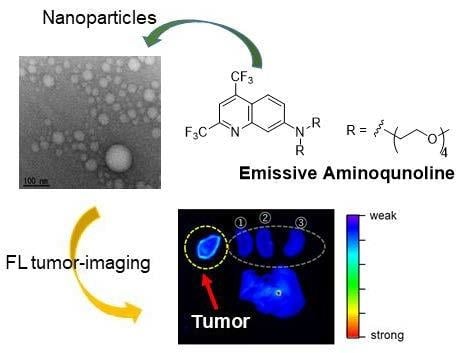
Fluorescence Tumor-Imaging Using a Thermo-Responsive Molecule with an Emissive Aminoquinoline Derivative
Abstract
:1. Introduction
2. Results and Discussion
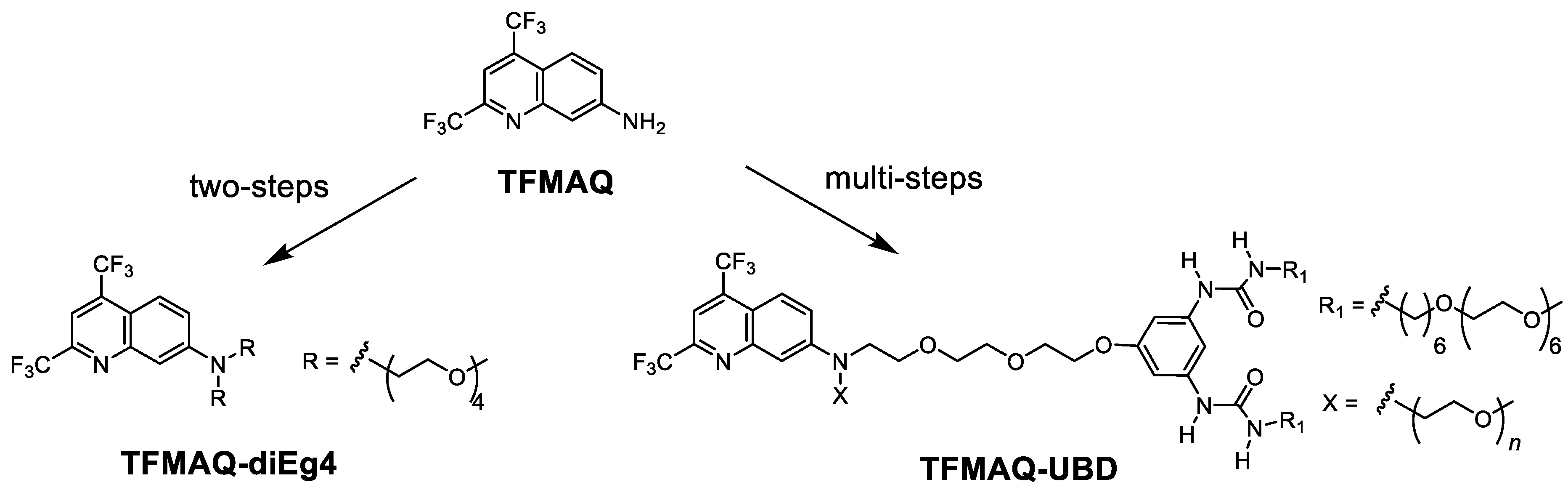
2.1. Synthesis
2.2. Solubility for Aqueous Solution
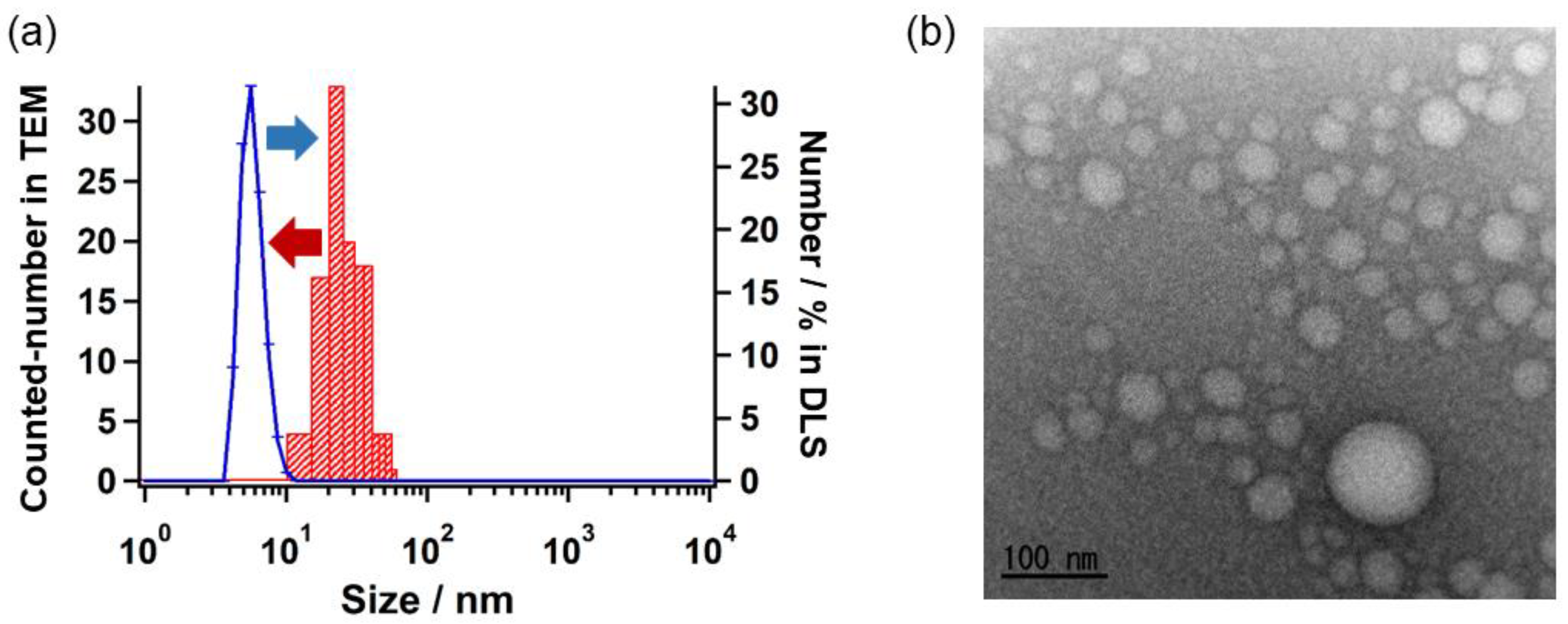
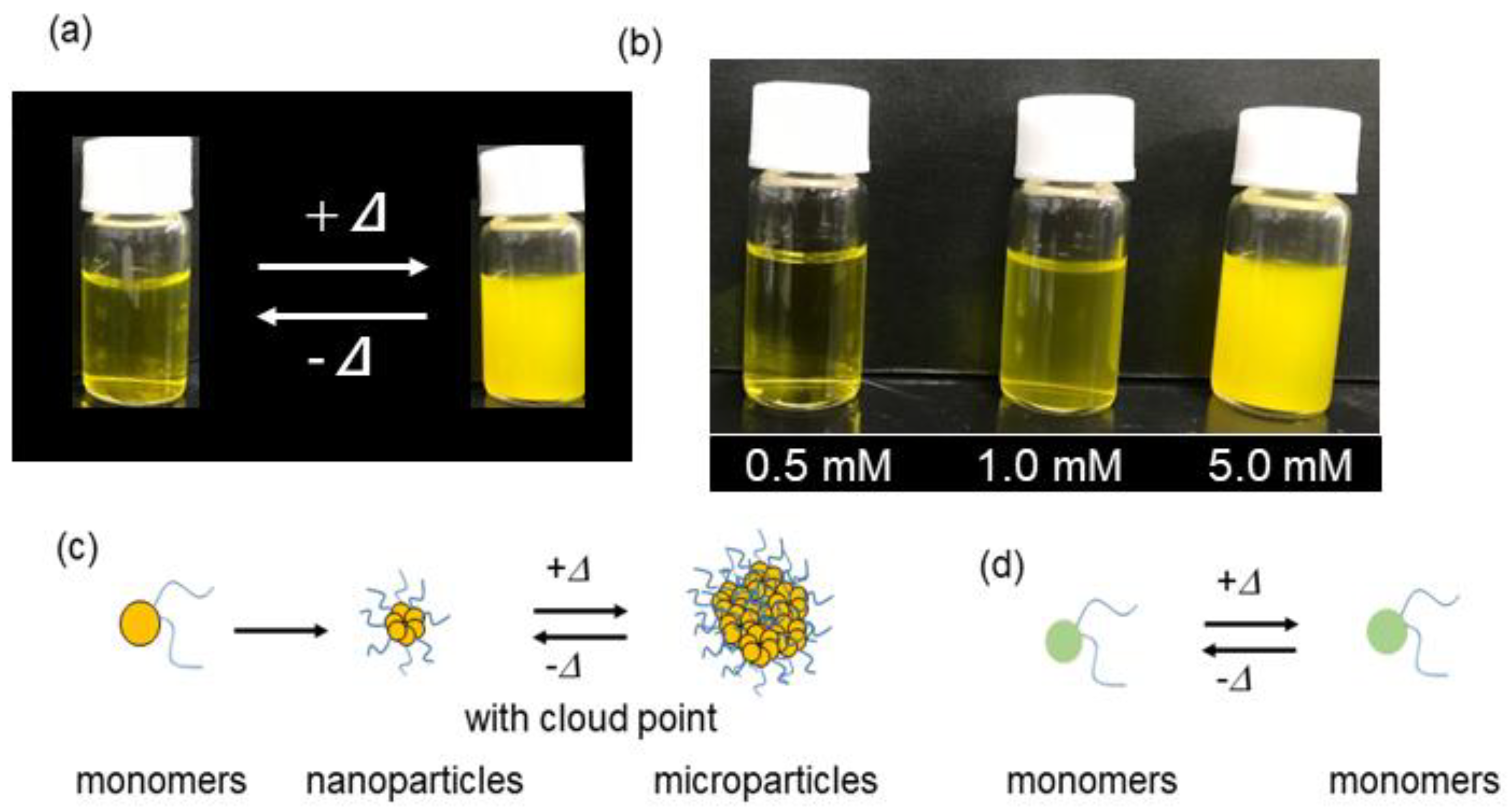
2.3. Self-Assembly Behavior
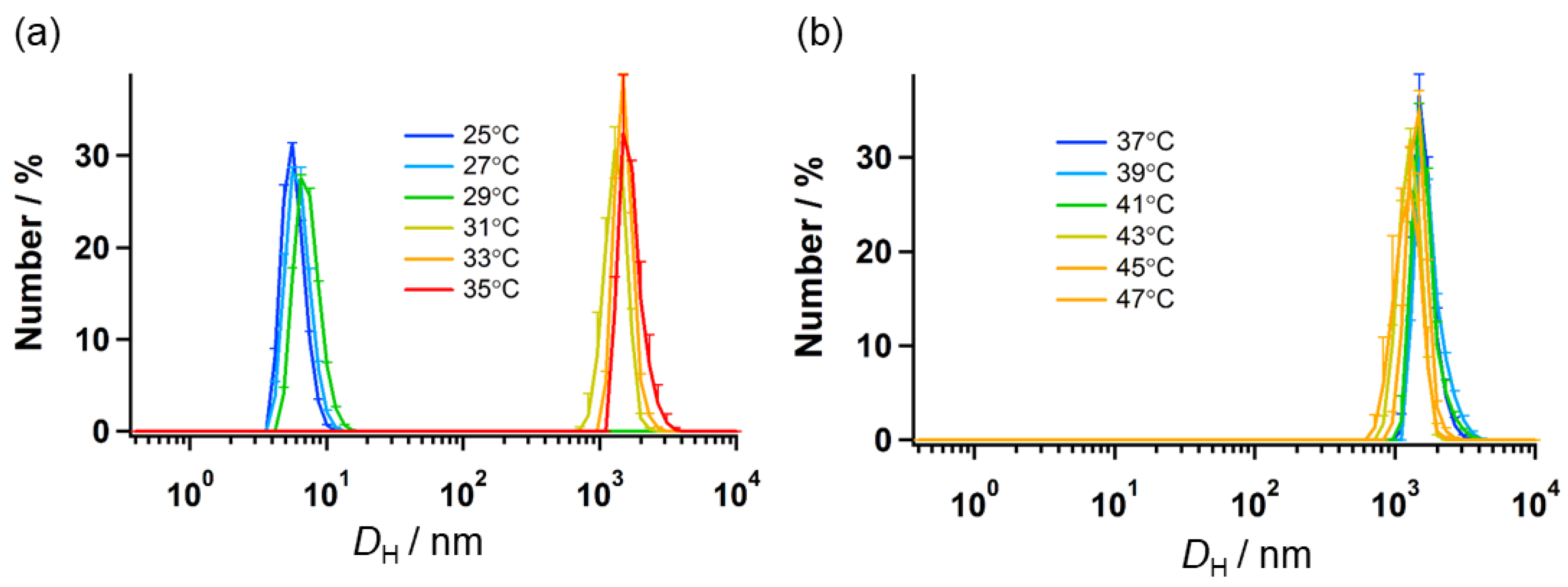
2.4. Temperature Dependence of Self-Assembly
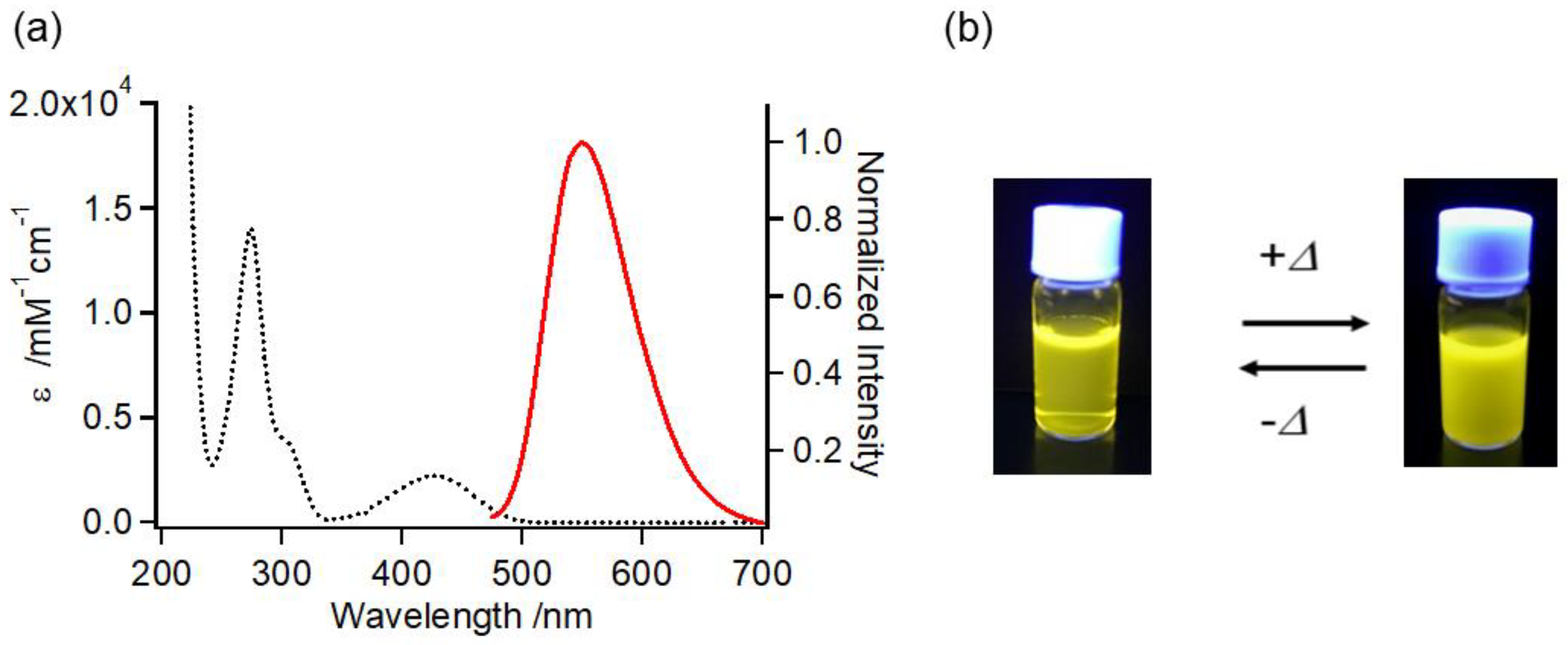
2.5. UV-Vis and Fluorescence Properties
2.6. Tumor-Imaging with TFMAQ-diEg4
- (1)
- Single-injection of 2.0 mM TFMAQ-diEg4 in saline, via the tail vein, without local heat.
- (2)
- Single-injection of 2.0 mM TFMAQ-diEg4 in saline, via the tail vein, with local heat.
- (3)
- Single-injection of 2.0 mM TFMAQ-diEg4 in saline, via the tail vein and drip via the tail vein, with local heat.
- (4)
- Single-injection of 0.2 mM TFMAQ-diEg4 in saline, via the tail vein and drip, without local heat.
