Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: In Vitro Cellular Uptake
Abstract
1. Introduction
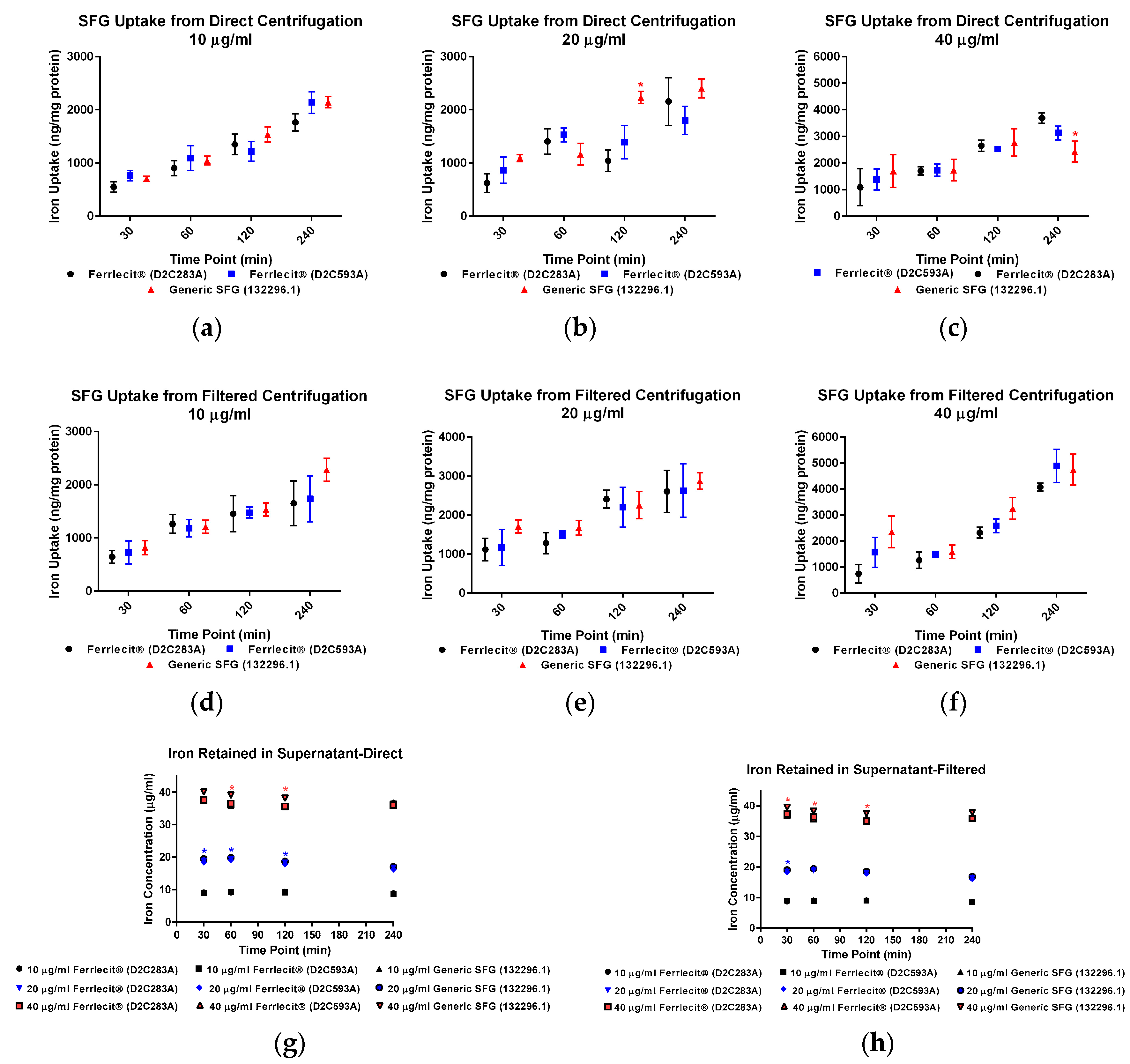
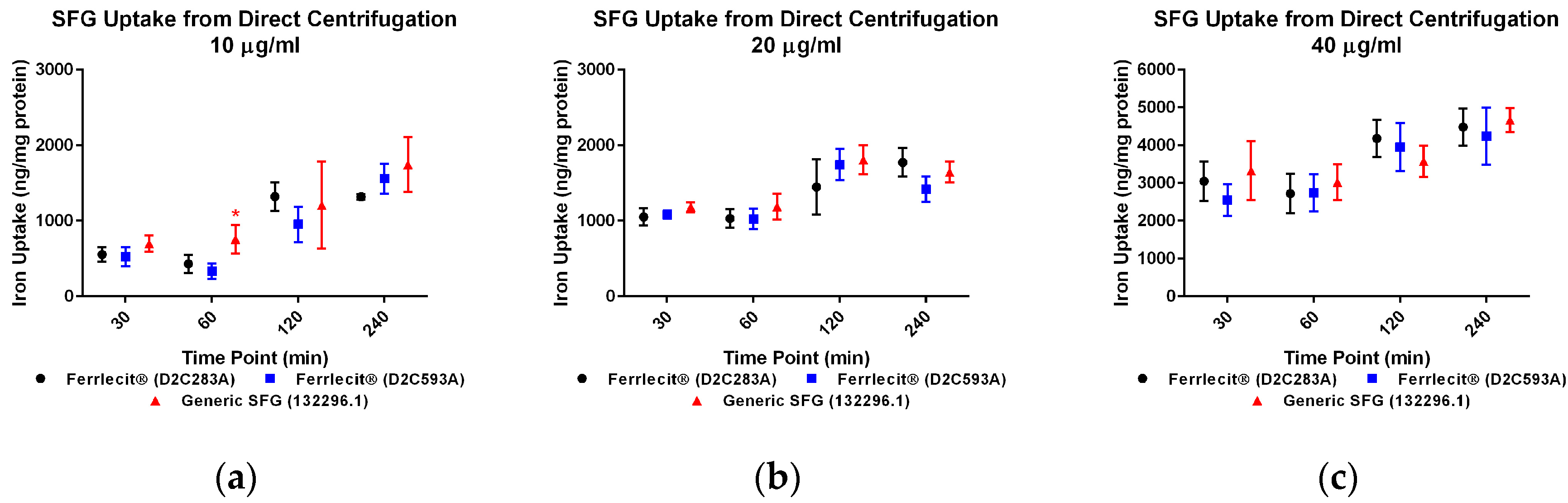
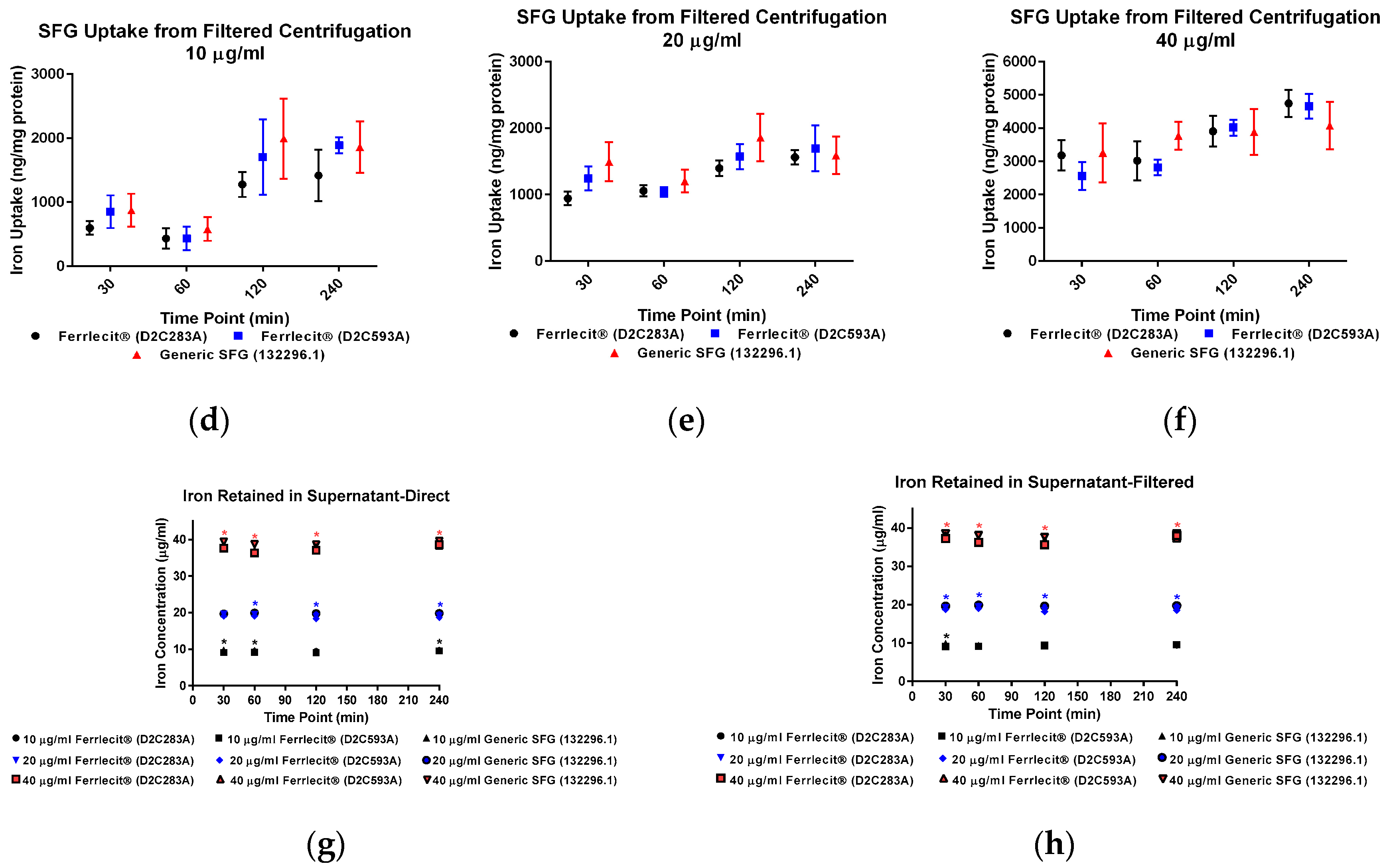
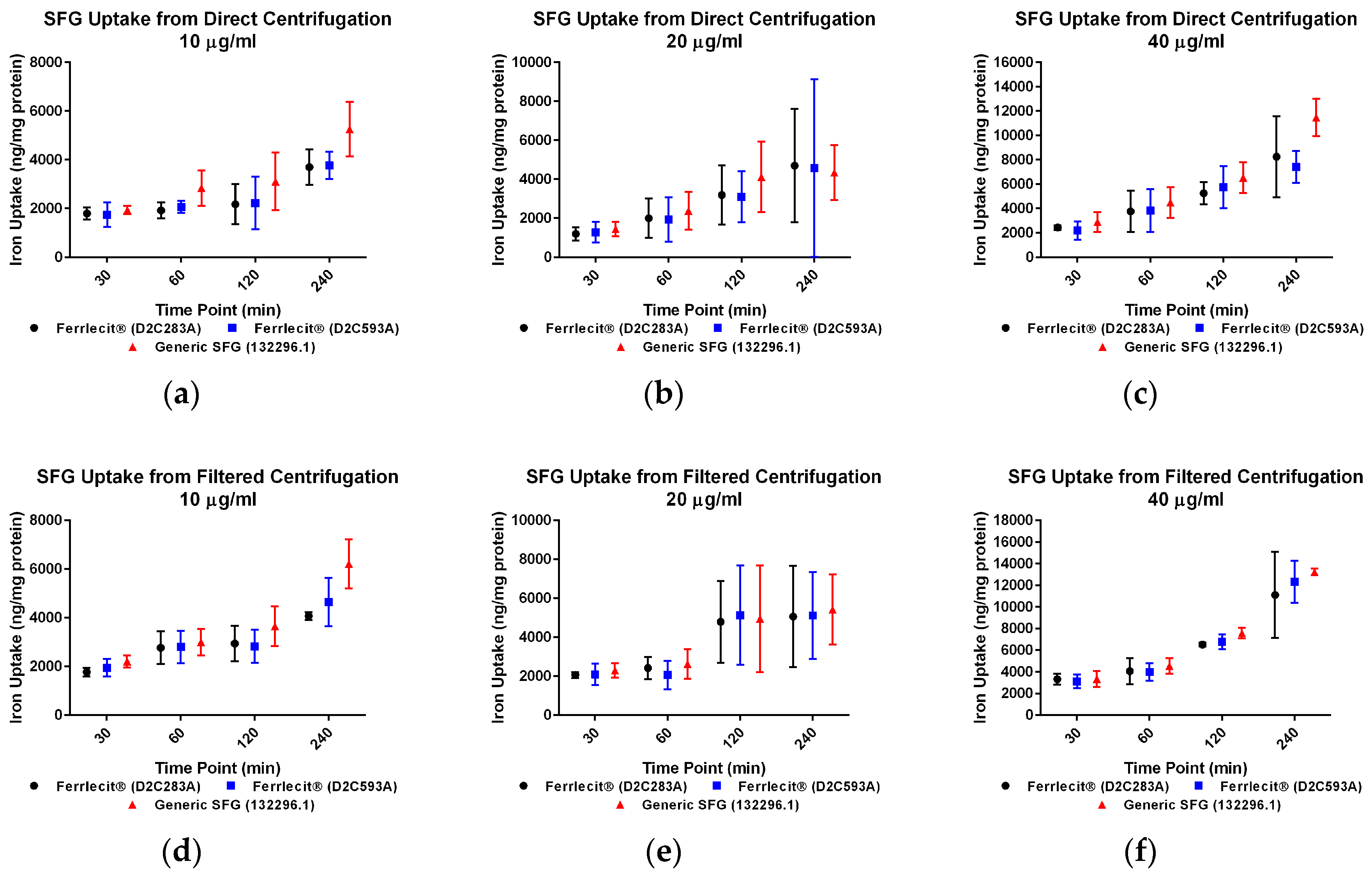
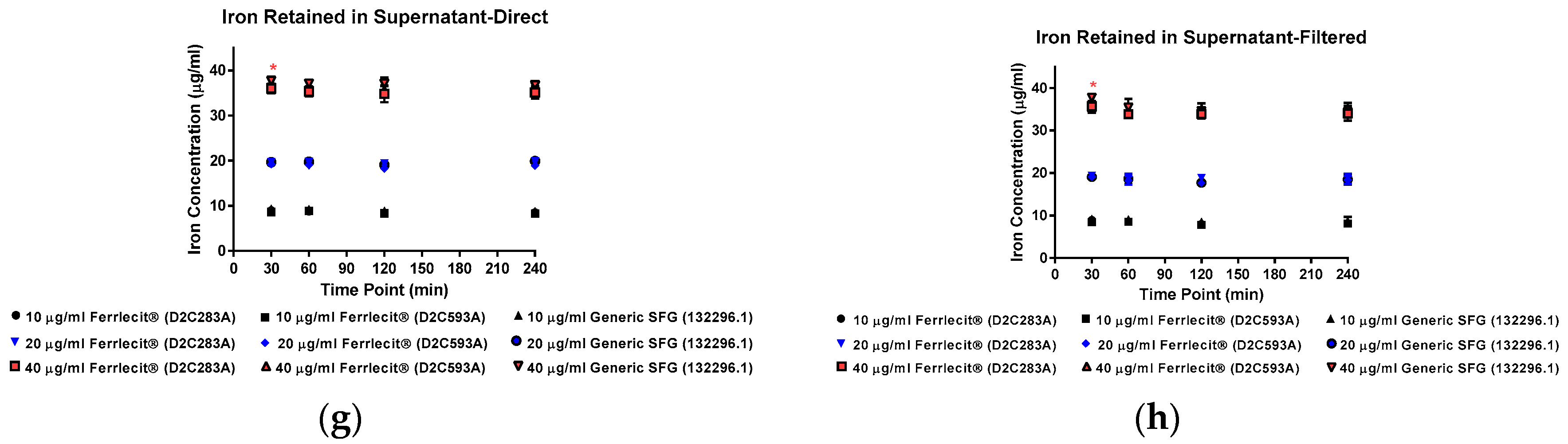
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Human Mononuclear Phagocyte Cell Lines
4.3. Drug Dose Selection and Cellular Uptake Study
4.4. Iron Uptake Assay
4.5. Calculation and Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Markowitz, G.S.; Kahn, G.A.; Feingold, R.E.; Coco, M.; Lynn, R.I. An evaluation of the effectiveness of oral iron therapy in hemodialysis patients receiving recombinant human erythropoietin. Clin. Nephrol. 1997, 48, 34–40. [Google Scholar] [PubMed]
- Qunibi, W.Y.; Martinez, C.; Smith, M.; Benjamin, J.; Mangione, A.; Roger, S.D. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol. Dial. Transplant. 2011, 26, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Gennaro, F.D. Switching Patients with Non-Dialysis Chronic Kidney Disease from Oral Iron to Intravenous Ferric Carboxymaltose: Effects on Erythropoiesis-Stimulating Agent Requirements, Costs, Hemoglobin and Iron Status. PLoS ONE 2015, 10, e0125528. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Bock, A.H.; Carrera, F.; Eckardt, K.U.; Gaillard, C.; Van Wyck, D.; Roubert, B.; Nolen, J.G.; Roger, S.D. FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol. Dial. Transpl. 2014, 29, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Rozen-Zvi, B.; Gafter-Gvili, A.; Paul, M.; Leibovici, L.; Shpilberg, O.; Gafter, U. Intravenous Versus Oral Iron Supplementation for the Treatment of Anemia in CKD: Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2008, 52, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C. Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: Oral or intravenous? Curr. Med. Res. Opin. 2010, 26, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.A.; Bhandari, S.; Saxena, S.; Agarwal, D.; Wirtz, G.; Kletzmayr, J.; Thomsen, L.L.; Coyne, D.W. A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia. Nephrol. Dial. Transplant. 2016, 31, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Staun, M.; Tandon, R.K.; Altorjay, I.; Thillainayagam, A.V.; Gratzer, C.; Nijhawan, S.; Thomsen, L.L. A Randomized, Open-Label., Non-Inferiority Study of Intravenous Iron Isomaltoside 1,000 (Monofer) Compared With Oral Iron for Treatment of Anemia in IBD (PROCEED). Am. J. Gastroenterol. 2013, 108, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C. Evolution of IV iron compounds over the last century. J. Ren. Care 2009, 35, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.B.; Garba, A.O. Ferumoxytol: A silver lining in the treatment of anemia of chronic kidney disease or another dark cloud? J. Blood Med. 2012, 3, 77–85. [Google Scholar] [PubMed]
- Keating, G.M. Ferric Carboxymaltose: A Review of Its Use in Iron Deficiency. Drugs 2015, 75, 101–127. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.T.; Regnier, C.E.; Loebertmann, C.L.; Bergstralh, E.J. Adverse events in chronic hemodialysis patients receiving intravenous iron dextran—A comparison of two products. Am. J. Nephrol. 2000, 20, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Mason, P.D.; Vaage-Nilsen, O.; Ahlmen, J. Update on adverse drug events associated with parenteral iron. Nephrol. Dial. Transplant. 2006, 21, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Wysowski, D.K.; Swartz, L.; Borders-Hemphill, B.V.; Goulding, M.R.; Dormitzer, C. Use of parenteral iron products and serious anaphylactic-type reactions. Am. J. Hematol. 2010, 85, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Fletes, R.; Lazarus, J.M.; Gage, J.; Chertow, G.M. Suspected iron dextran-related adverse drug events in hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 743–749. [Google Scholar] [CrossRef]
