Electrospun Poly(ethylene-co-vinyl alcohol)/Graphene Nanoplatelets Composites of Interest in Intelligent Food Packaging Applications
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Oxidation of Graphite
2.3. Reduction of Graphite Oxide
2.4. Preparation of Electrospun Fiber Mats
2.5. Morphology
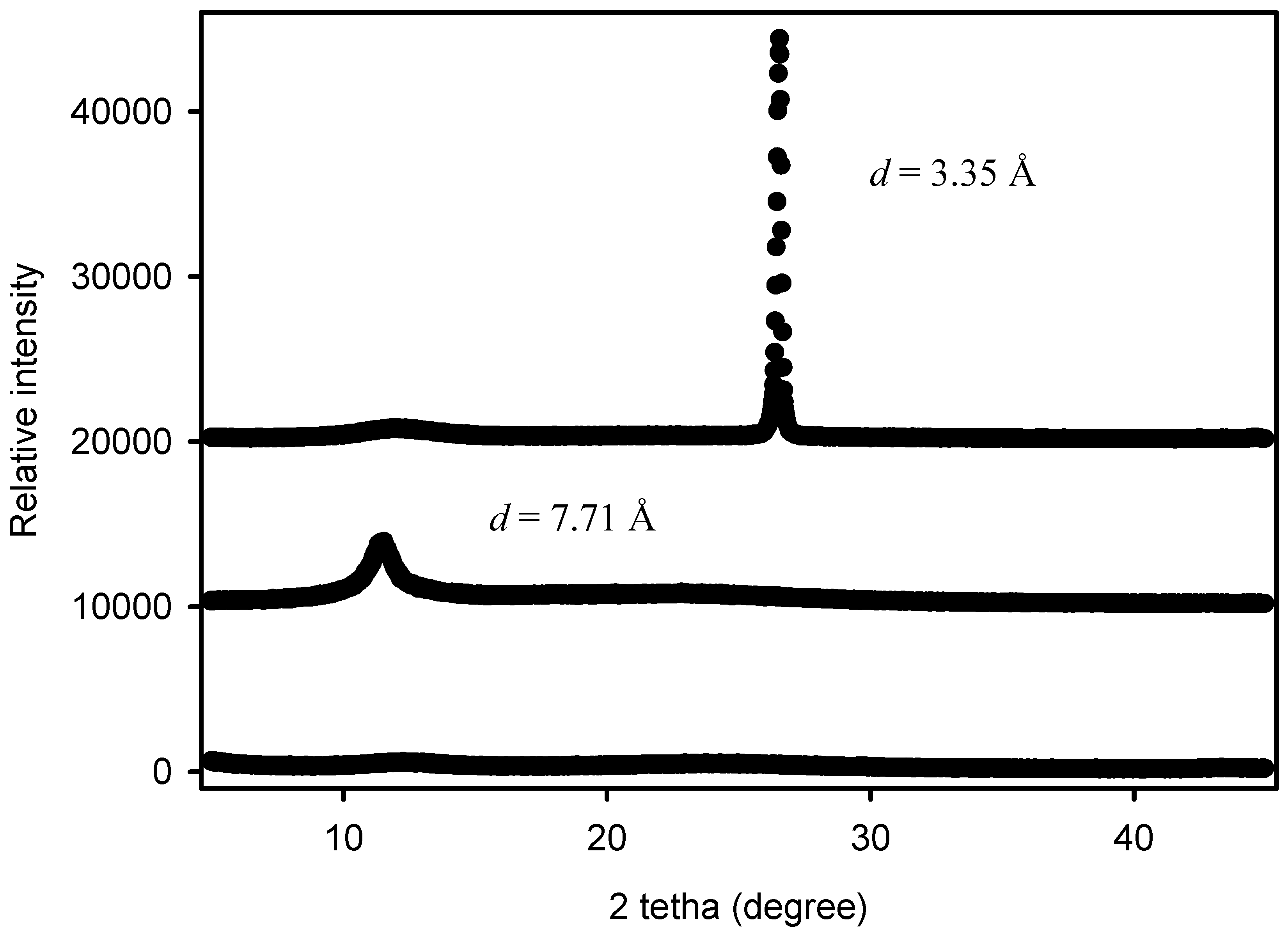
2.6. Wide Angle X-ray Scattering
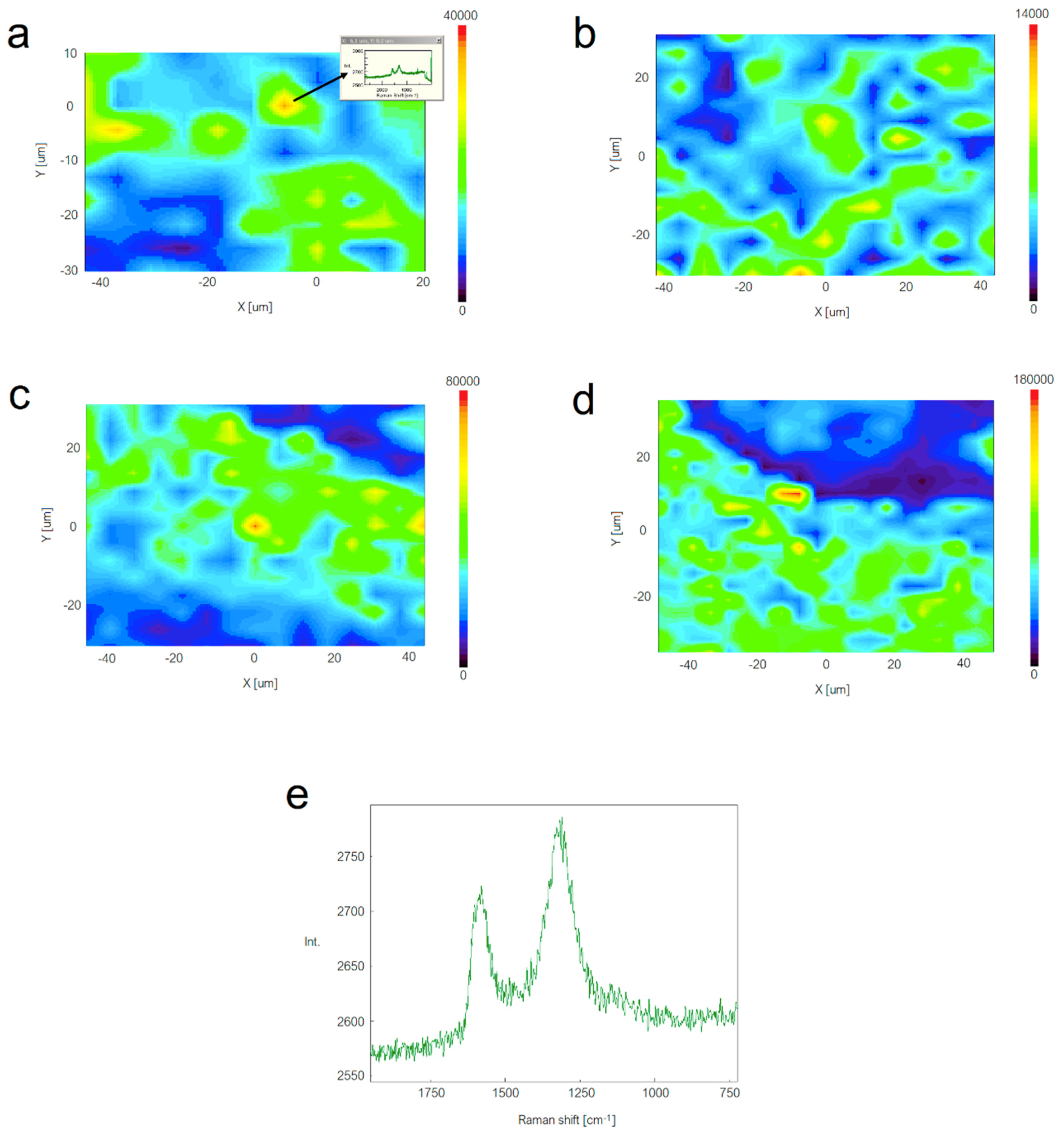
2.7. Raman Imaging
2.8. Thermal Analysis
2.9. Dielectrical Performance and Electrical Conductivity
3. Results and Discussion
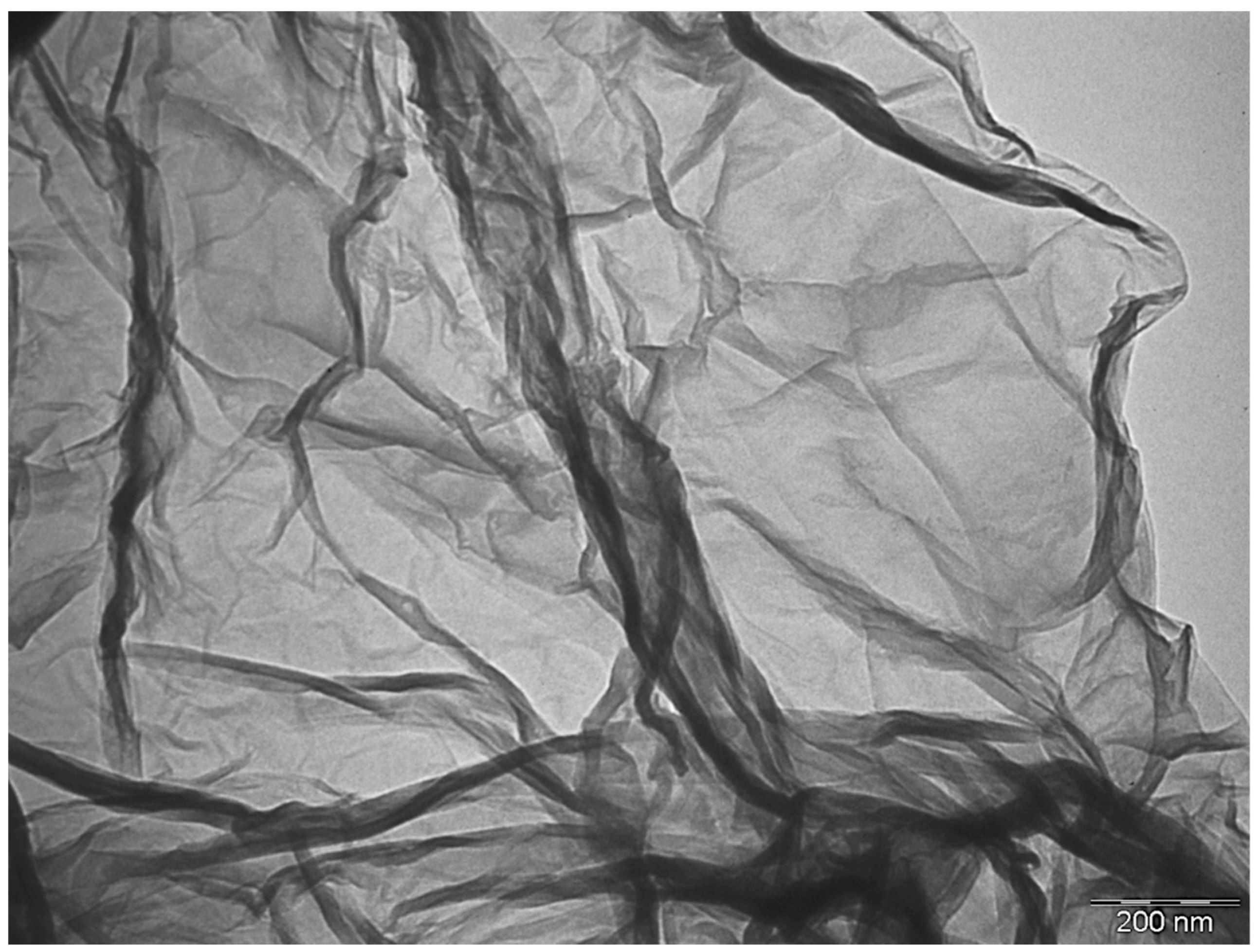
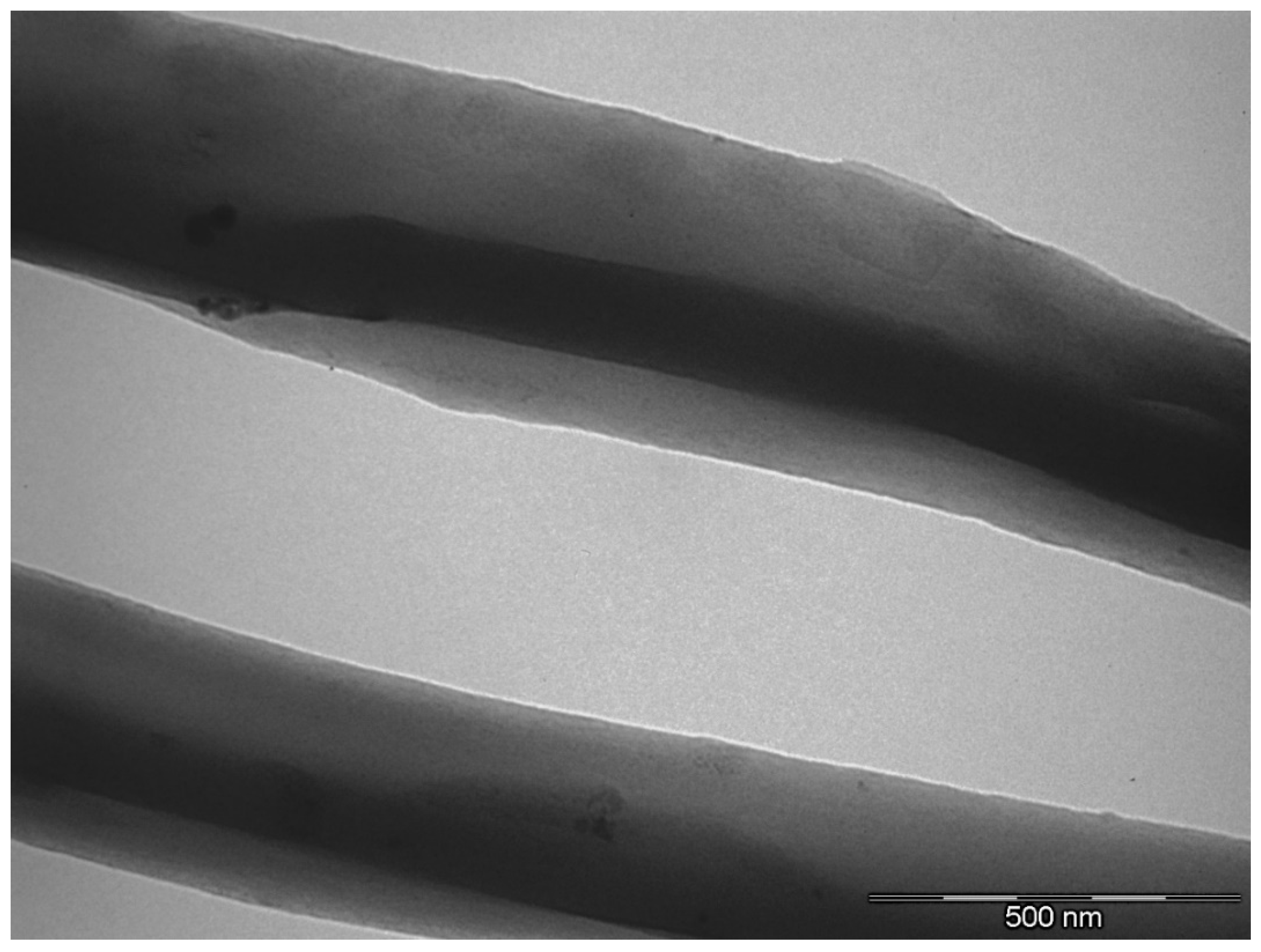
3.1. Characterization of GNPs
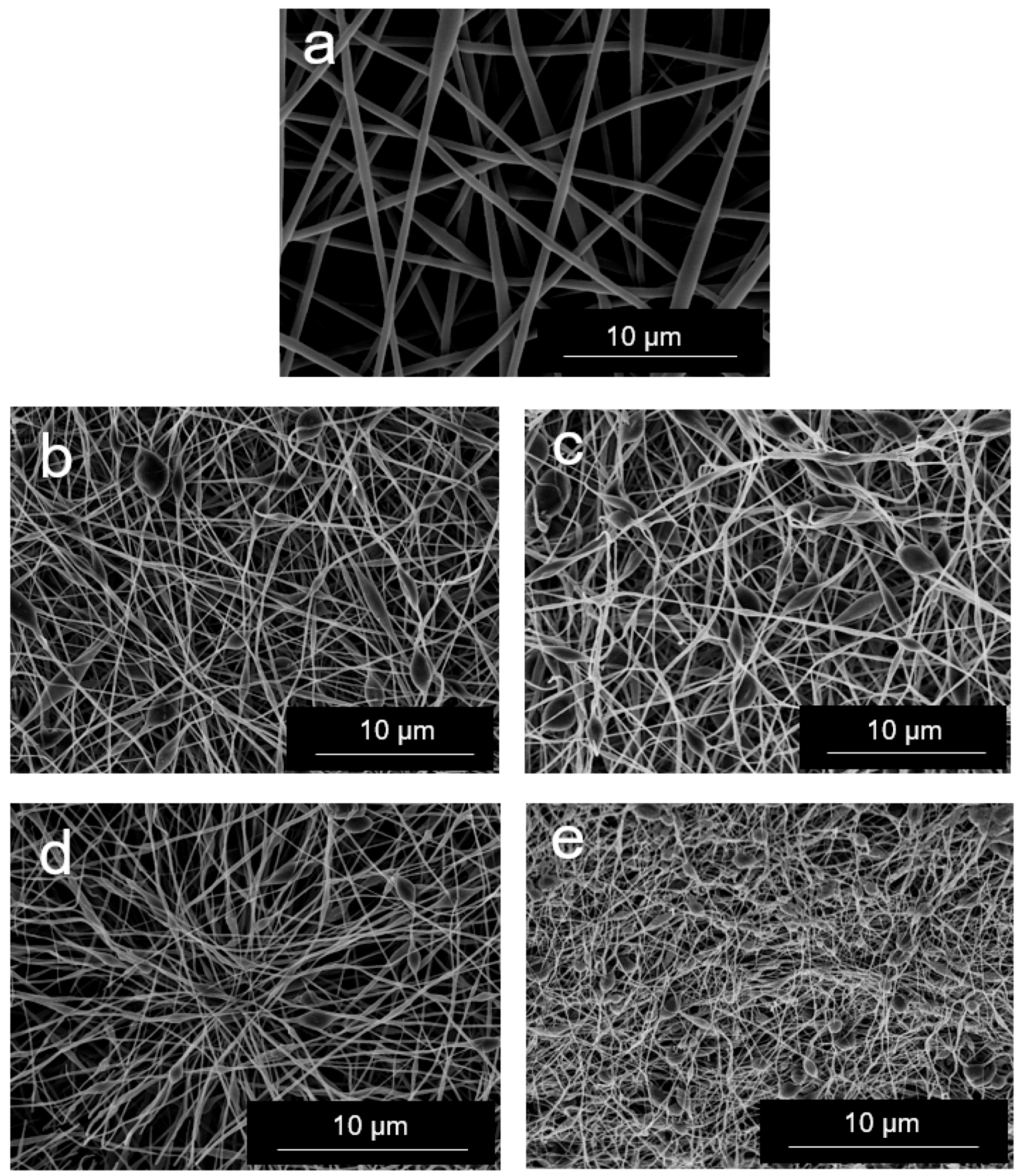
3.2. Morphology of EVOH/GNPs Fibers
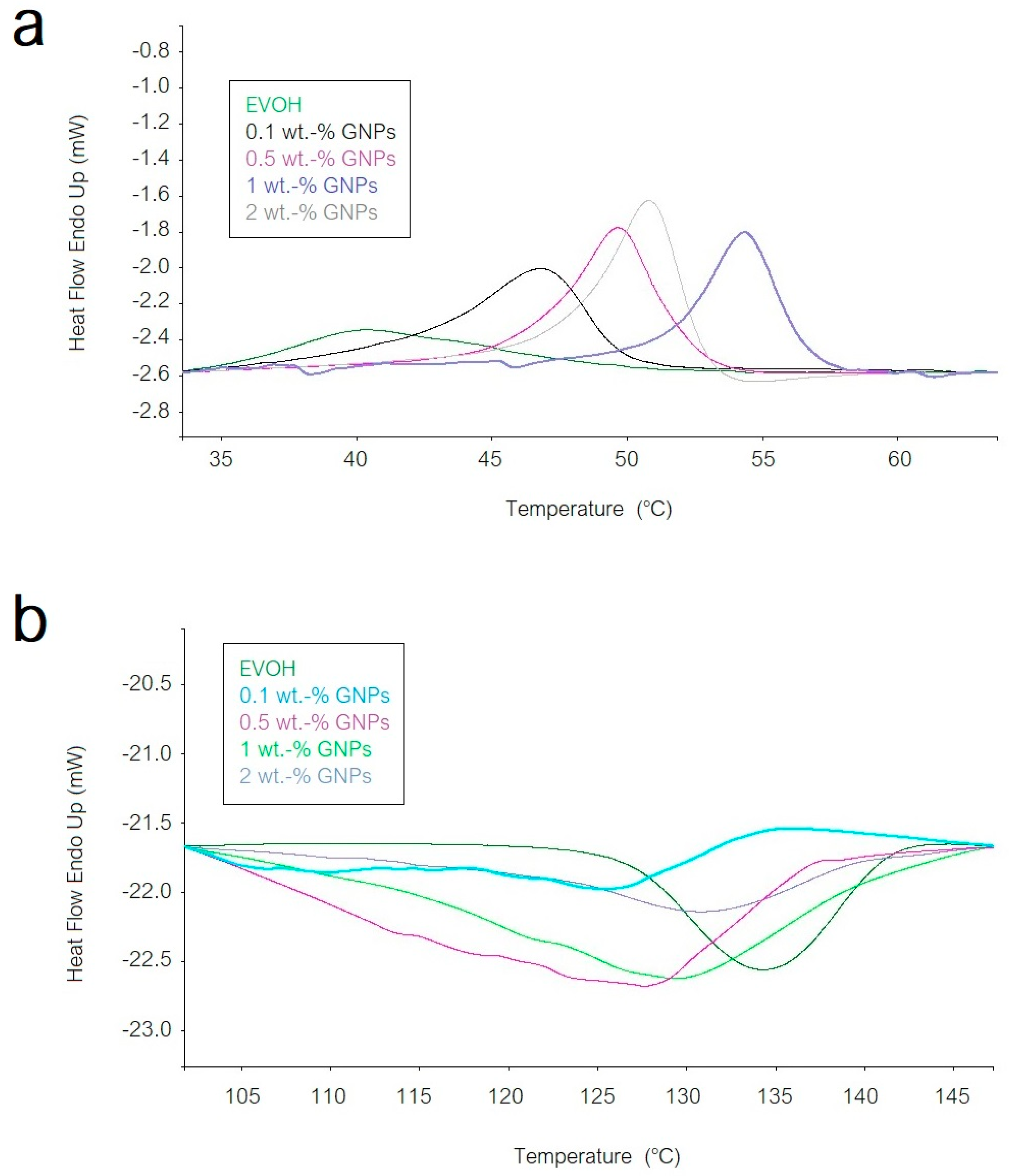
3.3. Thermal Properties of EVOH/GNPs Fibers
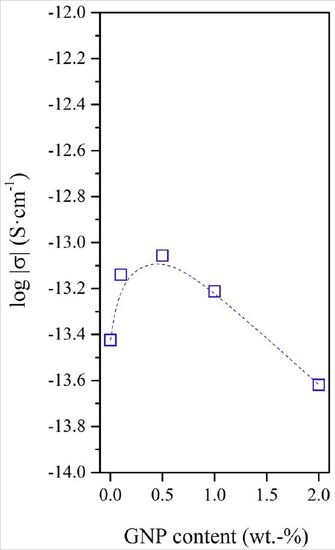
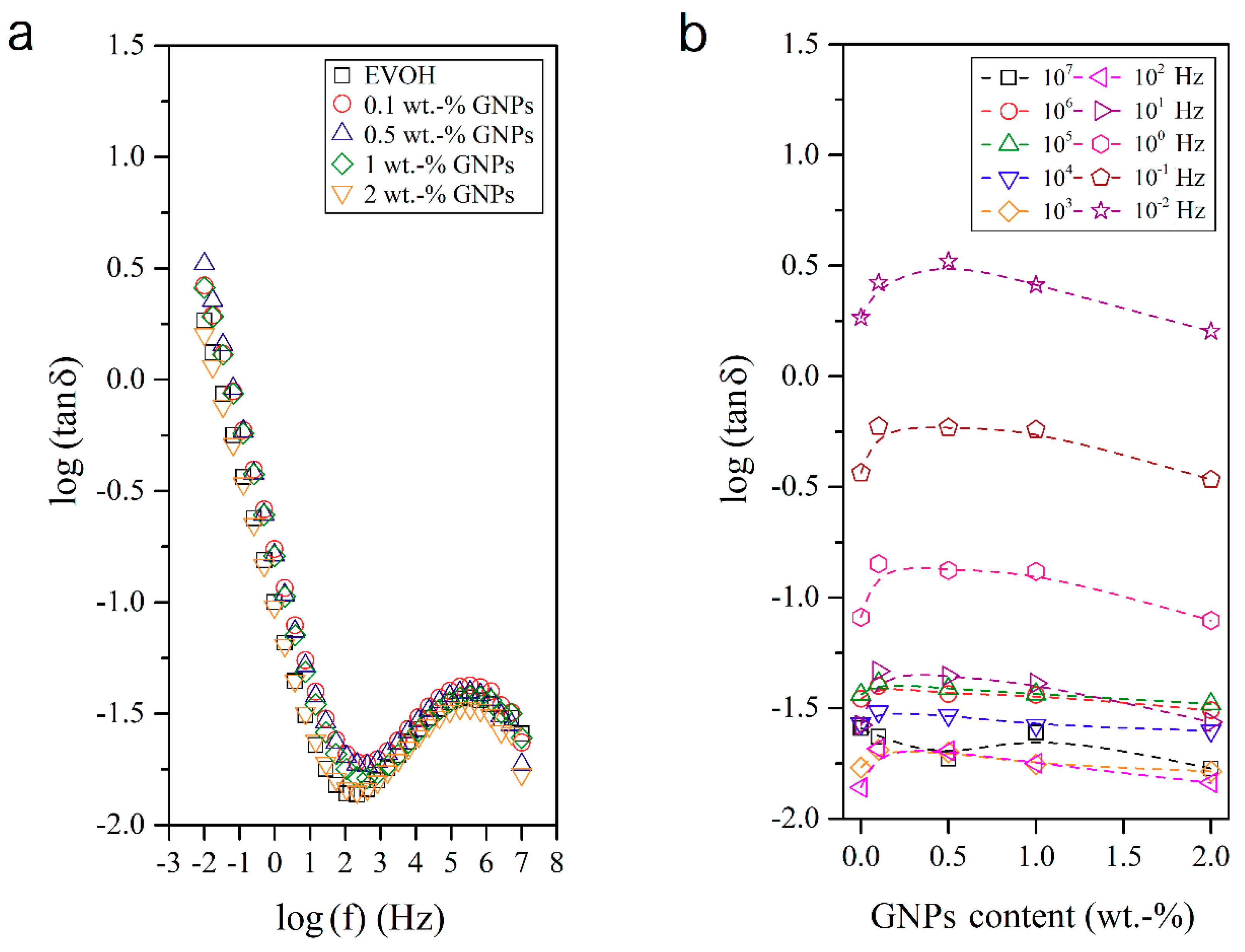
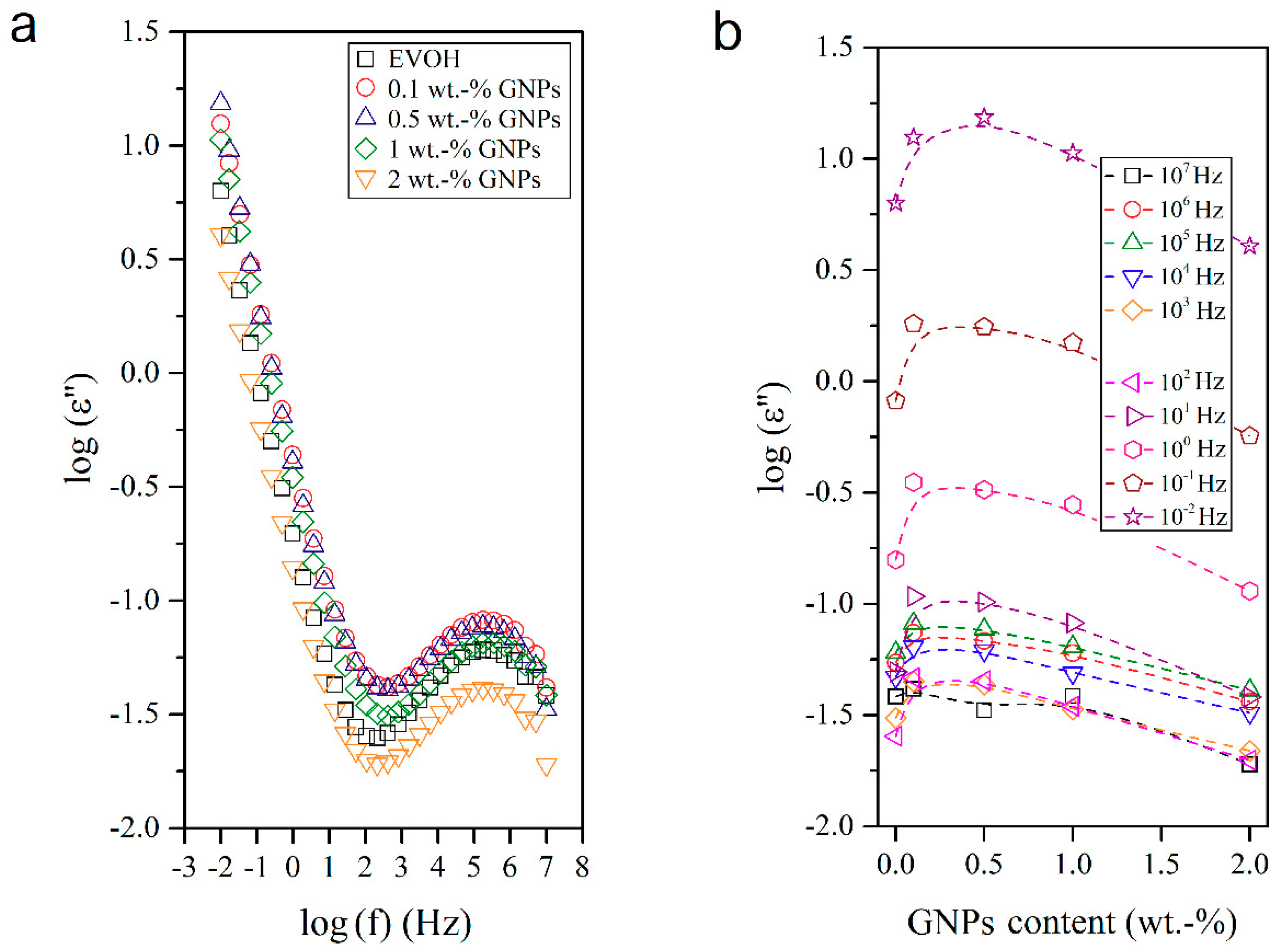
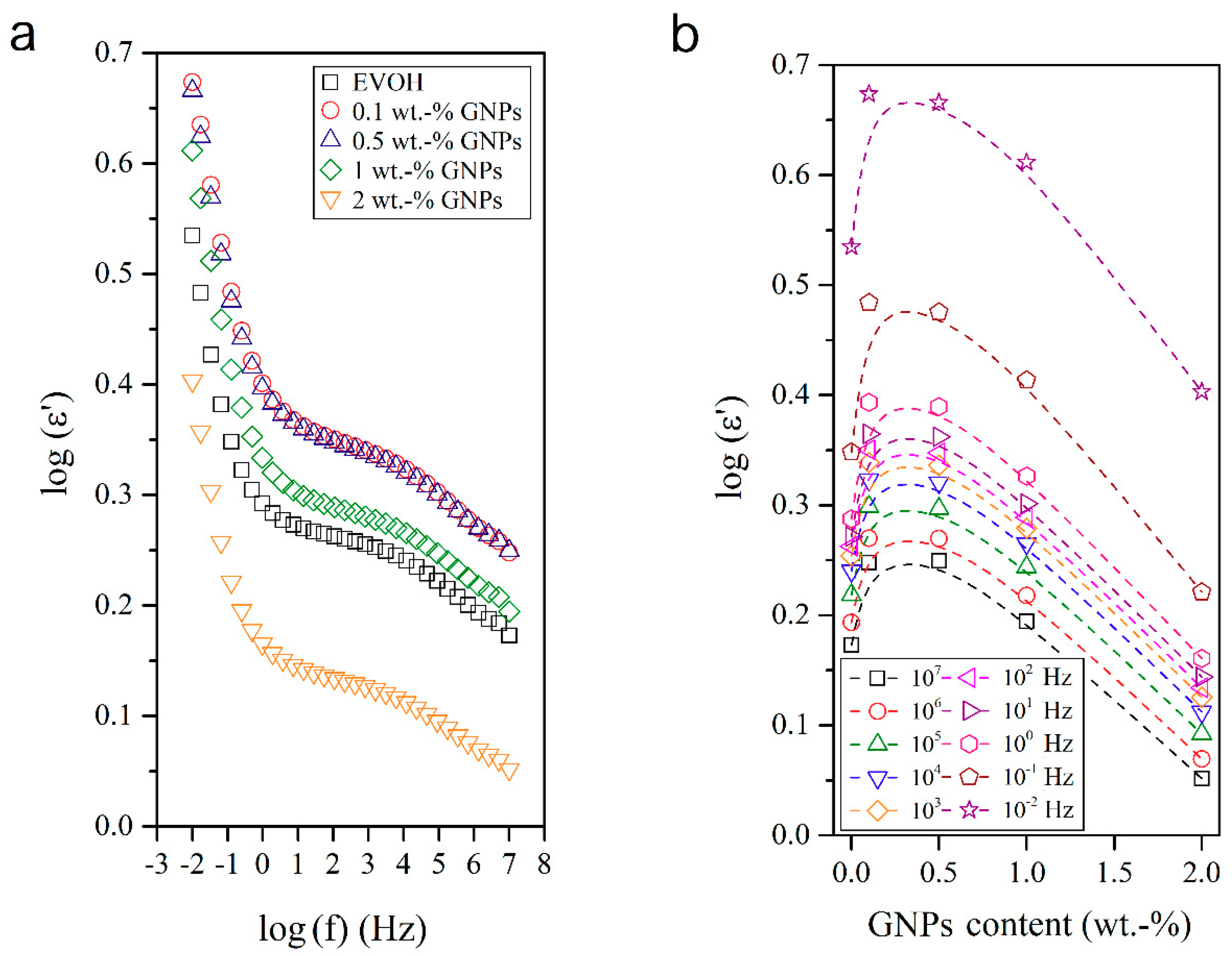
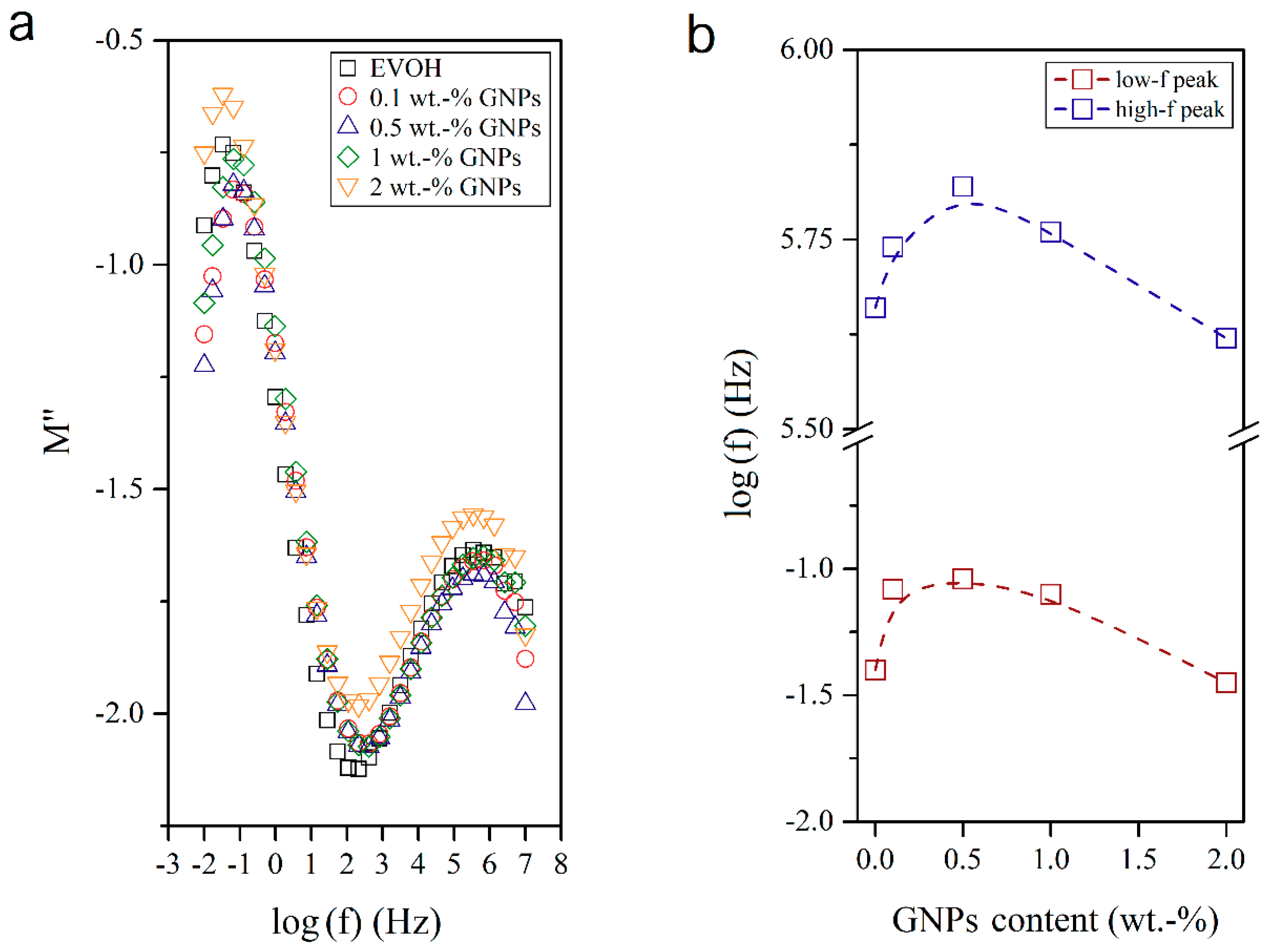
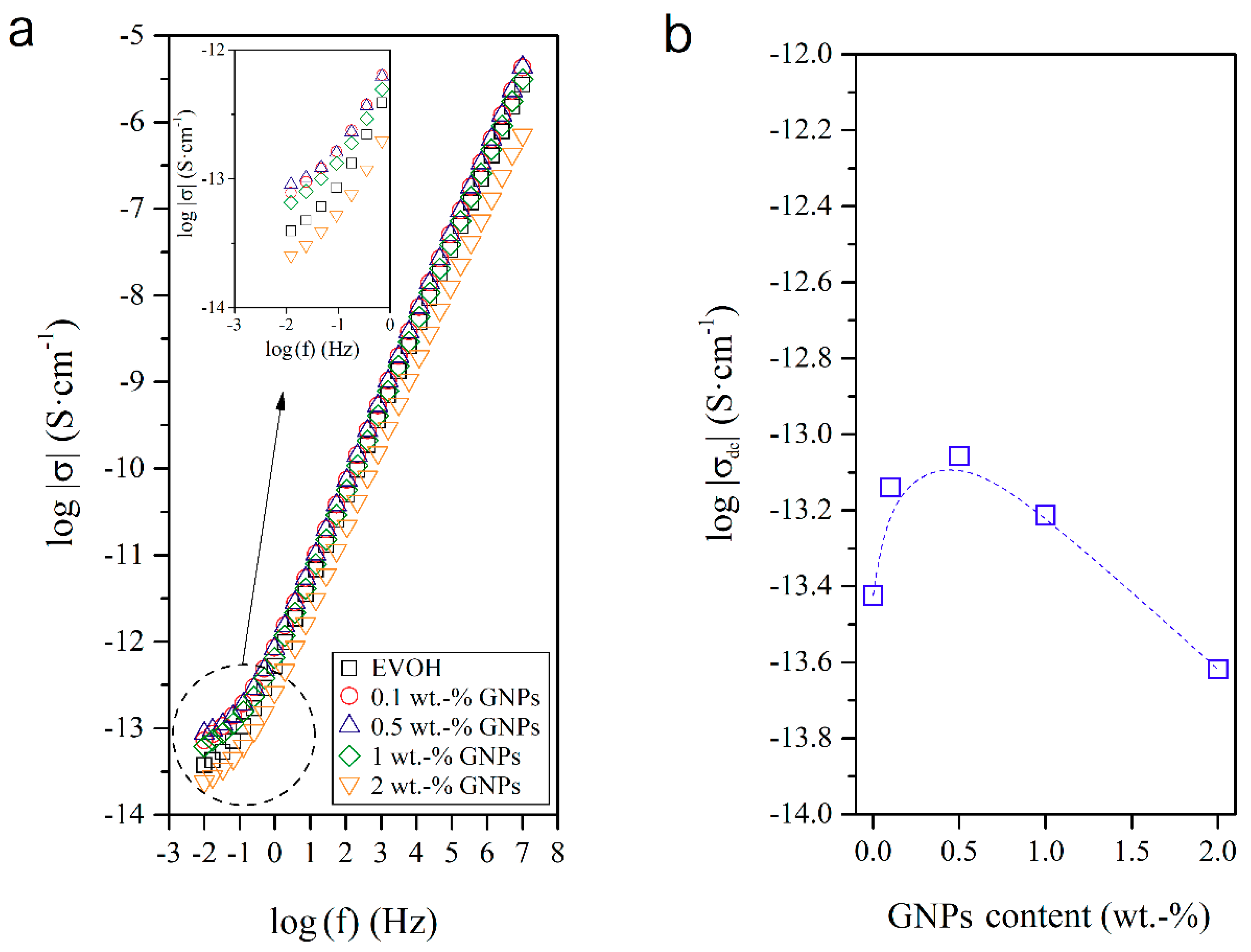
3.4. Dielectrical Performance and Electrical Conductivity of EVOH/GNPs Fibers
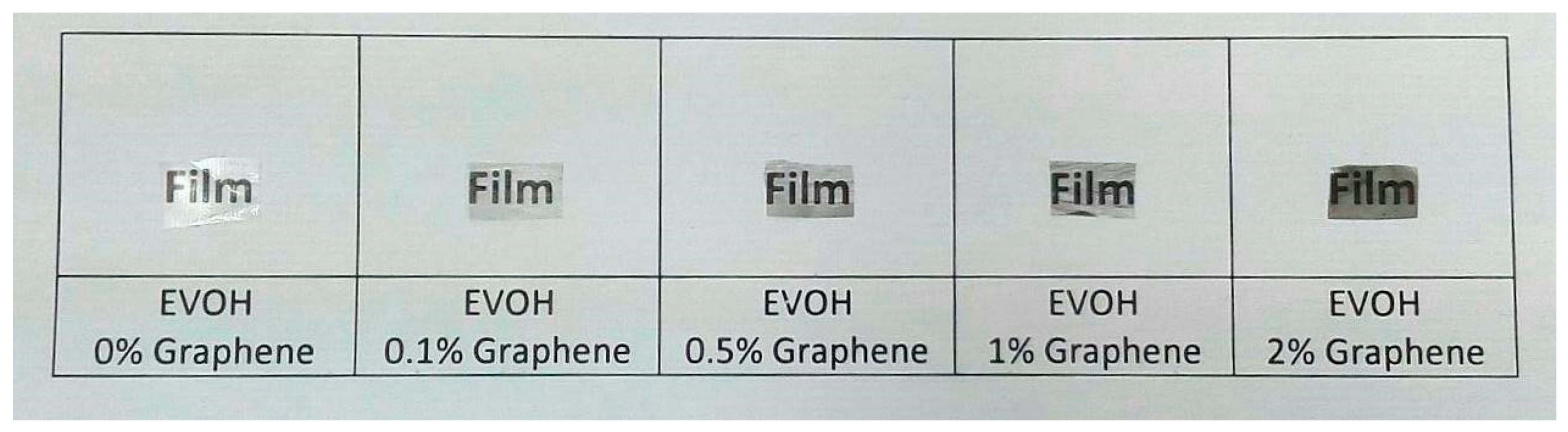
3.5. Electrospun EVOH/GNPs Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torres-Giner, S.; Gil, L.; Pascual-Ramírez, L.; Garde-Belza, J.A. Packaging: Food waste reduction. In Encyclopedia of Polymer Applications; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Echegoyen, Y. Nano-developments for food packaging and labeling applications. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Basel, Switzerland, 2015; pp. 141–166. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Liu, W.; Xue, M.; Xie, Z.; Zhao, D.; Zhang, M.; Chen, J.; Cao, T. Graphene as a conductive additive to enhance the high-rate capabilities of electrospun li4ti5o12 for lithium-ion batteries. Electrochim. Acta 2010, 55, 5813–5818. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S. Preparation of conductive carbon black-filled polymer nanocomposites via melt compounding. In Conductive Materials and Composites; Mitchell, V., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2016; pp. 117–164. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Pérez-Masiá, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Torres-Giner, S. Electrospun nanofibers for food packaging applications. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Lagaron, J.M., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 108–125. [Google Scholar]
- Torres-Giner, S.; Busolo, M.; Cherpinski, A.; Lagaron, J.M. Electrospinning in the packaging industry. In Electrospinning: From Basic Research to Commercialization; Kny, E., Ghosal, K., Thomas, S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 238–260. [Google Scholar]
- Torres-Giner, S.; Lagaron, J.M. Zein-based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. I. Morphology and thermal properties. J. Appl. Polym. Sci. 2010, 118, 778–789. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Lagaron, J.M. Zein-based ultrathin fibers containing ceramic nanofillers obtained by electrospinning. II. Mechanical properties, gas barrier, and sustained release capacity of biocide thymol in multilayer polylactide films. J. Appl. Polym. Sci. 2014, 131, 9270–9276. [Google Scholar] [CrossRef]
- Cherpinski, A.; Gozutok, M.; Sasmazel, H.T.; Torres-Giner, S.; Lagaron, J.M. Electrospun oxygen scavenging films of poly(3-hydroxybutyrate) containing palladium nanoparticles for active packaging applications. Nanomaterials 2018, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Asmatulu, R.; Ceylan, M.; Nuraje, N. Study of superhydrophobic electrospun nanocomposite fibers for energy systems. Langmuir 2011, 27, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhang, H.; Yang, J.; Wang, S.; Tang, D.Y.; Jose, R.; Ramakrishna, S.; Lim, C.T.; Loh, K.P. Graphene–polymer nanofiber membrane for ultrafast photonics. Adv. Funct. Mater. 2010, 20, 782–791. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhao, Y.; Lou, J.; Zhao, X.; Wang, R.; Liang, Q.; Huang, Z. Electrospinning fabrication and in situ mechanical investigation of individual graphene nanoribbon reinforced carbon nanofiber. Carbon 2017, 114, 717–723. [Google Scholar] [CrossRef]
- Moayeri, A.; Ajji, A. Core-shell structured graphene filled polyaniline/poly(methyl methacrylate) nanofibers by coaxial electrospinning. Nanosci. Nanotechnol. Lett. 2016, 8, 129–134. [Google Scholar] [CrossRef]
