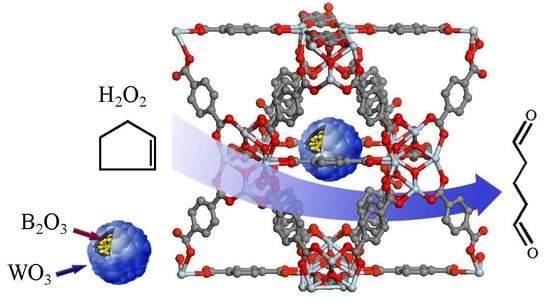
Modification Effects of B2O3 on The Structure and Catalytic Activity of WO3-UiO-66 Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.1.1. Synthesis of UiO-66
2.1.2. Synthesis of WO3/UiO-66
2.1.3. Synthesis of B2O3-WO3/UiO-66
2.2. Characterizations of Materials
2.3. Test of Catalytic activity
3. Results
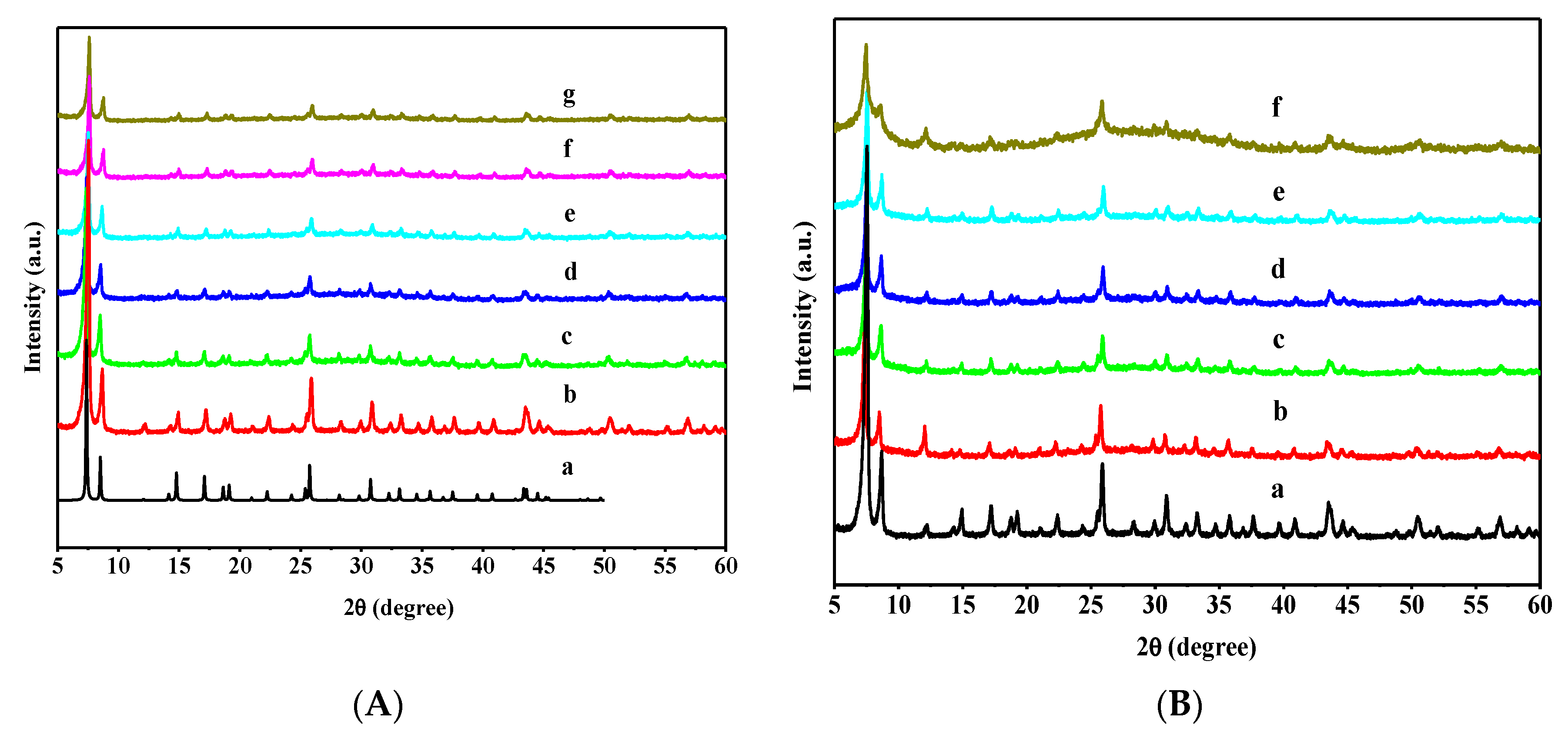
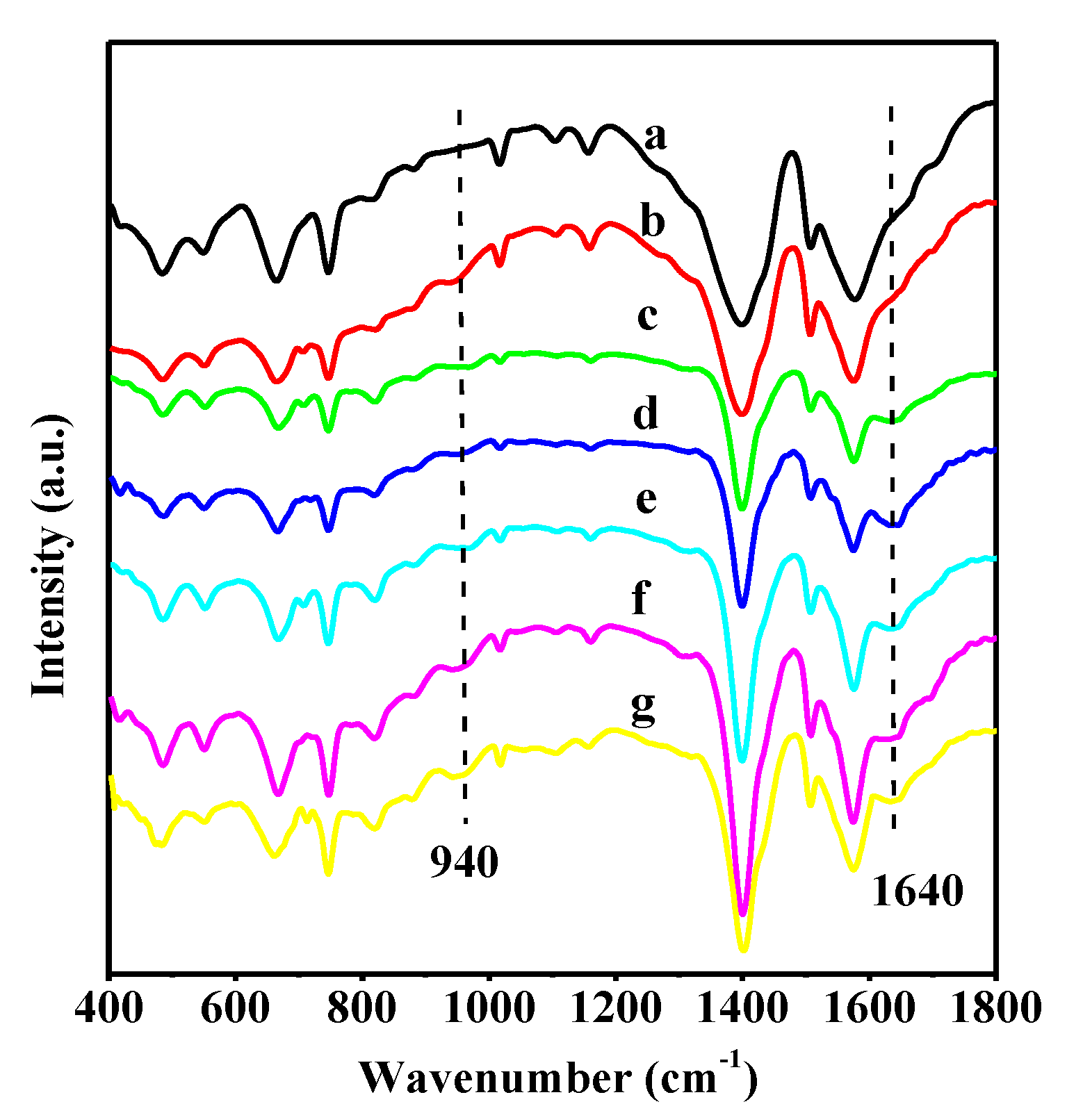
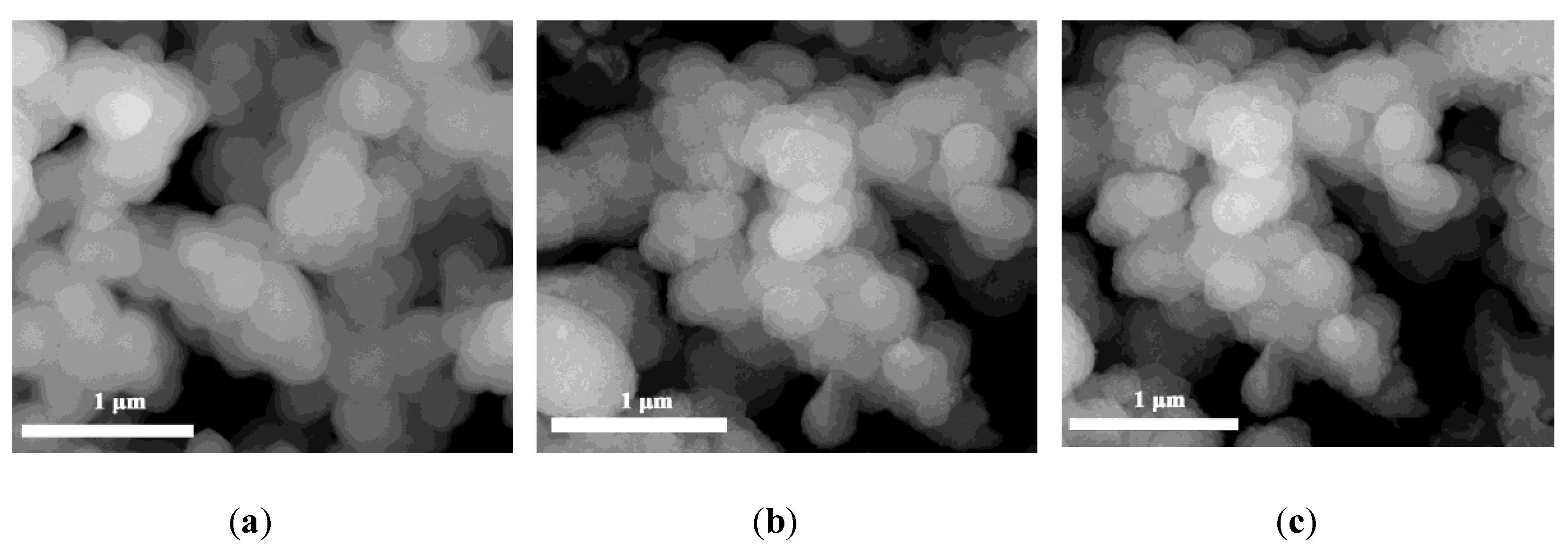
3.1. Catalyst Characterizations
3.2. Catalytic Activity and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lwin, S.; Wachs, I.E. Olefin Metathesis by Supported Metal Oxide Catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Debecker, D.P.; Praserthdam, P.; Panpranot, J.; Suriye, K.; Ayudhya, S.K.N. NaOH modified WO3/SiO2 catalysts for propylene production from 2-butene and ethylene metathesis. Chin. J. Catal. 2014, 35, 232–241. [Google Scholar] [CrossRef]
- Soultanidis, N.; Zhou, W.; Psarras, A.C.; Gonzalez, A.J.; Iliopoulou, E.F.; Kiely, C.J.; Wachs, I.E.; Wong, M.S. Relating n-Pentane Isomerization Activity to the Tungsten Surface Density of WOx/ZrO2. J. Am. Chem. Soc. 2010, 132, 13462–13471. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Zhang, H.B.; Zhang, Y.H.; Tang, Y.; Tang, K.J. Preparation and characterization of WOx/ZrO2 nanosized catalysts with high WOx dispersion threshold and acidity. J. Catal. 2013, 299, 119–128. [Google Scholar] [CrossRef]
- Yang, X.L.; Dai, W.L.; Gao, R.H.; Fan, K.N. Characterization and catalytic behavior of highly active tungsten-doped SBA-15 catalyst in the synthesis of glutaraldehyde using an anhydrous approach. J. Catal. 2007, 249, 278–288. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhu, Q.J.; Ding, J.; Liu, X.; Dai, W.L. Effect of calcination temperature of the support and the catalyst of WO3/SnO2 on the catalytic oxidation of 1,2-benzenedimethanol by H2O2. Appl. Catal. A 2014, 482, 171–178. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, Z.; Guo, C.; Ye, X.; Wu, Y. Study of the Alkylation of Isobutane with n-Butene over WO3/ZrO2 Strong Solid Acid. 1. Effect of the Preparation Method, WO3 Loading, and Calcination Temperature. Ind. Eng. Chem. Res. 2000, 39, 3717–3725. [Google Scholar] [CrossRef]