- (5)
- Single-injection of 0.2 mM TFMAQ-diEg4 in saline, via the tail vein and drip with local heat.
3. Materials and Methods
3.1. Instruments and Sample Preparation
3.2. Materials
3.3. Temperature Control in Mice with and without Local Heating around the Tumor Tissue
3.4. Single and Drip Administration via Tail Vein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, S.; Kang, E.; Kim, K.; Choi, K.; Kwon, I.C. New Generation of Multifunctional Nanoparticles for Cancer Imaging and Therapy. Adv. Funct. Mater. 2009, 19, 1553–1566. [Google Scholar] [CrossRef]
- Tannock, I.F.; Rotin, D. Acid pH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Brandt, L.; Ikomi-Kumm, J.; Olsson, H. Microcalorimetric Investigation of Cell Metabolism in Tumour Cells from Patients with Non-Hodgkin lymphoma. Scand. J. Haematol. 1986, 36, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular Temperature Mapping with a Fluorescent Polymeric Thermometer and Fluorescence Lifetime Imaging Microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of Molecules across Tumor Vasculature. Cancer Metastasis Rev. 1987, 6, 559–593. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihara, Y.; Kihara, Y.; Hamano, F.; Yanagida, K.; Morishita, Y.; Kunita, A.; Yamori, T.; Fukayama, M.; Aburatani, H.; Shimizu, T.; et al. The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc. Natl. Acad. Sci. USA 2010, 107, 17309–17314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Sato, K.; Tamaki, K.; Mochizuki, H.; Tamakuma, S.; Nishida, M.; Hirade, H. The Clinicopathological Significance of Measurement of the Temperaure in the Center of Brease Tumor. J. Jpn. Soc. Clin. Surg. 1997, 58, 2760–2764. [Google Scholar]
- Schild, H.G. Poly(NIPPAM): Experiment, Theory, and Applications. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Diab, C.; Akiyama, Y.; Kataoka, K.; Winnik, F.M. Microcalorimetric Study of the Temperature-induced Phase Separation in Aqueous Solutions of Poly(2-isopropyl-2-oxazolines). Macromolecules 2004, 37, 2556–2562. [Google Scholar] [CrossRef]