- Moayeri, A.; Ajji, A. High capacitance carbon nanofibers from poly(acrylonitrile) and poly(vinylpyrrolidone)-functionalized graphene by electrospinning. J. Nanosci. Nanotechnol. 2017, 17, 1820–1829. [Google Scholar] [CrossRef]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-film particles of graphite oxide 1: High-yield synthesis and flexibility of the particles. Carbon 2004, 42, 2929–2937. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- van Turnhout, J.; Wübbenhorst, M. Analysis of complex dielectric spectra. Ii: Evaluation of the activation energy landscape by differential sampling. J. Non-Cryst. Solids 2002, 305, 50–58. [Google Scholar] [CrossRef]
- Macedo, P.B.; Moynihan, C.T.; Bose, R. Role of ionic diffusion in polarization in vitreous ionic conductors. Phys. Chem. Glasses 1972, 13, 171–179. [Google Scholar]
- Dyre, J.C. Some remarks on ac conduction in disordered solids. J. Non-Cryst. Solids 1991, 135, 219–226. [Google Scholar] [CrossRef]
- Yasmin, A.; Daniel, I.M. Mechanical and thermal properties of graphite platelet/epoxy composites. Polymer 2004, 45, 8211–8219. [Google Scholar] [CrossRef]
- Simon, D.A.; Bischoff, E.; Buonocore, G.G.; Cerruti, P.; Raucci, M.G.; Xia, H.; Schrekker, H.S.; Lavorgna, M.; Ambrosio, L.; Mauler, R.S. Graphene-based masterbatch obtained via modified polyvinyl alcohol liquid-shear exfoliation and its application in enhanced polymer composites. Mater. Des. 2017, 134, 103–110. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Olsson, R.T.; Lopez-Rubio, A.; Lagaron, J.M. Development of electrospun evoh fibres reinforced with bacterial cellulose nanowhiskers. Part i: Characterization and method optimization. Cellulose 2011, 18, 335–347. [Google Scholar] [CrossRef]
