ZnO Nanostructures for Tissue Engineering Applications
Abstract
1. Introduction
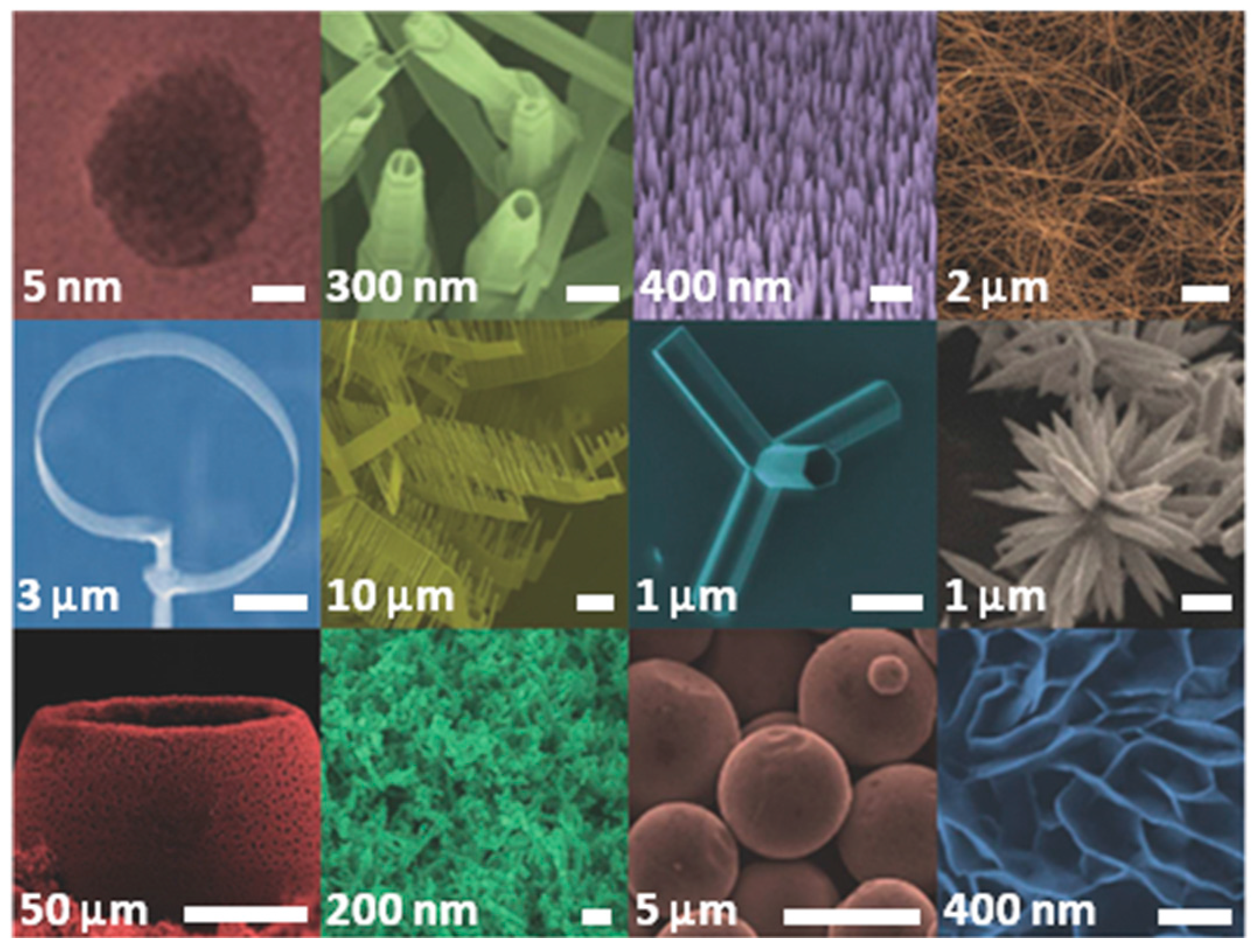