- Hayashi, H.; Ohkubo, K.; Karasawa, S.; Koga, N. Assemblies of Functional Small-Sized Molecules Having 4-Amino-2,2,6,6-tetramethylpiperidine-1-oxyl Responsive to Heat and pH in Water and Their Water Proton Relaxivities. Langmuir 2011, 27, 12709–12719. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Morishita, K.; Fuchi, Y.; Kobayashi, S.; Karasawa, S. Effect of Hydrophobicity on the Self-Assembly Behavior of Urea Benzene Derivatives in Aqueous Solution. Appl. Sci. 2018, 8, 1080. [Google Scholar] [CrossRef]
- Morishita, K.; Murayama, S.; Araki, T.; Aoki, I.; Karasawa, S. Thermal- and pH-Dependent Size Variable Radical Nanoparticles and Its Water Proton Relaxivity for Metal-Free MRI Functional Contrast Agents. J. Org. Chem. 2016, 81, 8351–8362. [Google Scholar] [CrossRef] [PubMed]
- Morishita, K.; Okamoto, O.; Murayama, S.; Usui, K.; Ohashi, E.; Hirai, G.; Aoki, I.; Karasawa, S. Water-Proton Relaxivities of Radical Nanoparticles Self-Assembled via Hydration or Dehydration Processes. Langmuir 