- Sukumaran, S.S.; Rekha, C.R.; Resmi, A.N.; Jinesh, K.B.; Gopchandran, K.G. Raman and scanning tunneling spectroscopic investigations on graphene-silver nanocomposites. J. Sci. Adv. Mater. Devices 2018. [Google Scholar] [CrossRef]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Gouanvé, F.; Espuche, E. Effect of humidity on mechanical, thermal and barrier properties of evoh films. J. Membr. Sci. 2017, 540, 1–9. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Harris, S.; McEwen, I.J. Physical aging in poly(vinyl acetate). 2. Relative rates of volume and enthalpy relaxation. Macromolecules 1998, 31, 2611–2615. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Smith, S.; Horne, B.; Gourlay, G.M. Physical aging of polycarbonate: Enthalpy relaxation, creep response, and yielding behavior. Macromolecules 1999, 32, 5046–5061. [Google Scholar] [CrossRef]
- Dhawan, S.; Barbosa-Cànovas, G.V.; Tang, J.; Sablani, S.S. Oxygen barrier and enthalpy of melting of multilayer evoh films after pressure-assisted thermal processing and during storage. J. Appl. Polym. Sci. 2011, 122, 1538–1545. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Santonja-Blasco, L.; Badia, J.D.; Imrie, C.T.; Ribes-Greus, A. Characterization of functionalized side-chain liquid crystal methacrylates containing nonmesogenic units by dielectric spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 8722–8731. [Google Scholar] [CrossRef]