2. Zinc Oxide: Synthesis, Properties and Applications
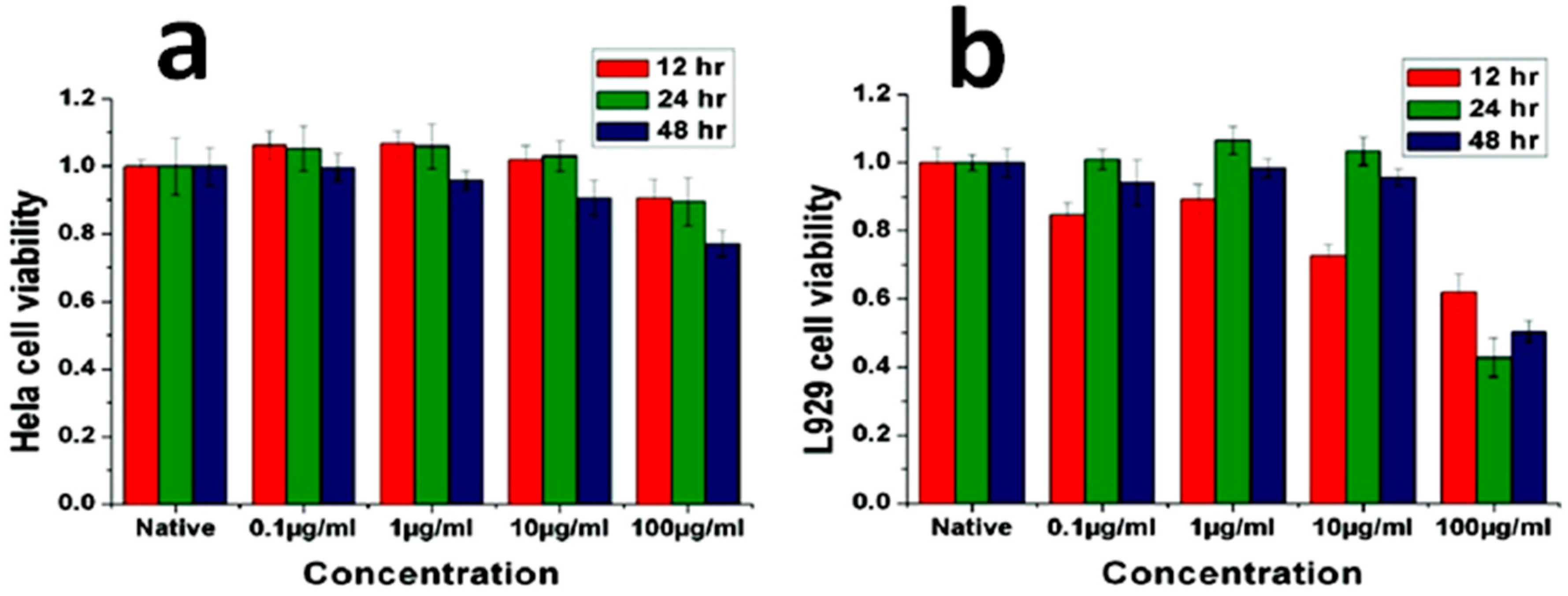