2017, 33, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Llower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Fan, W.; Zhao, Y. Ultrasensitive pH-Induced Water Solubility Switch Using UCST Polymers. Macromolecules 2016, 49, 1424–1433. [Google Scholar] [CrossRef]
- Araki, T.; Murayama, S.; Usui, K.; Shimada, T.; Aoki, I.; Karasawa, S. Self-Assembly Behavior of Emissive Urea Benzene Derivatives Enables Heat-Induced Accumulation in Tumor Tissue. Nano Lett. 2017, 17, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Karasawa, S.; Koga, N. Crystal Structures and Emitting Properties of Trifluoromethylaminoquinoline Derivatives: Thermal Single-Crystal-to-Single-Crystal Transformation of Polymorphic Crystals That Emit Different Colors. Chem. Eur. J. 2012, 18, 15038–15048. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, S.; Hagihara, R.; Abe, Y.; Harada, N.; Todo, J.; Koga, N. Crystal Structures, Thermal Properties, and Emission Behaviors of N,N-R-Phenyl-7-amino-2,4-trifluoromethylquinoline Derivatives: Supercooled Liquid-to-Crystal Transformation Induced by Mechanical Stimuli. Cryst. Growth Des. 2014, 14, 2468–2478. [Google Scholar] [CrossRef]