- Badia, J.D.; Reig-Rodrigo, P.; Teruel-Juanes, R.; Kittikorn, T.; Strömberg, E.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Effect of sisal and hydrothermal ageing on the dielectric behaviour of polylactide/sisal biocomposites. Compos. Sci. Technol. 2017, 149, 1–10. [Google Scholar] [CrossRef]
- Salaberría, A.M.; Teruel-Juanes, R.; Badia, J.D.; Fernandes, S.C.M.; de Juano-Arbona, V.S.; Labidi, J.; Ribes-Greus, A. Influence of chitin nanocrystals on the dielectric behaviour and conductivity of chitosan-based bionanocomposites. Compos. Sci. Technol. 2018, 167, 323–330. [Google Scholar] [CrossRef]
- Badia, J.D.; Monreal, L.; de Juano-Arbona, V.S.; Ribes-Greus, A. Dielectric spectroscopy of recycled polylactide. Polym. Degrad. Stab. 2014, 107, 21–27. [Google Scholar] [CrossRef]
- Bizet, A.; Nakamura, N.; Teramoto, Y.; Hatakeyama, T. The influence of moisture on the dielectric properties of poly(ethylene-co-vinyl alcohol). Thermochim. Acta 1994, 241, 191–198. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Chiva-Flor, A.; Feijoo, J.L. Injection-molded parts of polypropylene/multi-wall carbon nanotubes composites with an electrically conductive tridimensional network. Polym. Compos. 2016, 37, 488–496. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Pissis, P.; Kyritsis, A. Electrical conductivity studies in hydrogels. Solid State Ionics 1997, 97, 105–113. [Google Scholar] [CrossRef]