3. Biocompatible and Antibacterial Properties of Pure ZnO Nanostructures
4. ZnO-Based Composite Materials
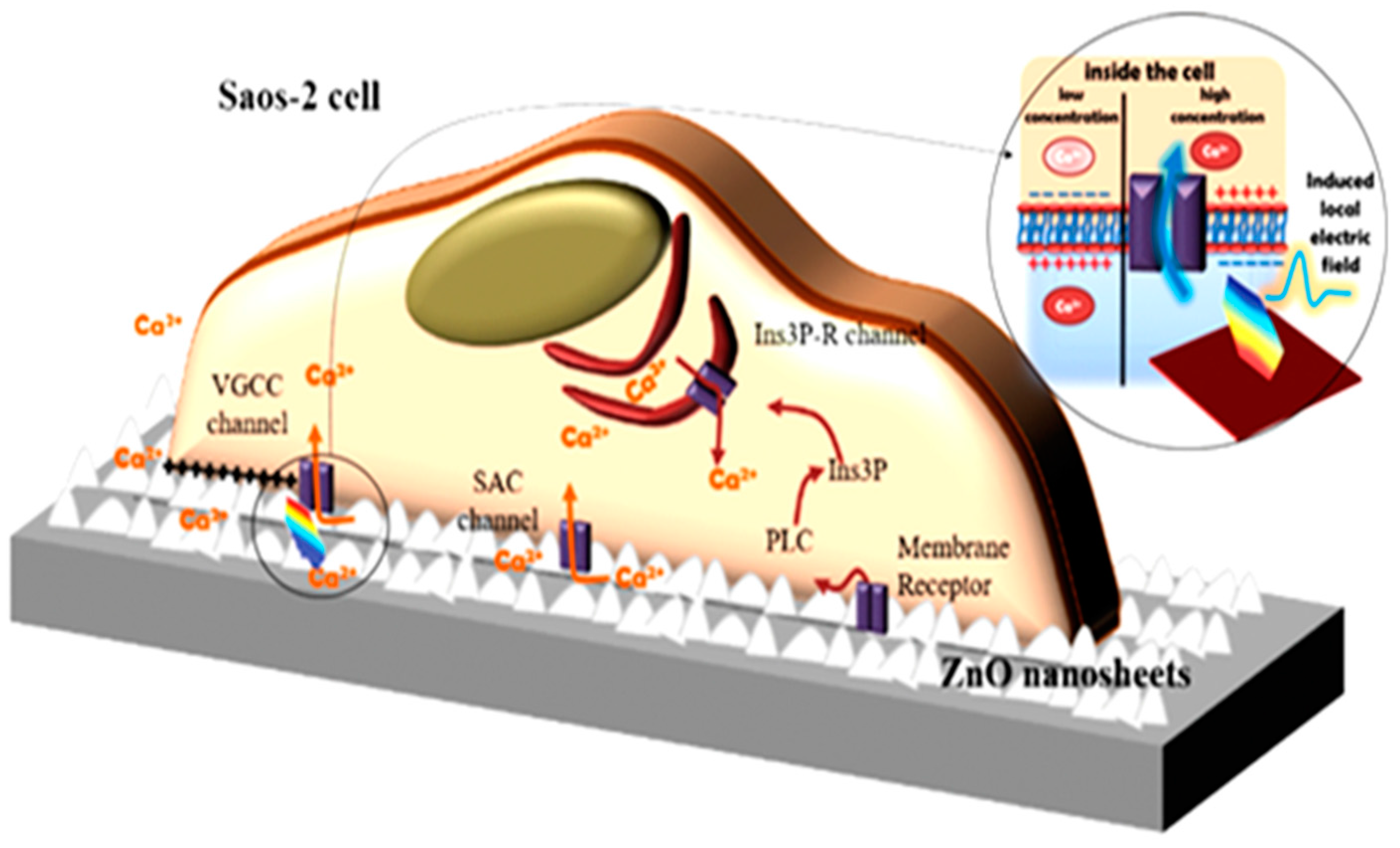
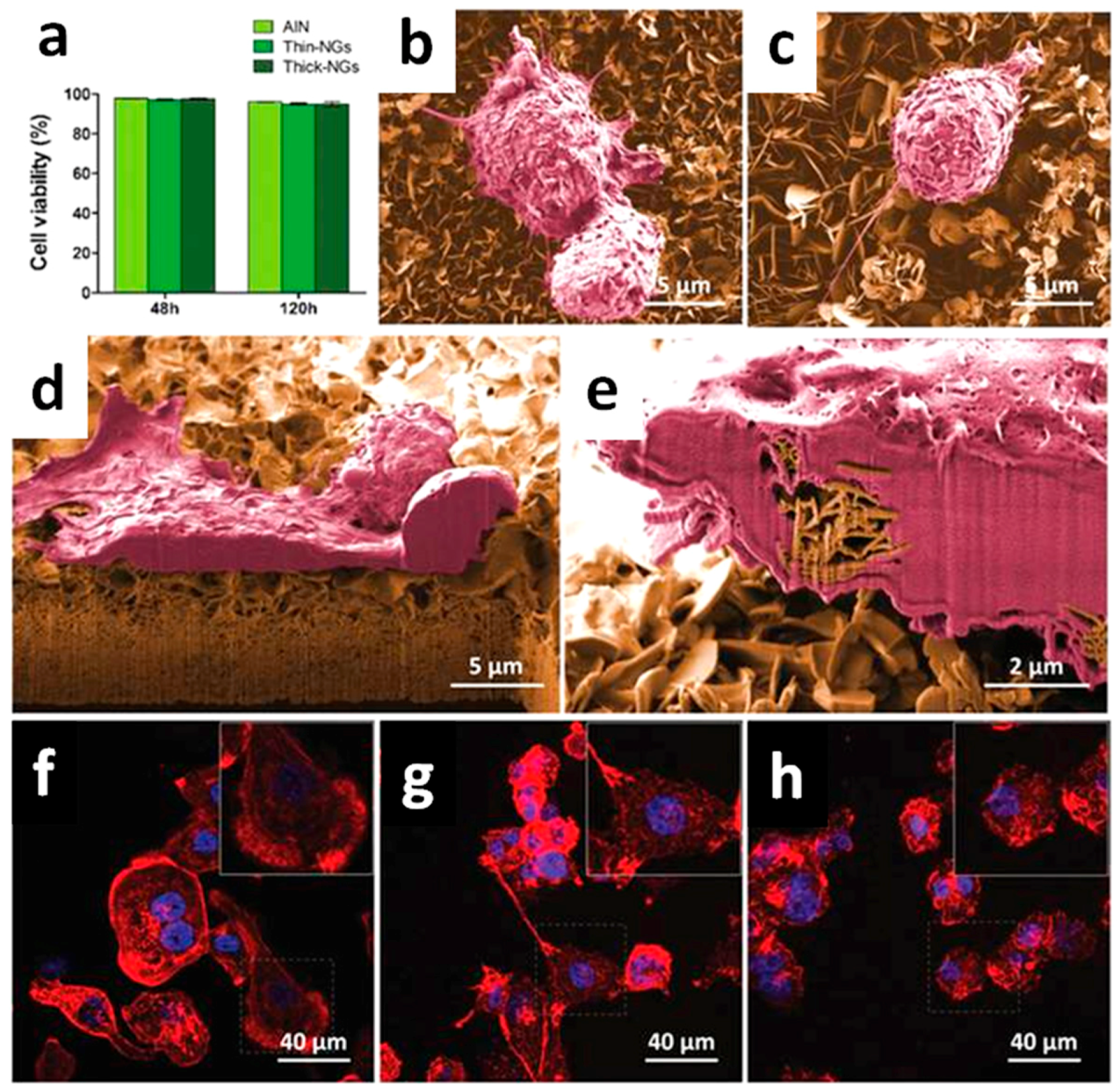
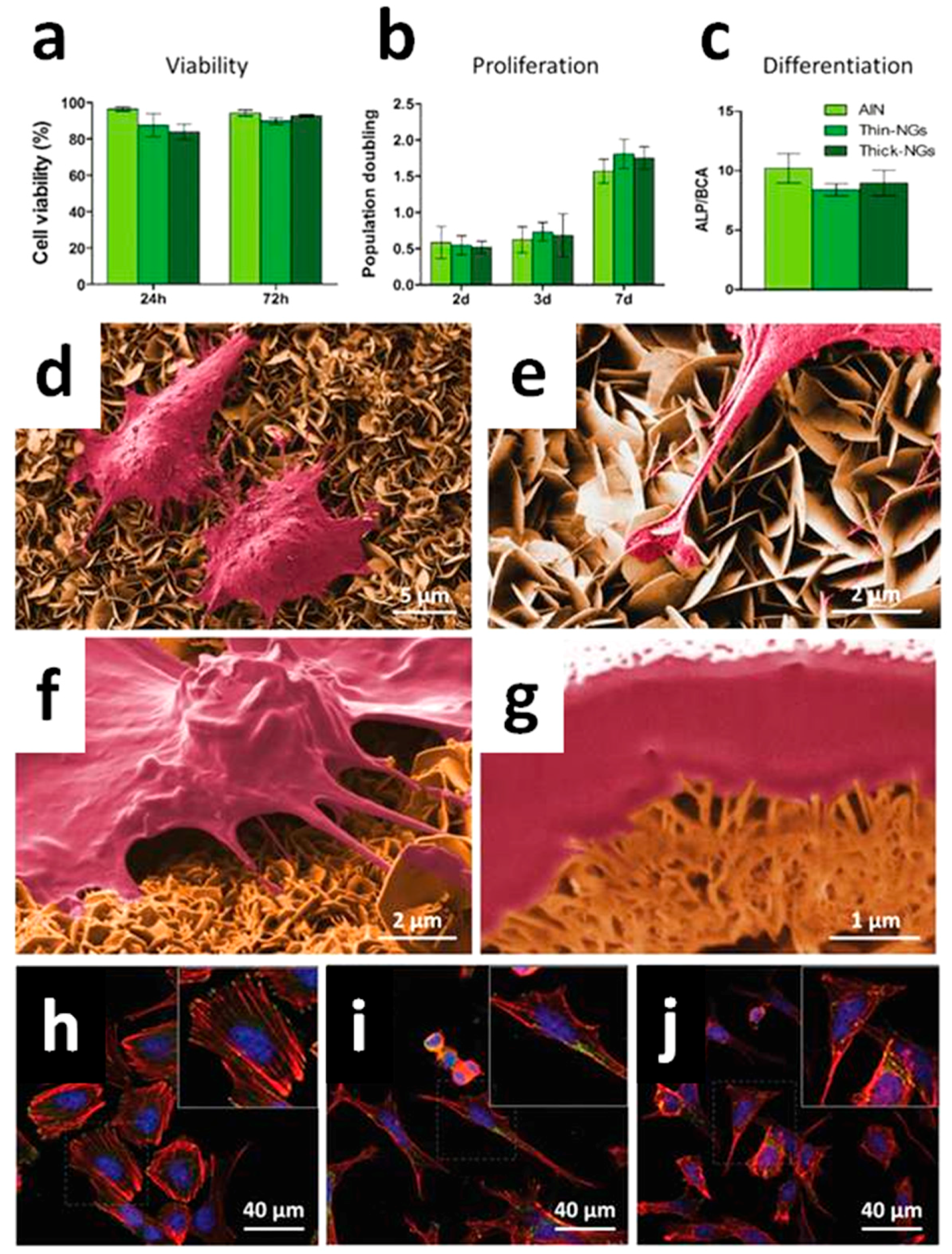
