nanomaterials. 2018; 8(11):884. https://doi.org/10.3390/nano8110884
Chicago/Turabian StyleGreish, Khaled, Valeria Pittalà, Sebastien Taurin, Safa Taha, Fatemah Bahman, Aanchal Mathur, Anfal Jasim, Fatima Mohammed, Ibrahim M. El-Deeb, Salim Fredericks, and et al. 2018. "Curcumin–Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer" Nanomaterials 8, no. 11: 884. https://doi.org/10.3390/nano8110884
APA StyleGreish, K., Pittalà, V., Taurin, S., Taha, S., Bahman, F., Mathur, A., Jasim, A., Mohammed, F., El-Deeb, I. M., Fredericks, S., & Rashid-Doubell, F. (2018). Curcumin–Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer. Nanomaterials, 8(11), 884. https://doi.org/10.3390/nano8110884