- Mutlu, V.N.; Yilmaz, S. Esterification of cetyl alcohol with palmitic acid over WO3/Zr-SBA-15 and Zr-SBA-15 catalysts. Appl. Catal. A 2016, 522, 194–200. [Google Scholar] [CrossRef]
- Can, F.; Berland, S.; Royer, S.; Courtois, X.; Duprez, D. Composition-Dependent Performance of CexZr1–xO2 Mixed-Oxide-Supported WO3 Catalysts for the NOx Storage Reduction–Selective Catalytic Reduction Coupled Process. ACS Catal. 2013, 3, 1120–1132. [Google Scholar] [CrossRef]
- Liu, Z.M.; Su, H.; Chen, B.H.; Li, J.H.; Woo, S.I. Activity enhancement of WO3 modified Fe2O3 catalyst for the selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2016, 299, 255–262. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Hirai, T. Visible light-induced partial oxidation of cyclohexane on WO3 loaded with Pt nanoparticles. Catal. Sci. Technol. 2012, 2, 400–405. [Google Scholar] [CrossRef]
- Dong, P.Y.; Xu, N.; Xu, Y.; Wang, X.F. A study of Pt/WO3-carrier catalysts for photocatalytic purification of NO gas. Catal. Commun. 2016, 84, 142–146. [Google Scholar] [CrossRef]
- Hino, M.; Arata, K. Synthesis of solid superacid of tungsten oxide supported on zirconia and its catalytic action for reactions of butane and pentane. J. Chem. Soc. Chem. Commun. 1987, 18, 407–425. [Google Scholar] [CrossRef]
- Jimenez-Morales, I.; Santamaria-Gonzalez, J.; Maireles-Torres, P.; Jimenez-Lopez, A. Zirconium doped MCM-41 supported WO3 solid acid catalysts for the esterification of oleic acid with methanol. Appl. Catal. A 2010, 379, 61–68. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.G.; Chang, C.R.; Zhu, X.L.; Liu, X.; Han, J.Y.; Ge, Q.F. Enhancing tungsten oxide/SBA-15 catalysts for hydrolysis of cellobiose through doping ZrO2. Appl. Catal. A 2016, 523, 182–192. [Google Scholar] [CrossRef]