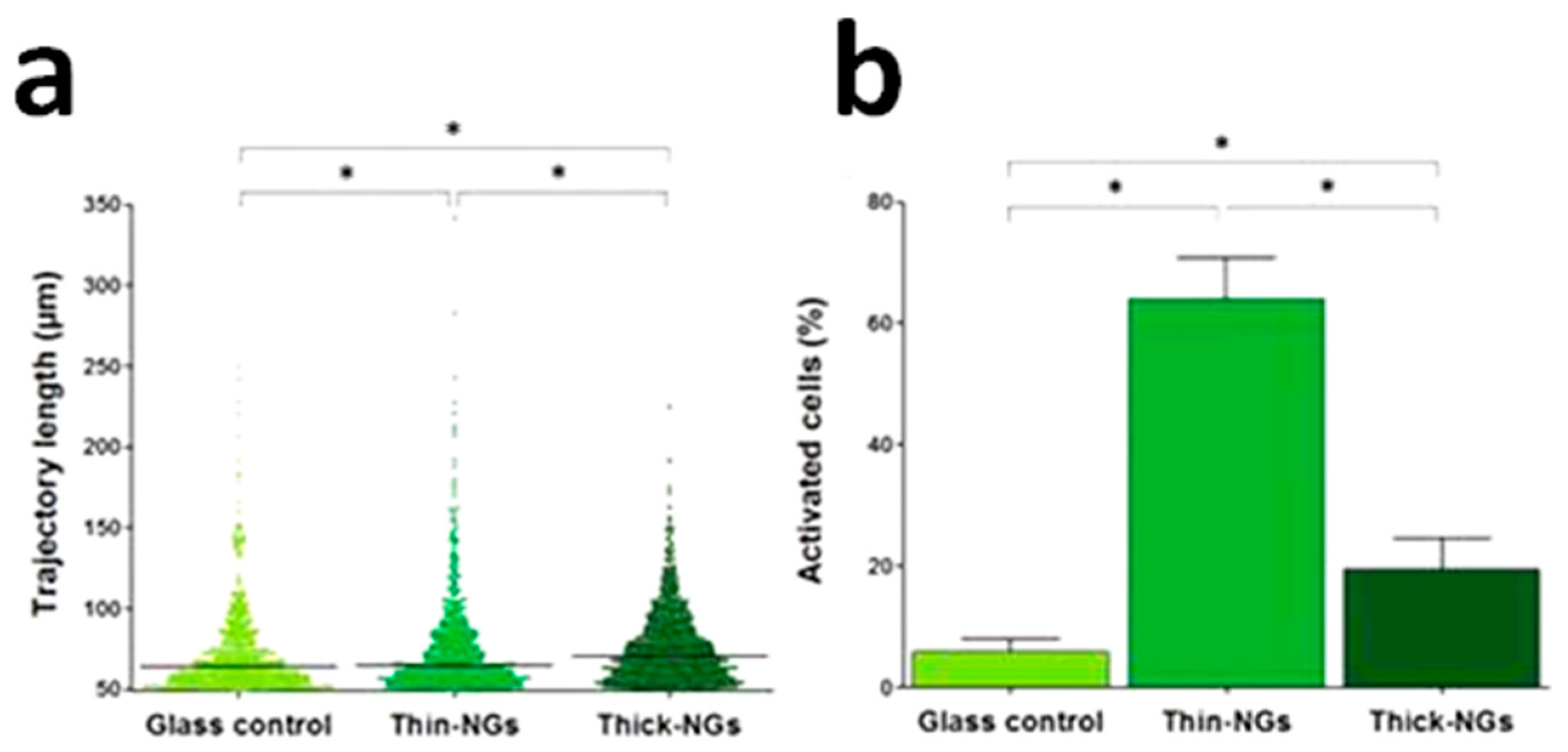
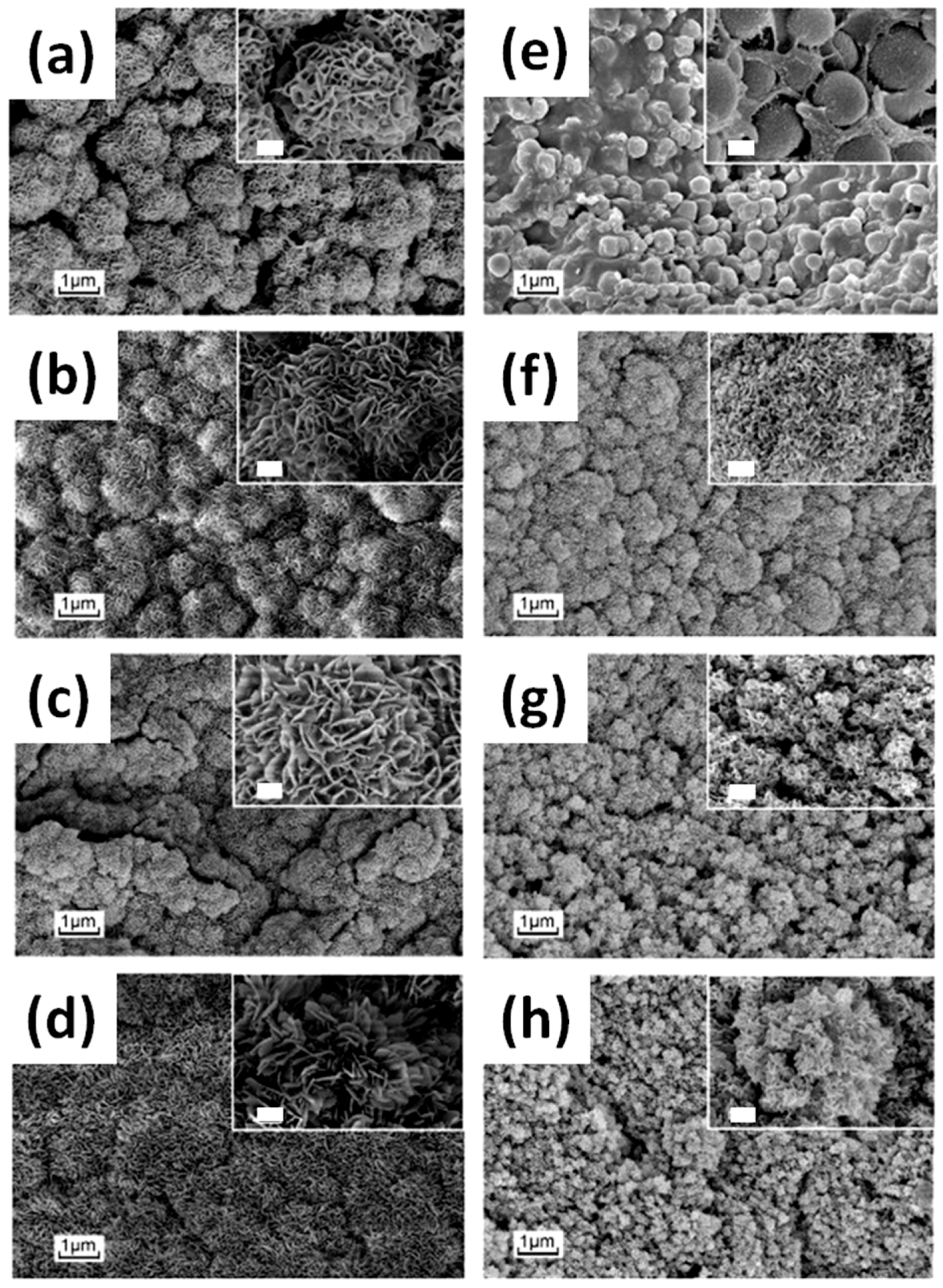
4.1. Bioactivity and Bone Tissue