- Karasawa, S.; Todo, J.; Usui, K.; Harada, N.; Yoza, K.; Suemune, H.; Koga, N. Regioselective Photocyclizations of Di(quinolinyl)arylamines and Tri(quinolinyl)amine with Emission Color Changes and Photoreaction-Induced Self-Assemblies. Chem. Eur. J. 2016, 22, 7771–7781. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, R.; Usui, K.; Karasawa, S. Two-step Transformation of p-Anisolylaminoquinoline Derivatives Induced by Conformation- and Packing-Dominated Processes. Dyes Pigments 2017, 143, 401–408. [Google Scholar] [CrossRef]
- Richter, C.; Schaepe, K.; Glorius, F.; Ravoo, B.J. Tailor-made N-heterocyclic Carbenes for Nanoparticle Stabilization. Chem. Commun. 2014, 50, 3204–3207. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, H.; Abe, S.; Mino, T.; Nomura, W.; Tamamura, H. Intense Blue Fluorescence in a Leucine Zipper Assembly. ChemBioChem 2011, 12, 691–694. [Google Scholar] [CrossRef] [PubMed]
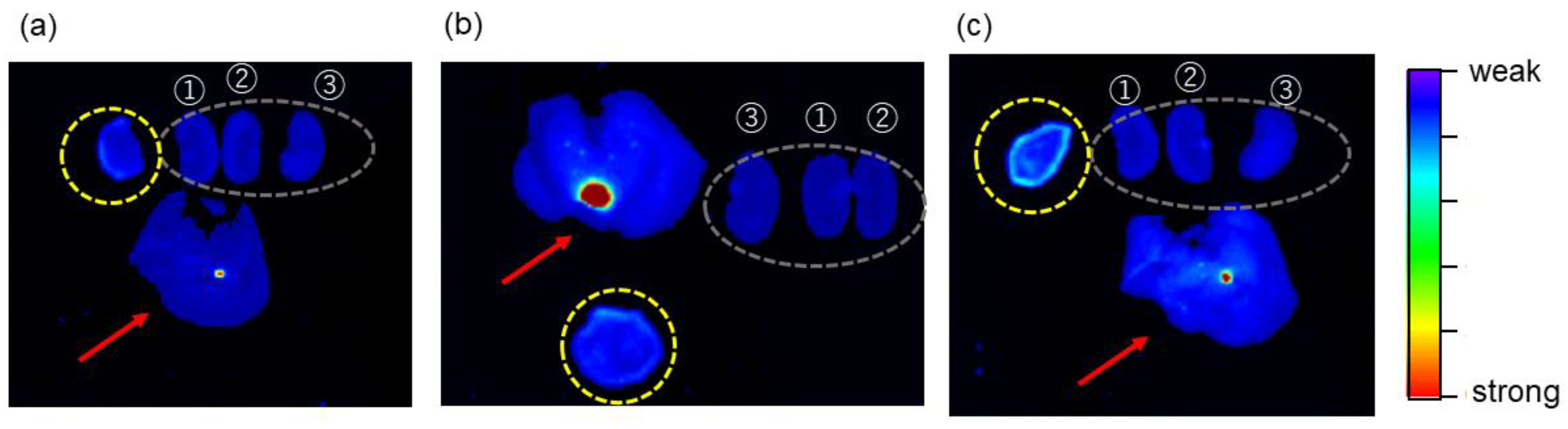
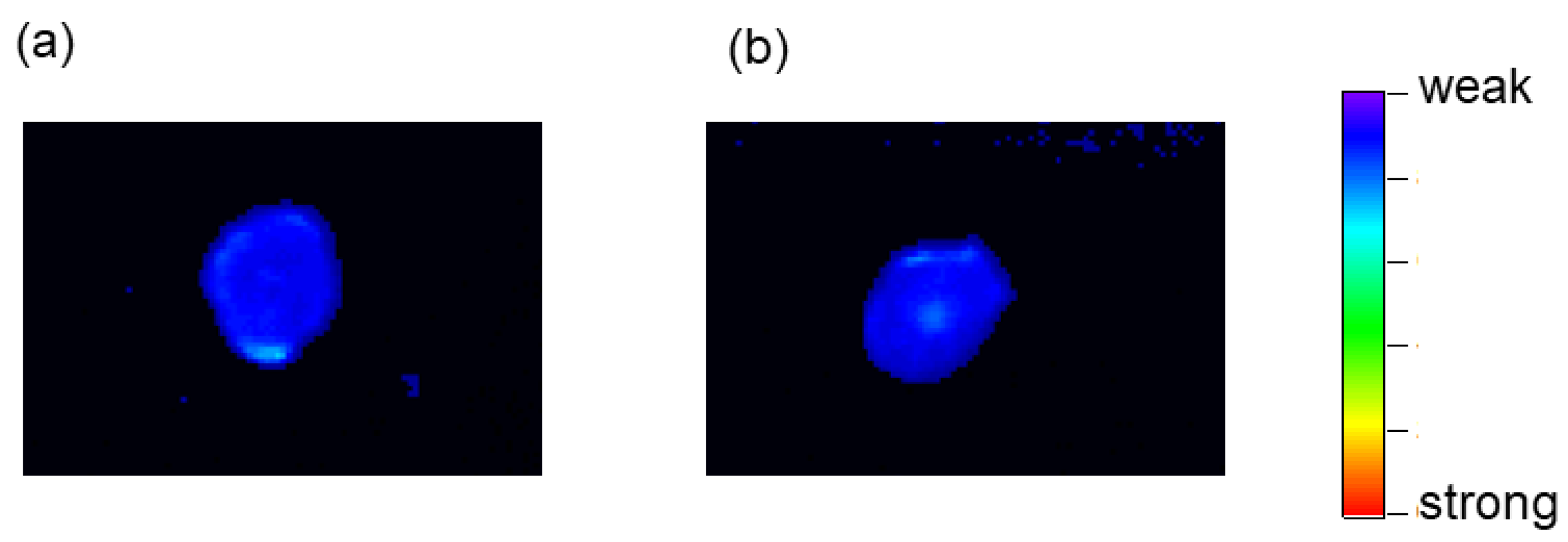
| Groups * | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Concentration of the vial | 2.0 mM | 2.0 mM | 2.0 mM | 0.2 mM | 0.2 mM |
| Concentration in the body # | 0.5 mM | 0.5 mM | Over 1.0 mM | Over 0.1 mM | Over 0.1 mM |
| Heat at tumor | No | Yes | Yes | No | Yes |
| Intravenous (i.v.) administration via the tail vein | Single-i.v. of 200 μL | Single-i.v. of 200 μL | Single-i.v. of 200 μL and then drip of 100 μL/h for 2 h | Single-i.v. of 200 μL and then drip of 100 μL/h for 2 h | Single-i.v. of 200 μL and then drip of 100 μL/h for 2 h |
| Emission intensity | Weak ** | Weak ** | Strong | Weak | Weak |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araki, T.; Fuchi, Y.; Murayama, S.; Shiraishi, R.; Oyama, T.; Aso, M.; Aoki, I.; Kobayashi, S.; Yamada, K.-i.; Karasawa, S. Fluorescence Tumor-Imaging Using a Thermo-Responsive Molecule with an Emissive Aminoquinoline Derivative. Nanomaterials 2018, 8, 782. https://doi.org/10.3390/nano8100782