- Raistrick, I.D.; Franceschetti, D.R.; Macdonald, J.R. Theory. In Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 27–128. [Google Scholar]
- Miyairi, K. Low-frequency dielectric response of polyethylene terephthalate (pet) films. J. Phys. D Appl. Phys. 1986, 19, 1973–1980. [Google Scholar] [CrossRef]
- Neagu, E.; Pissis, P.; Apekis, L. Electrical conductivity effects in polyethylene terephthalate films. J. Appl. Phys. 2000, 87, 2914–2922. [Google Scholar] [CrossRef]
- Yamamoto, K.; Namikawa, H. Conduction current relaxation of inhomogeneous conductor I. Jpn. J. Appl. Phys. 1988, 27, 1845–1851. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Torres-Giner, S.; Pardo-Figuerez, M.; Álvarez-Láinez, M.; Lagaron, J.M. Superhydrophobic bilayer coating based on annealed electrospun ultrathin poly(ε-caprolactone) fibers and electrosprayed nanostructured silica microparticles for easy emptying packaging applications. Coatings 2018, 8, 173. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Post-processing optimization of electrospun submicron poly(3-hydroxybutyrate) fibers to obtain continuous films of interest in food packaging applications. Food Addit. Contam. 2017, 34, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and characterization of electrospun food biopackaging films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) derived from fruit pulp biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
| GNPs Content (wt.-%) | dIG-ID (cm−1) | ID/IG |