Regeneration Properties
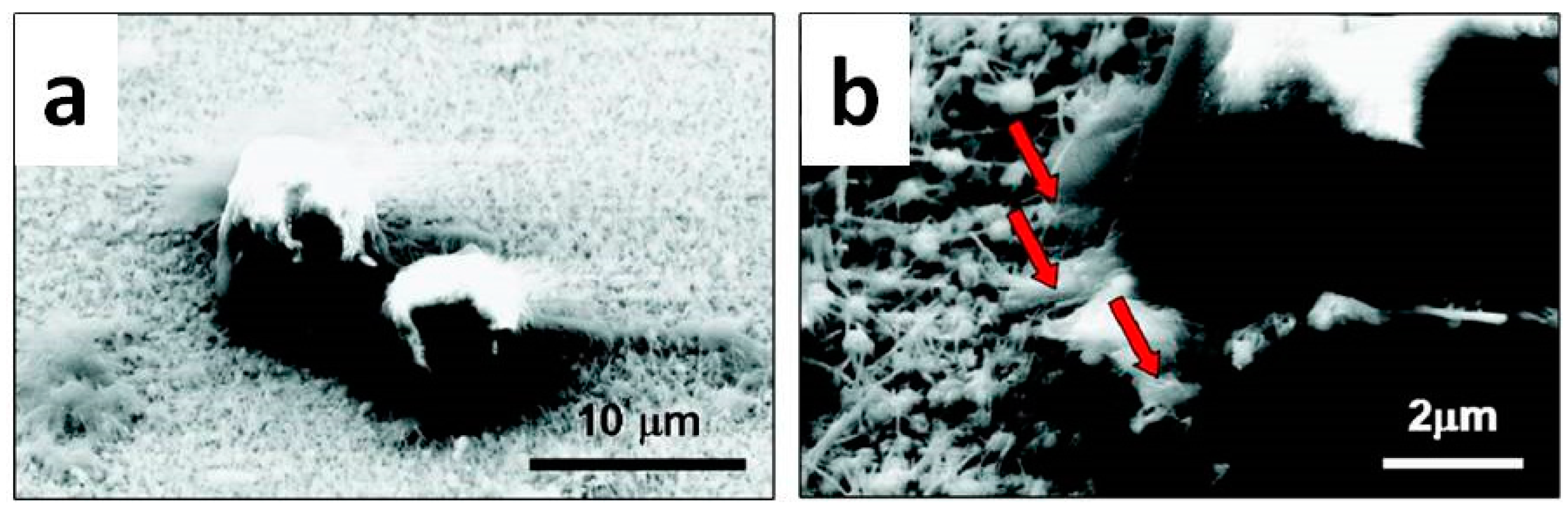
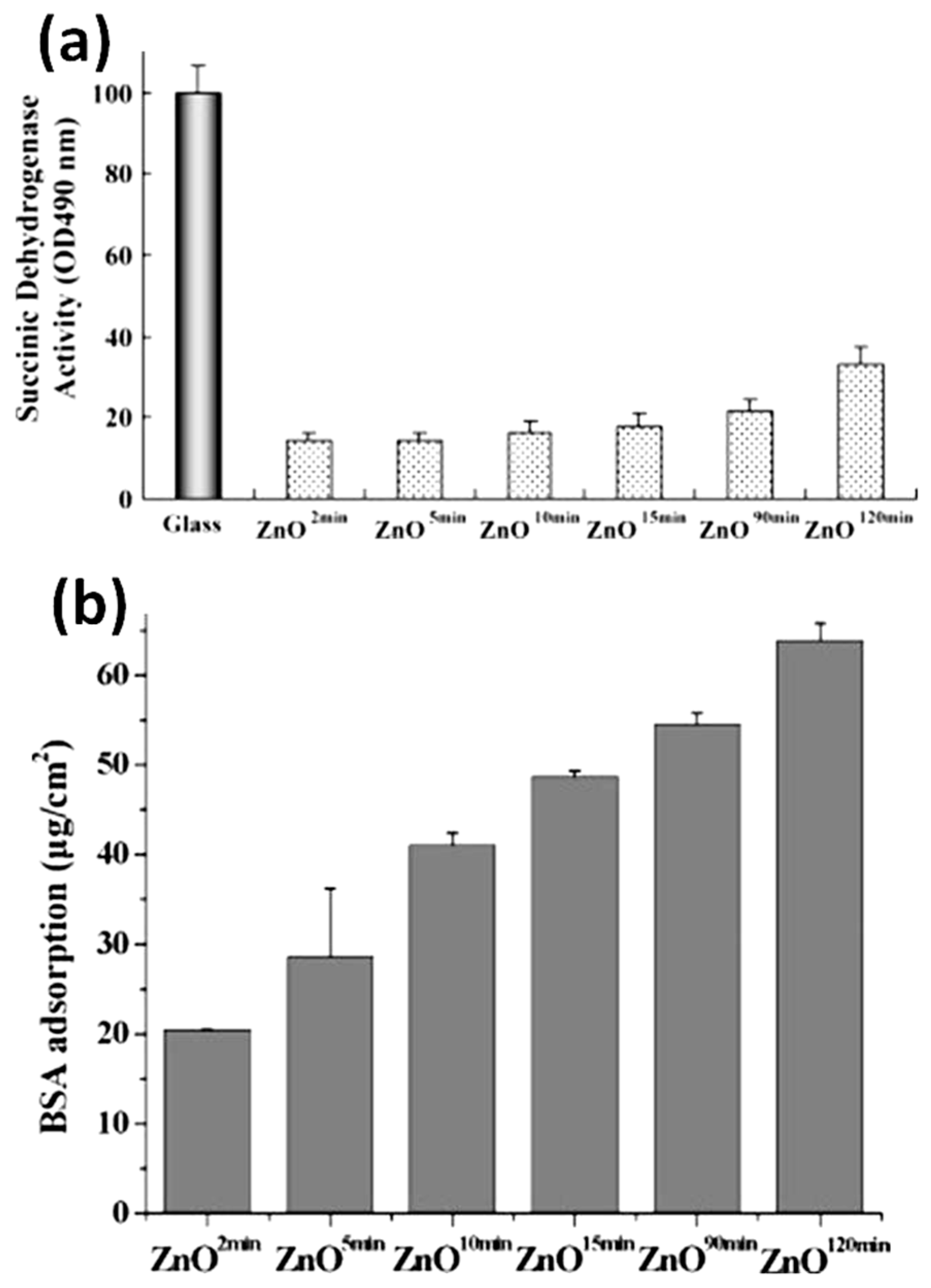