- He, Y.Y.; Ford, M.E.; Zhu, M.H.; Liu, Q.C. Wu, Z.L.; Wachs, I.E. Selective catalytic reduction of NO by NH3 with WO3-TiO2 catalysts: Influence of catalyst synthesis method. Appl. Catal. B 2016, 188, 123–133. [Google Scholar] [CrossRef]
- Kamata, K.; Yonehara, K.; Sumida, Y.; Hirata, K.; Nojima, S.; Mizuno, N. Efficient Heterogeneous Epoxidation of Alkenes by a Supported Tungsten Oxide Catalyst. Angew. Chem. Int. Ed. 2011, 50, 12062–12066. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Gupta, V.K. Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci. 2011, 362, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O'Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H.; Xamena, F.X.L.I. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Hybrid porous solids: past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Zhang, B.Y.; Fang, Y.H.; Sun, W.J.; Qi, Z.Y.; Pei, Y.C.; Qi, S.Y.; Yuan, P.Y.; Luan, X.C.; Goh, T.W.; et al. Metal-Organic Framework Derived Carbons: Applications as Solid Base Catalyst and Support for Pd Nanoparticles in Tandem Catalysis. Chem. Eur. J. 2017, 23, 4266–4270. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Burgal, J.D.S.; Szekely, G.; Davies, R.P.; Braddock, D.C.; Livingston, A. Hybrid Polymer/MOF membranes for Organic Solvent Nanofiltration (OSN): Chemical Modification and the Quest for Perfection. J. Membr. Sci. 2016, 503, 166–176. [Google Scholar] [CrossRef]
- Masih, D.; Chernikova, V.; Shekhah, O.; Eddaoudi, M.; Mohammed, O.F. Zeolite-like Metal-organic Framework (MOF) Encaged Pt(II)-porphyrin for Anion-selective Sensing. ACS Appl. Mater. Interfaces 2018, 10, 11399–11405. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Peters, A.W.; Liu, J.; Zhang, X.; Schweitzer, N.M.; Hupp, J.T.; Farha, O.K. Size effect of the active sites in UiO-66-supported nickel catalysts synthesized via atomic layer deposition for ethylene hydrogenation. Inorg. Chem. Front. 2017, 4, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Dai, W.L.; Chen, H.; Xu, J.H.; Cao, Y.; Li, H.X.; Fan, K.N. Novel tungsten-containing mesoporous HMS material: its synthesis, characterization and catalytic application in the selective oxidation of cyclopentene to glutaraldehyde by aqueous H2O2. Appl. Catal. A 2005, 283, 1–8. [Google Scholar] [CrossRef]