- Chertow, G.M.; Mason, P.D.; Vaage-Nilsen, O.; Ahlmen, J. On the relative safety of parenteral iron formulations. Nephrol. Dial. Transplant. 2004, 19, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Coyne, D.W.; Fishbane, S.; Folkert, V.; Lynn, R.; Nissenson, A.R.; Agarwal, R.; Eschbach, J.W.; Fadem, S.Z.; Trout, J.R.; et al. Sodium ferric gluconate complex in hemodialysis patients: Adverse reactions compared to placebo and iron dextran. Kidney Int. 2002, 61, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Bailie, G.R.; Horl, W.H.; Verhoef, J.J. Differences in spontaneously reported hypersensitivity and serious adverse events for intravenous iron preparations: Comparison of Europe and North America. Arzneimittel-Forschung-Drug Research. Arzneimittelforschung 2011, 61, 267–275. [Google Scholar] [PubMed]
- Van Wyck, D.; Anderson, J.; Johnson, K. Labile iron in parenteral iron formulations: A quantitative and comparative study. Nephrol. Dial. Transplant. 2004, 19, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Transferrin saturation with intravenous irons: An in vitro study. Kidney Int. 2004, 66, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.B.; Boyd, A.V.; McQuade, C.R.; Harford, A.; Norenberg, J.P.; Zager, P.G. Comparison