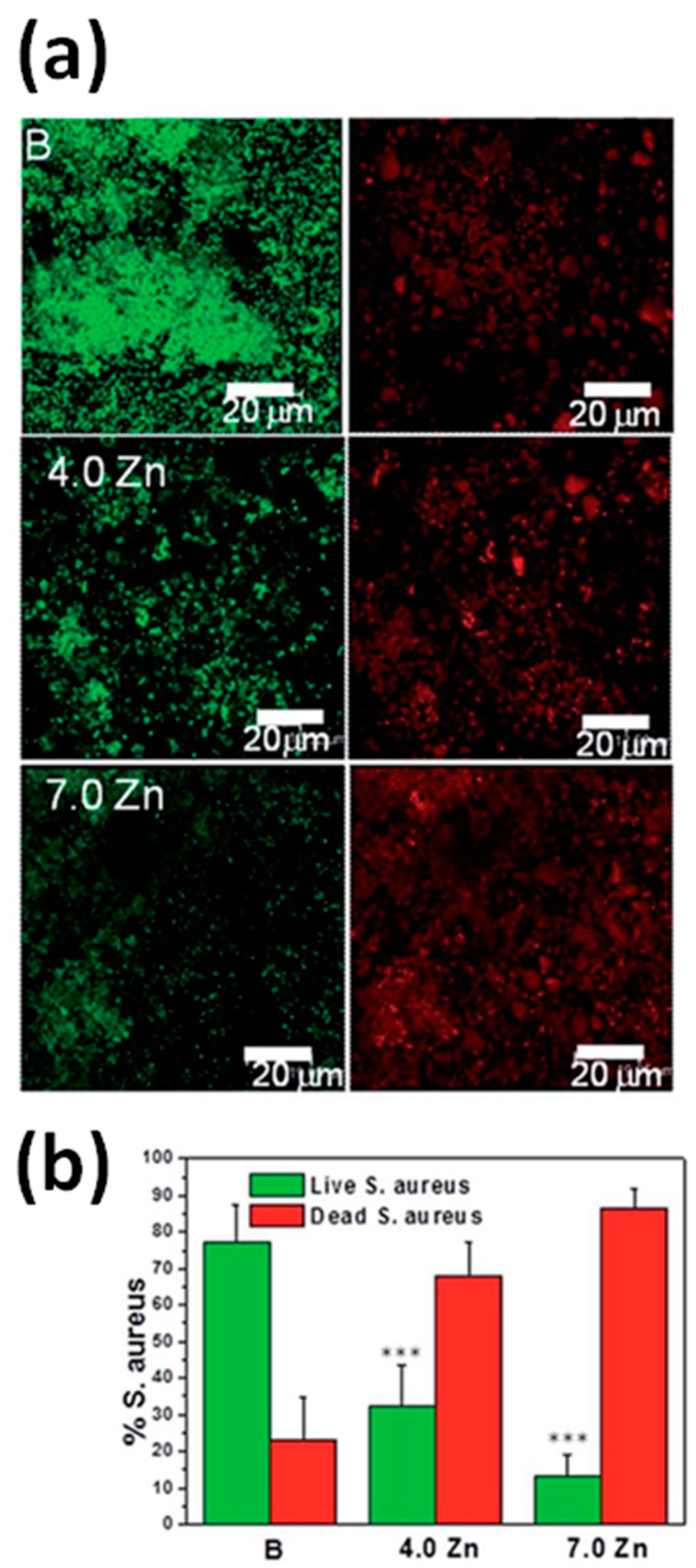
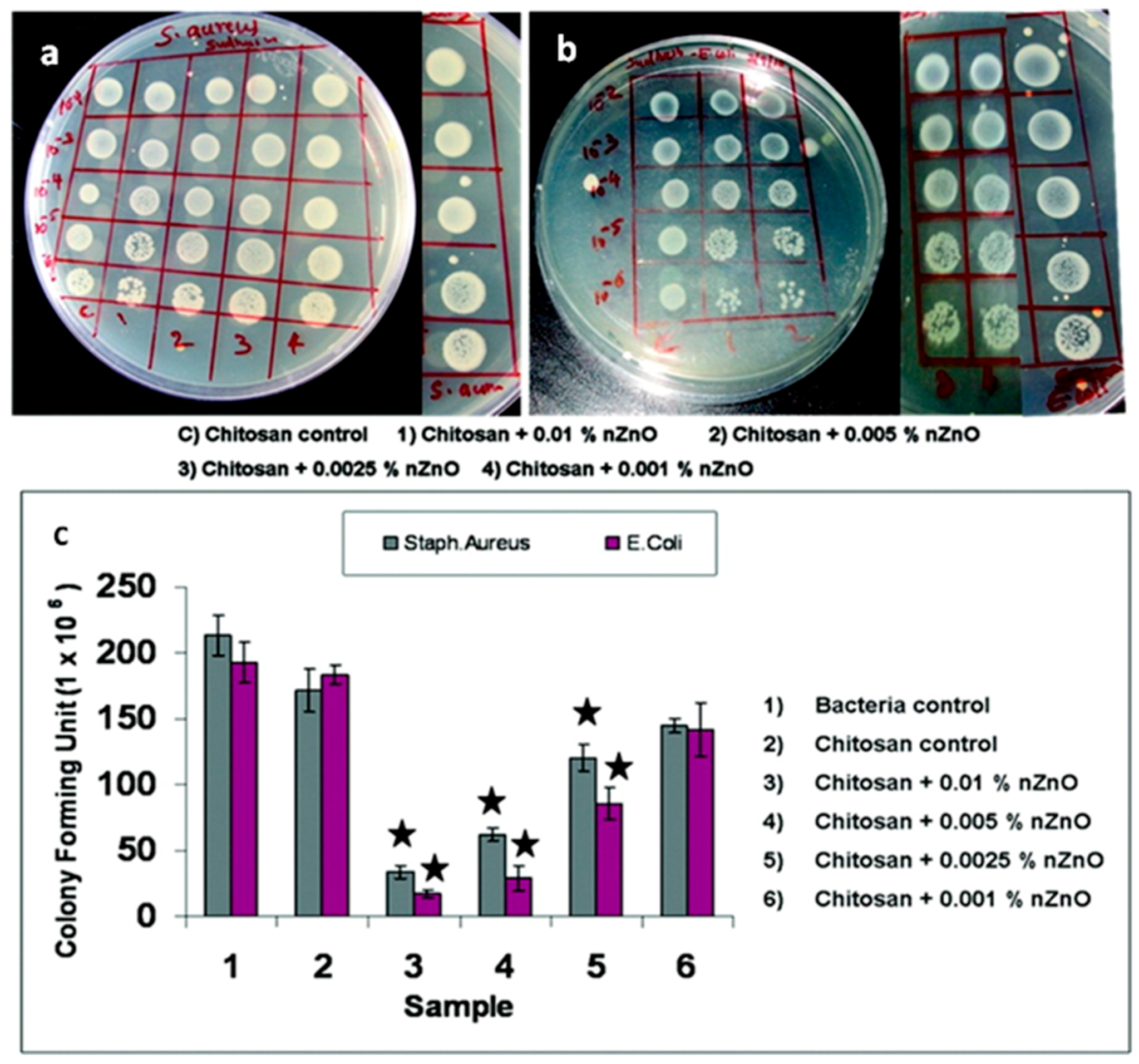
4.2. Antibacterial Properties