- Yang, X.L.; Dai, W.L.; Gao, R.H.; Chen, H.; Li, H.X.; Cao, Y.; Fan, K.N. Synthesis, characterization and catalytic application of mesoporous W-MCM-48 for the selective oxidation of cyclopentene to glutaraldehyde. J. Mol. Catal. A Chem. 2005, 241, 205–214. [Google Scholar] [CrossRef]
- Yang, X.L.; Dai, W.L.; Chen, H.; Cao, Y.; Li, H.X.; He, H.Y.; Fan, K.N. Novel efficient and green approach to the synthesis of glutaraldehyde over highly active W-doped SBA-15 catalyst. J. Catal. 2005, 229, 259–263. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colon, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycle in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Fodi, T.; Didaskalou, C.; Kupai, J.; Balogh, G.T.; Huszthy, P.; Szekely, G. Nanofiltration-Enabled In Situ Solvent and Reagent Recycle for Sustainable Continuous-Flow Synthesis. ChemSusChem 2017, 10, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dai, W.L.; Deng, J.F.; Fan, K.N. Novel Heterogeneous W-Doped MCM-41 Catalyst for Highly Selective Oxidation of Cyclopentene to Glutaraldehyde by Aqueous H2O2. Catal. Lett. 2002, 81, 131–136. [Google Scholar] [CrossRef]
- Dai, W.L.; Chen, H.; Cao, Y.; Li, H.X.; Xie, S.H.; Fan, K.N. Novel economic and green approach to the synthesis of highly active W-MCM41 catalyst in oxidative cleavage of cyclopentene. Chem. Commun. 2003, 9, 892–893. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Juan-Alcaniz, J.; Ramos-Fernandez, E.V.; Lafont, U.; Gascon, J.; Kapteijn, F. Building MOF bottles around phosphotungstic acid ships: One-pot synthesis of bi-functional polyoxometalate-MIL-101 catalysts. J. Catal. 2010, 269, 229–241. [Google Scholar] [CrossRef]