Araki T, Fuchi Y, Murayama S, Shiraishi R, Oyama T, Aso M, Aoki I, Kobayashi S, Yamada K-i, Karasawa S. Fluorescence Tumor-Imaging Using a Thermo-Responsive Molecule with an Emissive Aminoquinoline Derivative. Nanomaterials. 2018; 8(10):782. https://doi.org/10.3390/nano8100782
Chicago/Turabian StyleAraki, Takeru, Yasufumi Fuchi, Shuhei Murayama, Ryoma Shiraishi, Tokimi Oyama, Mariko Aso, Ichio Aoki, Shigeki Kobayashi, Ken-ichi Yamada, and Satoru Karasawa. 2018. "Fluorescence Tumor-Imaging Using a Thermo-Responsive Molecule with an Emissive Aminoquinoline Derivative" Nanomaterials 8, no. 10: 782. https://doi.org/10.3390/nano8100782
APA StyleAraki, T., Fuchi, Y., Murayama, S., Shiraishi, R., Oyama, T., Aso, M., Aoki, I., Kobayashi, S., Yamada, K.-i., & Karasawa, S. (2018). Fluorescence Tumor-Imaging Using a Thermo-Responsive Molecule with an Emissive Aminoquinoline Derivative. Nanomaterials, 8(10), 782. https://doi.org/10.3390/nano8100782