|---|---|---|
| 0.1 | 261 | 0.986 |
| 0.5 | 257 | 0.991 |
| 1 | 267 | 0.981 |
| 2 | 270 | 0.962 |
| GNPs Content (wt.-%) | σdc (S cm−1) × 1014 |
|---|---|
| 0.0 | 3.77 |
| 0.1 | 7.25 |
| 0.5 | 8.78 |
| 1.0 | 6.14 |
| 2.0 | 2.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Giner, S.; Echegoyen, Y.; Teruel-Juanes, R.; Badia, J.D.; Ribes-Greus, A.; Lagaron, J.M. Electrospun Poly(ethylene-co-vinyl alcohol)/Graphene Nanoplatelets Composites of Interest in Intelligent Food Packaging Applications. Nanomaterials 2018, 8, 745. https://doi.org/10.3390/nano8100745
Torres-Giner S, Echegoyen Y, Teruel-Juanes R, Badia JD, Ribes-Greus A, Lagaron JM. Electrospun Poly(ethylene-co-vinyl alcohol)/Graphene Nanoplatelets Composites of Interest in Intelligent Food Packaging Applications. Nanomaterials. 2018; 8(10):745. https://doi.org/10.3390/nano8100745
Chicago/Turabian StyleTorres-Giner, Sergio, Yolanda Echegoyen, Roberto Teruel-Juanes, Jose D. Badia, Amparo Ribes-Greus, and Jose M. Lagaron. 2018. "Electrospun Poly(ethylene-co-vinyl alcohol)/Graphene Nanoplatelets Composites of Interest in Intelligent Food Packaging Applications" Nanomaterials 8, no. 10: 745. https://doi.org/10.3390/nano8100745
APA StyleTorres-Giner, S., Echegoyen, Y., Teruel-Juanes, R., Badia, J. D., Ribes-Greus, A., & Lagaron, J. M. (2018). Electrospun Poly(ethylene-co-vinyl alcohol)/Graphene Nanoplatelets Composites of Interest in Intelligent Food Packaging Applications. Nanomaterials, 8(10), 745. https://doi.org/10.3390/nano8100745