- Hu, X.F.; Lu, Y.K.; Dai, F.N.; Liu, C.G.; Liu, Y.Q. Host–guest synthesis and encapsulation of phosphotungstic acid in MIL-101 via “bottle around ship”: An effective catalyst for oxidative desulfurization. Micropor. Mesopor. Mater. 2013, 170, 36–44. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H.; Moghadam, M.; Masoudinia, M. Nano-rod catalysts: Building MOF bottles (MIL-101 family as heterogeneous single-site catalysts) around vanadium oxide ships. J. Mol. Catal. A 2013, 374–375, 46–52. [Google Scholar] [CrossRef]
- Doweidar, H.; El-Damrawi, G.; Al-Zaibani, M. Distribution of species in Na2O–CaO–B2O3 glasses as probed by FTIR. Vib. Spectrosc. 2013, 68, 91–95. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems—With Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wee, L.H.; Bonino, F.; Lamberti, C.; Bordiga, S.; Martens, J.A. Cr-MIL-101 encapsulated Keggin phosphotungstic acid as active nanomaterial for catalysing the alcoholysis of styrene oxide. Green Chem. 2014, 16, 1351–1357. [Google Scholar] [CrossRef]
- Yang, X.L.; Qiao, L.M.; Dai, W.L. Phosphotungstic acid encapsulated in metal-organic framework UiO-66: An effective catalyst for the selective oxidation ofcyclopentene to glutaraldehyde. Micropor. Mesopor. Mater. 2015, 211, 73–81. [Google Scholar] [CrossRef]
- Ramanathan, A.; Maheswari, R.; Grady, B.P.; Moore, D.S.; Barich, D.H.; Subramaniam, B. Tungsten-incorporated cage-type mesoporous silicate: W-KIT-5. Micropor. Mesopor. Mater. 2013, 175, 43–49. [Google Scholar] [CrossRef]
- Bhuiyan, T.I.; Arudra, P.; Akhtar, M.N.; Aitani, A.M.; Abudawoud, R.H.; Al-Yami, M.A.; Al-Khattaf, S.S. Metathesis of 2-butene to propylene over W-mesoporous molecular sieves: A comparative study between tungsten containing MCM-41 and SBA-15. Appl. Catal. A 2013, 467, 224–234. [Google Scholar] [CrossRef]
- Hu, B.; Liu, H.; Tao, K.; Xiong, C.R.; Zhou, S.H. Highly Active Doped Mesoporous KIT-6 Catalysts for Metathesis of 1-Butene and Ethene to Propene: The Influence of Neighboring Environment of W Species. J. Phys. Chem. C 2013, 117, 26385–26395. [Google Scholar] [CrossRef]