of oxidative stress markers after intravenous administration of iron dextran, sodium ferric gluconate, and iron sucrose in patients undergoing hemodialysis. Pharmacotherapy 2007, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Brookhart, M.A.; Freburger, J.K.; Ellis, A.R.; Winkelmayer, W.C.; Wang, L.; Kshirsagar, A.V. Comparative short-term safety of sodium ferric gluconate versus iron sucrose in hemodialysis patients. Am. J. Kidney Dis. 2016, 67, 119–127. [Google Scholar] [CrossRef] [PubMed]
- FDA, Draft Guidance on Ferumoxytol. 2012. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM333051.pdf (accessed on 29 November 2017).
- FDA, Draft Guidance on Sodium Ferric Gluconate Complex. 2013. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM358142.pdf (accessed on 30 November 2017).
- FDA, Draft Guidance on Iron Sucrose. 2013. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM297630.pdf (accessed on 30 November 2017).
- FDA, Draft Guidance on Iron Dextran. 2016. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM520240.pdf (accessed on 30 November 2017).
- FDA, Draft Guidance on Ferric Carboxymaltose. 2016. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM495022.pdf (accessed on 30 November 2017).
- Jahn, M.R.; Andreasen, H.B.; Futterer, S.; Nawroth, T.; Schunemann, V.; Kolb, U.; Hofmeister, W.; Munoz, M.; Bock, K.; Meldal, M.; et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 2011, 78, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Danielson, B.G. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J. Am. Soc. Nephrol. 2004, 15, S93–S98. [Google Scholar] [PubMed]
- Stefansson, B.V.; Haraldsson, B.; Nilsson, U. Acute oxidative stress following intravenous iron injection in patients on chronic hemodialysis: A comparison of iron-sucrose and iron-dextran. Nephron Clin. Pract. 2011, 118, c249–c256. [Google Scholar] [CrossRef] [PubMed]
- Michelis, R.; Sela, S.; Kristal, B. Intravenous iron-gluconate during haemodialysis modifies plasma beta(2)-microglobulin properties and levels. Nephrol. Dial. Transplant. 2005, 20, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Van Wyck, D.B. Labile iron: Manifestations and clinical implications. J. Am. Soc. Nephrol. 2004, 15, S107–S111. [Google Scholar] [PubMed]
- EMA, Reflection Paper on the Data Requirements For Intravenous Iron-Based Nano-Colloidal Products Developed with Reference to an Innovator Medicinal Product. 2015. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184922.pdf (accessed on 26 October 2016).