- Weber, R.S. Effect of Local Structure on the UV-Visible Absorption Edges of Molybdenum Oxide Clusters and Supported Molybdenum Oxides. J. Catal. 1995, 151, 470–474. [Google Scholar] [CrossRef]
- Klepel, O.; Bohlmann, W.; Ivanov, E.B.; Riede, V.; Papp, H. Incorporation of tungsten into MCM-41 framework. Micropor. Mesopor. Mater. 2004, 76, 105–112. [Google Scholar] [CrossRef]
- Valigi, M.; Gazzoli, D.; Pettiti, I.; Mattei, G.; Colonna, S.; De Rossi, S.; Ferraris, G. WOx/ZrO2 catalysts: Part 1. Preparation, bulk and surface characterization. Appl. Catal. A 2002, 231, 159–172. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.Q.; He, P.; Yuan, Y.Z. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Vimont, A.; Goupil, J.-M.; Lavalley, J.-C.; Daturi, M.; Surble, S.; Serre, C.; Millange, F.; Ferey, G.; Audebrand, N. Investigation of Acid Sites in a Zeotypic Giant Pores Chromium(III) Carboxylate. J. Am. Chem. Soc. 2006, 128, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Colorio, G.G.; Vedrine, J.C.; Auroux, A.; Bonnetotb, B. Partial oxidation of ethane over alumina-boria catalysts. Appl. Catal. A 1996, 137, 55–68. [Google Scholar] [CrossRef]
- Colorio, G.G.; Bonnetotb, B.; Vedrine, J.C.; Auroux, A. Characteristics of alumina boria catalysts used in ethane partial oxidation. Stud. Surf. Sci. Catal. 1994, 82, 143–149. [Google Scholar]
- Zheng, J.B.; Xia, Z.Q.; Li, J.J.; Lai, W.K.; Yi, X.D.; Chen, B.H.; Fang, W.P.; Wan, H.L. Promoting effect of boron with high loading on Ni-based catalyst for hydrogenation of thiophene-containing ethylbenzene. Catal. Commun. 2012, 21, 18–21. [Google Scholar] [CrossRef]
- Zhu, S.H.; Gao, X.Q.; Zhu, Y.L.; Zhu, Y.F.; Zheng, H.Y.; Li, Y.W. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Furukawa, H.; Nakamura, T.; Inagaki, H.; Nishikawa, E.; Imai, C.; Misong, M. Oxidation of Cyclopentene with Hydrogen Peroxide Catalyzed by 12-Heteropoly Acids. Chem. Lett. 1988, 17, 877–880. [Google Scholar] [CrossRef]
- Deng, J.F.; Xu, X.H.; Chen, H.Y.; Jang, A.R. A new process for preparing dialdehydes by catalytic oxidation of cyclic olefins with aqueous hydrogen peroxide. Tetrahedron 1992, 48, 3503–3514. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q.Q.; Zhang, Z.Y.; Liu, X.; Zhao, J.Q.; Cheng, S.B.; Zong, B.N.; Dai, W.L. Carbon nitride nanosheets decorated with WO3 nanorods: Ultrasonic-assisted facile synthesis and catalytic application in the green manufacture of dialdehydes. Appl. Catal. B 2015, 165, 511–518. [Google Scholar] [CrossRef]
- Zhang, J.S.; Yu, F.L.; Tao, R.Q.; Xie, C.X.; Yuan, B.; Yu, S.T. Selective Oxidation of Cyclopentene to Gluraraldehyde Catalyzed by Heteropolyphosphatotungstate Ionic Liquid. Chem. J. Chin. Univ. 2017, 38, 2248–2254. [Google Scholar]
- Didaskalou, C.; Kupai, J.; Cseri, L.; Barabas, J.; Vass, E.; Holtzl, T.; Szekely, G. Membrane-grafted asymmetric organocatalyst for an integrated synthesis-separation platform. ACS Catal. 2018, 8, 7430–7438. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Motamedi, A.; Marvdashti, Z.; Hosseini, S.H. Magnetic nanocomposite based on functionalized salep as a green support for immobilization of palladium nanoparticles: Reusable heterogeneous catalyst for Suzuki coupling reactions. Catal. Commun. 2017, 97, 27–31. [Google Scholar] [CrossRef]

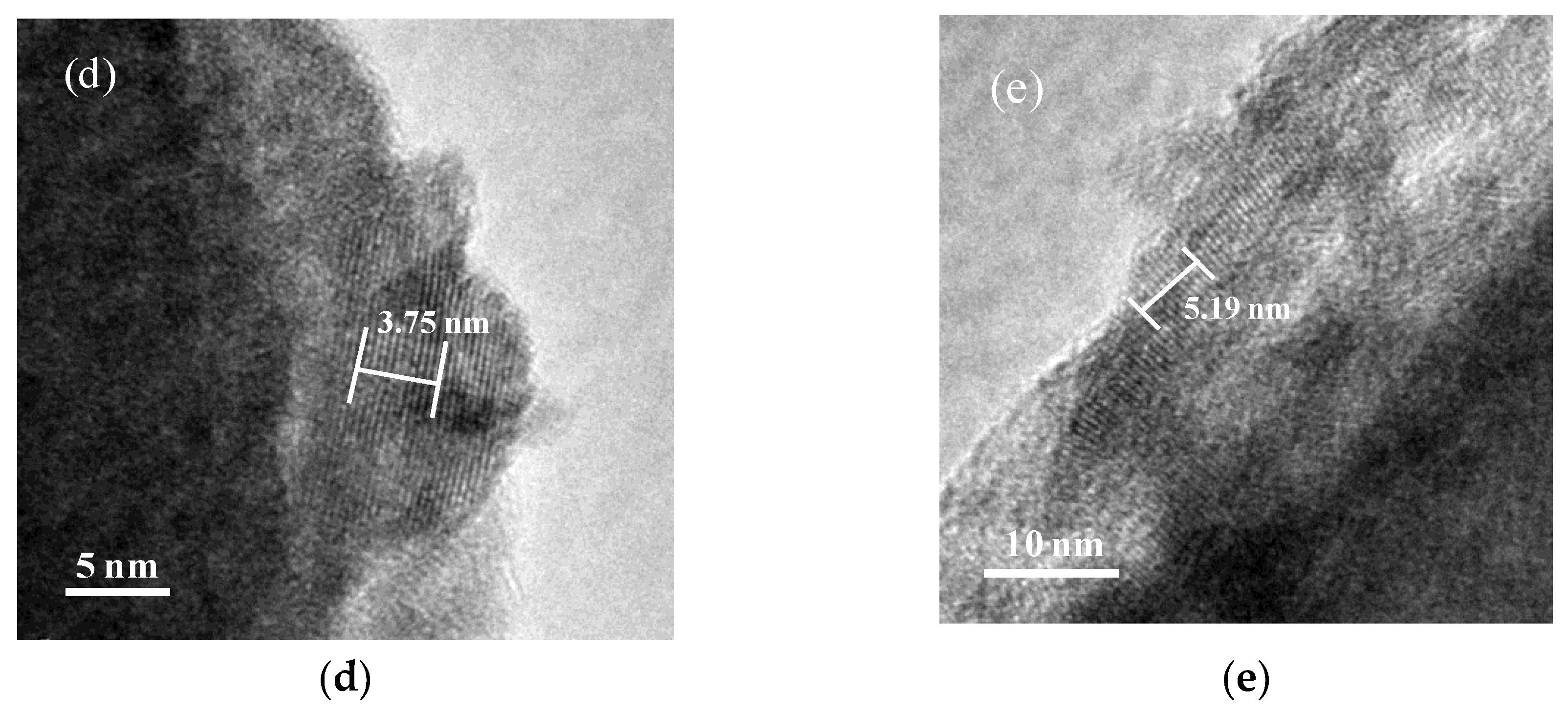
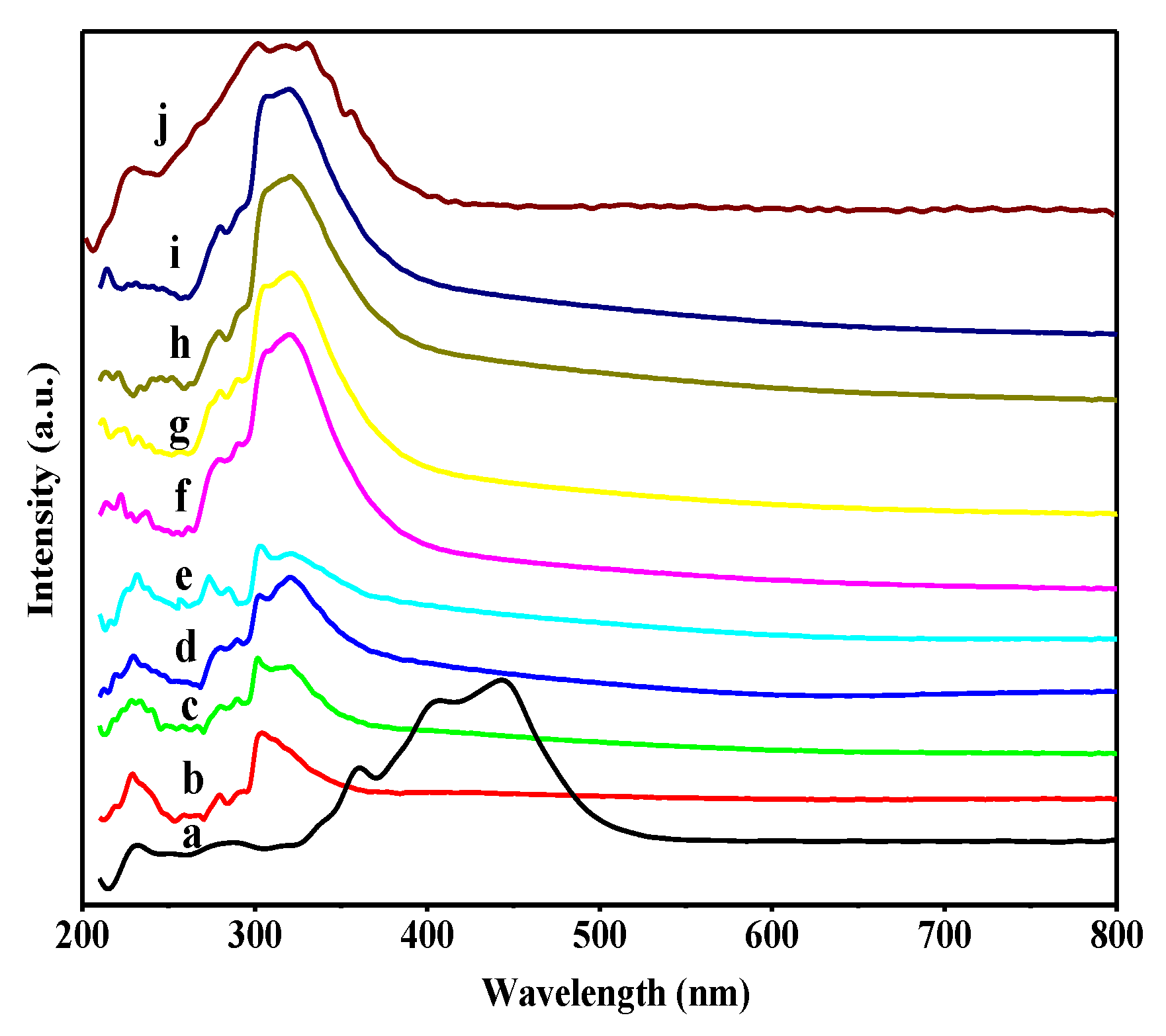
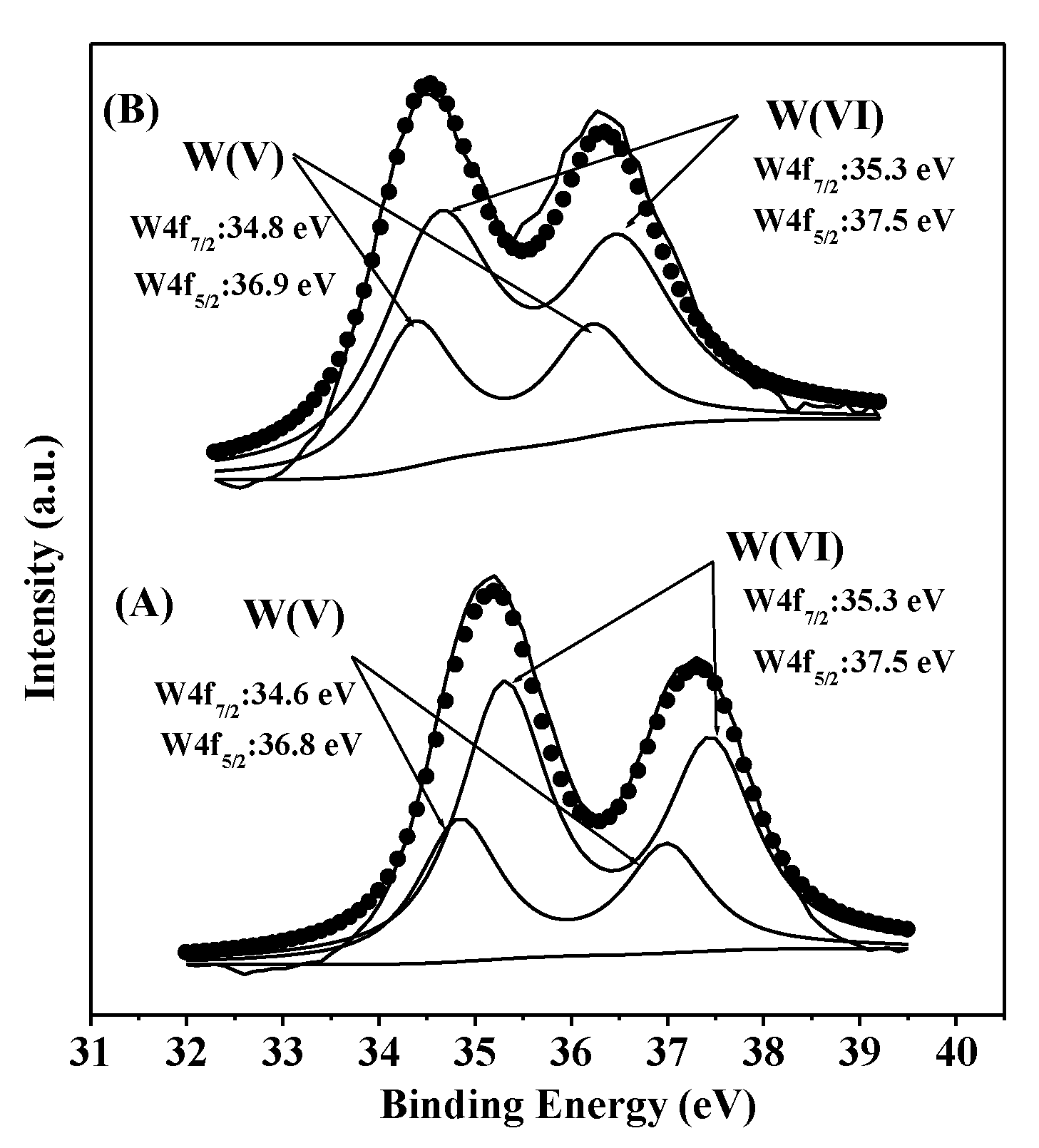

| Sample | SLangmuir (m2·g−1) | SBET (m2·g−1) | Pore Diameter (nm) | Pore Volume (cm3·g−1) | W6+/W5+ a |
|---|---|---|---|---|---|
| UiO-66 | 1344 | 1062 | 1.9 | 0.46 | - |
| 40 wt %WO3/UiO-66 | 817 | 684 | 2.0 | 0.34 | 2.1 |
| 45 wt % WO3/UiO-66 | 736 | 592 | 2.1 | 0.32 | - |
| 15 wt %B2O3-40 wt %WO3/UiO-66 | 809 | 677 | 2.1 | 0.36 | 2.2 |
| Sample (WO3/UiO-66 or B2O3-WO3/UiO-66) | WO3 Contents wt % b | B2O3 Contents wt % b | Conversion of CPE (%) | GA Yield (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|---|
| GA | CPDL | CPLE | Others c | |||||
| UiO-66 | - | - | 21.2 | 3.8 | 18.0 | 13.6 | 12.3 | 56.1 |