- Sun, D.; Rouse, R.; Patel, V.; Wu, Y.; Zheng, J.; Patri, A.; Chitranshi, P.; Keire, D.; Ma, J.; Jiang, W. Comparative evaluation of U.S. brand and generic intravenous sodium ferric gluconate complex in sucrose injection: Physicochemical characterization. Nanomaterials 2017. submitted. [Google Scholar]
- Liu, J.; Qiu, Z.Y.; Wang, S.Q.; Zhou, L.; Zhang, S.M. A modified double-emulsion method for the preparation of daunorubicin-loaded polymeric nanoparticle with enhanced in vitro anti-tumor activity. Biomed. Mater. 2010, 5, 065002. [Google Scholar] [CrossRef] [PubMed]
- Praschberger, M.; Cornelius, C.; Schitegg, M.; Goldenberg, H.; Scheiber-Mojdehkar, B.; Sturm, B. Bioavailability and stability of intravenous iron sucrose originator versus generic iron sucrose AZAD. Pharm. Dev. Technol. 2015, 20, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Laskar, A.; Ghosh, M.; Khattak, S.I.; Li, W.; Yuan, X.M. Degradation of superparamagnetic iron oxide nanoparticle-induced ferritin by lysosomal cathepsins and related immune response. Nanomedicine 2012, 7, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delecher, M.; Tron, K.; Nienhaus, G.U.; Musyanovych, A.; Mailander, V.; Landfester, K.; et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Sanz-Ortega, L.; Mulens-Arias, V.; Gutierrez, L.; Perez-Yague, S.; Barber, D.F. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Beekman, C.; Matta, M.K.; Thomas, C.; Mohammad, A.; Stewart, S.; Xu, L.; Chockalingam, A.; Shea, K.; Sun, D.; Jiang, W.; et al. Comparative evaluation of U.S. Brand and generic intravenous sodium ferric gluconate complex in sucrose injection: Biodistribution after intravenous dosing in rats. Nanomaterials 2017. submitted. [Google Scholar]
- Cho, E.C.; Zhang, Q.; Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011, 6, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bancos, S.; Tyner, K.M. Evaluating the effect of assay preparation on the uptake of gold nanoparticles by RAW264.7 cells. J. Nanobiotechnol. 2014, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Herd, H.L.; Bartlett, K.T.; Gustafson, J.A.; McGill, L.D.; Ghandehari, H. Macrophage silica nanoparticle response is phenotypically dependent. Biomaterials 2015, 53, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007, 134, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Safi, M.; Courtois, J.; Seigneuret, M.; Conjeaud, H.; Berret, J.F. The effects of aggregation and protein corona on the cellular internalization of iron oxide nanoparticles. Biomaterials 2011, 32, 9353–9363. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Burtea, C.; Thirifays, C.; Rezaee, F.; Mahmoudi, M. Significance of cell “observer” and protein source in nanobiosciences. J. Colloid Interface Sci. 2013, 392, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Schottler, S.; Klein, K.; Landfester, K.; Mailander, V. Protein source and choice of anticoagulant decisively affect nanoparticle protein corona and cellular uptake. Nanoscale 2016, 8, 5526–5536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.J.; Tian, X.; Wu, A.Q.; Li, J.X.; Tian, J.; Chong, Y.; Chai, Z.F.; Zhao, Y.L.; Chen, C.Y.; Ge, C.C. Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner. ACS Appl. Mater. Interfaces 2015, 7, 20568–20575. [Google Scholar] [CrossRef] [PubMed]
- Baribeault, D. Sodium ferric gluconate (SFG) in complex with sucrose for IV infusion: bioequivalence of a new generic product with the branded product in healthy volunteers. Curr. Med. Res. Opin. 2011, 27, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Sun, D.; Tyner, K.; Jiang, W.; Rouse, R. Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: In Vitro Cellular Uptake. Nanomaterials 2017, 7, 451. https://doi.org/10.3390/nano7120451
Wu M, Sun D, Tyner K, Jiang W, Rouse R. Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: In Vitro Cellular Uptake. Nanomaterials. 2017; 7(12):451. https://doi.org/10.3390/nano7120451
Chicago/Turabian StyleWu, Min, Dajun Sun, Katherine Tyner, Wenlei Jiang, and Rodney Rouse. 2017. "Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: In Vitro Cellular Uptake" Nanomaterials 7, no. 12: 451. https://doi.org/10.3390/nano7120451
APA StyleWu, M., Sun, D., Tyner, K., Jiang, W., & Rouse, R. (2017). Comparative Evaluation of U.S. Brand and Generic Intravenous Sodium Ferric Gluconate Complex in Sucrose Injection: In Vitro Cellular Uptake. Nanomaterials, 7(12), 451. https://doi.org/10.3390/nano7120451