| 20 wt %WO3 | 19.3 | - | 75.5 | 24.8 | 32.9 | 9.5 | 32.0 | 25.6 |
| 30 wt %WO3 | 30.7 | - | 90.5 | 35.3 | 39.0 | 10.1 | 29.5 | 21.4 |
| 35 wt %WO3 | 34.8 | - | 96.7 | 47.0 | 48.6 | 12.1 | 27.1 | 12.2 |
| 40 wt %WO3 | 41.3 | - | 100 | 64.9 | 64.9 | 13.5 | 14.2 | 7.4 |
| 45 wt %WO3 | 45.9 | - | 100 | 63.8 | 63.8 | 12.9 | 13.6 | 9.7 |
| 5 wt %B2O3-40 wt %WO3 | 40.8 | 4.6 | 100 | 70.0 | 70.0 | 5.8 | 14.7 | 9.5 |
| 10 wt %B2O3-40 wt %WO3 | 41.3 | 9.3 | 100 | 71.6 | 71.6 | 6.1 | 13.6 | 8.7 |
| 15 wt %B2O3-40 wt %WO3 | 40.9 | 14.5 | 100 | 73.4 | 73.4 | 4.5 | 12.1 | 10.0 |
| 20 wt %B2O3-40 wt %WO3 | 41.1 | 20.3 | 100 | 69.6 | 69.6 | 5.2 | 15.2 | 10.0 |
| 11.4 wt %W-HMS | - | - | 100 | 76.3 | 76.3 | 14.6 | 7.8 | 1.3 |
| Entry | Conversion of CPE (%) | GA Yield (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| GA | CPDL | CPLE | Others | |||
| 1 | 100 | 73.8 | 73.8 | 4.3 | 11.6 | 10.3 |
| 2 | 100 | 72.9 | 72.9 | 4.1 | 10.9 | 12.1 |
| 3 | 98.2 | 68.9 | 70.2 | 4.6 | 9.8 | 15.4 |
| 4 | 97.3 | 67.0 | 68.9 | 4.8 | 9.2 | 17.1 |
| 5 | 96.8 | 65.8 | 68.0 | 5.9 | 9.6 | 16.5 |
| 6 | 96.0 | 64.7 | 67.4 | 5.7 | 9.8 | 17.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wu, N.; Miao, Y.; Li, H. Modification Effects of B2O3 on The Structure and Catalytic Activity of WO3-UiO-66 Catalyst. Nanomaterials 2018, 8, 781. https://doi.org/10.3390/nano8100781
Yang X, Wu N, Miao Y, Li H. Modification Effects of B2O3 on The Structure and Catalytic Activity of WO3-UiO-66 Catalyst. Nanomaterials. 2018; 8(10):781. https://doi.org/10.3390/nano8100781
Chicago/Turabian StyleYang, Xinli, Nan Wu, Yongxia Miao, and Haobo Li. 2018. "Modification Effects of B2O3 on The Structure and Catalytic Activity of WO3-UiO-66 Catalyst" Nanomaterials 8, no. 10: 781. https://doi.org/10.3390/nano8100781
APA StyleYang, X., Wu, N., Miao, Y., & Li, H. (2018). Modification Effects of B2O3 on The Structure and Catalytic Activity of WO3-UiO-66 Catalyst. Nanomaterials, 8(10), 781. https://doi.org/10.3390/nano8100781