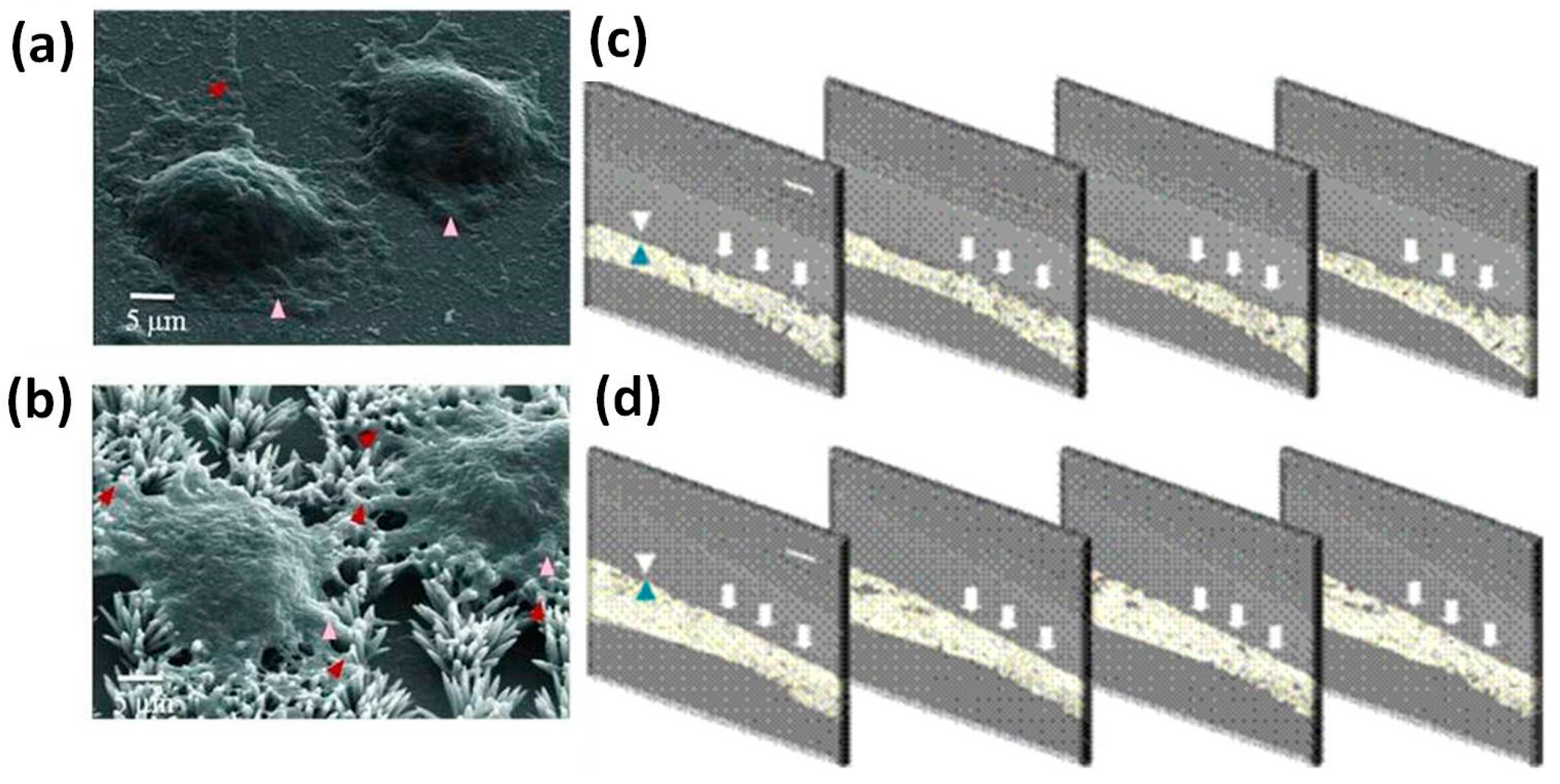
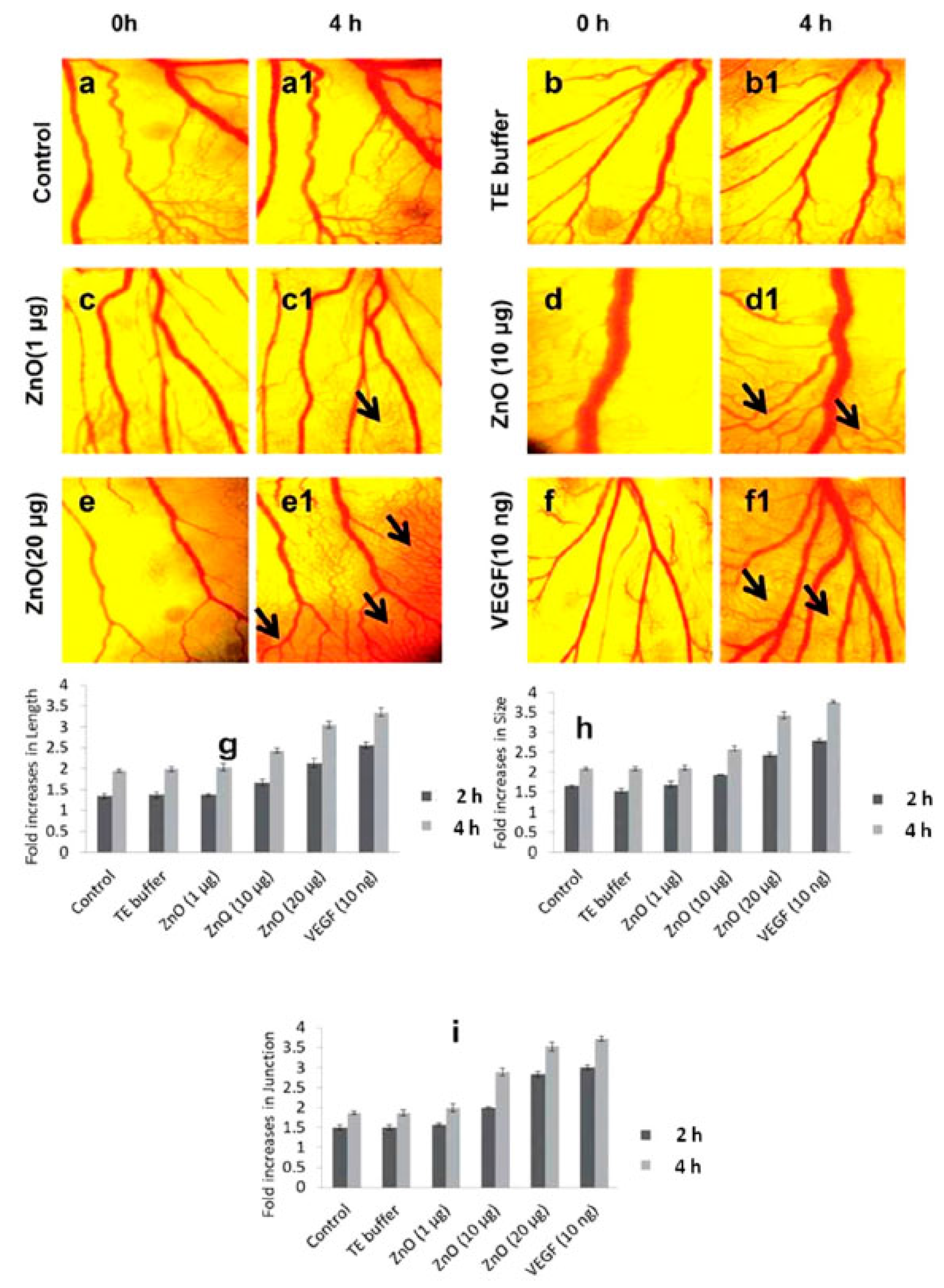
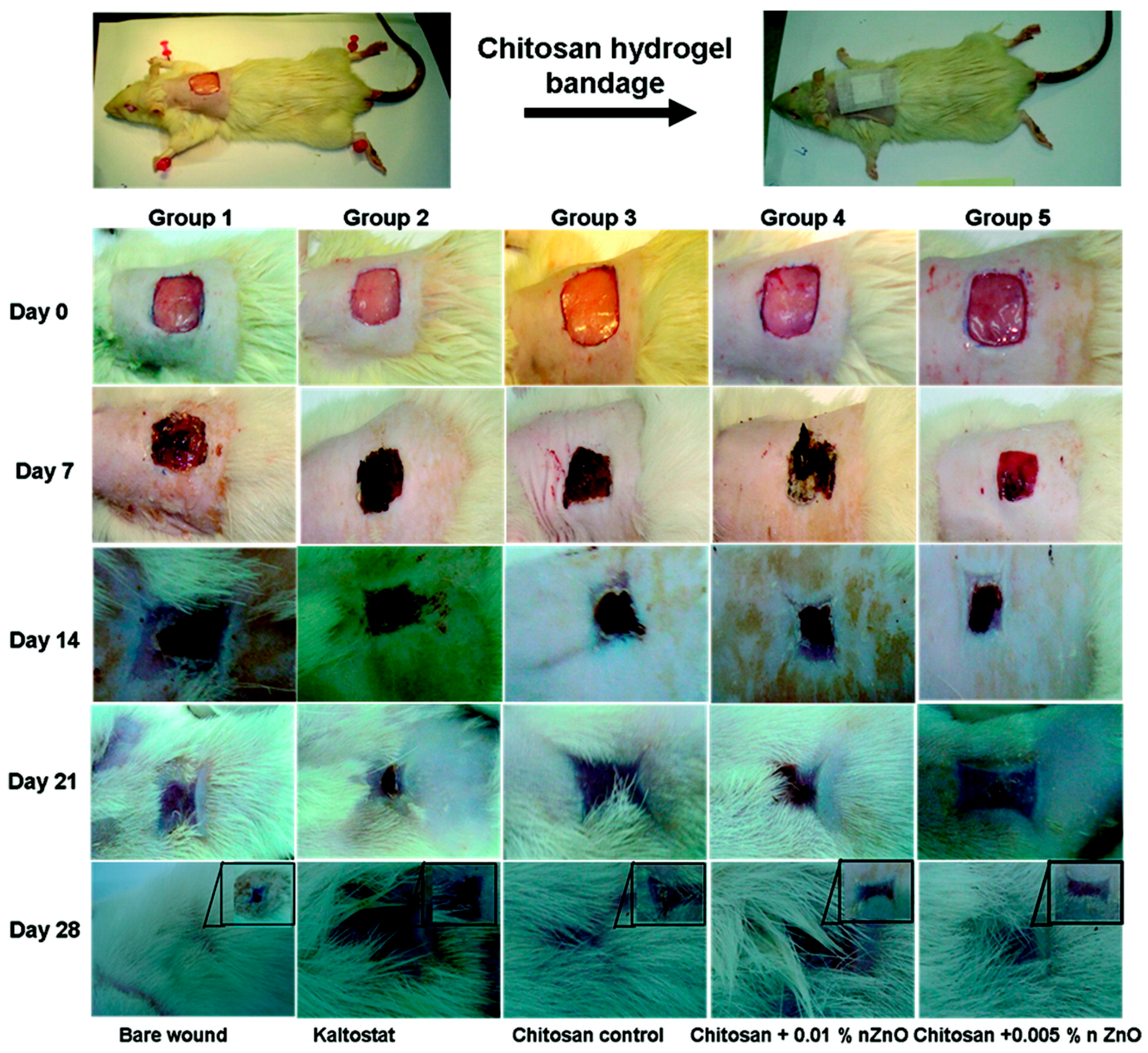
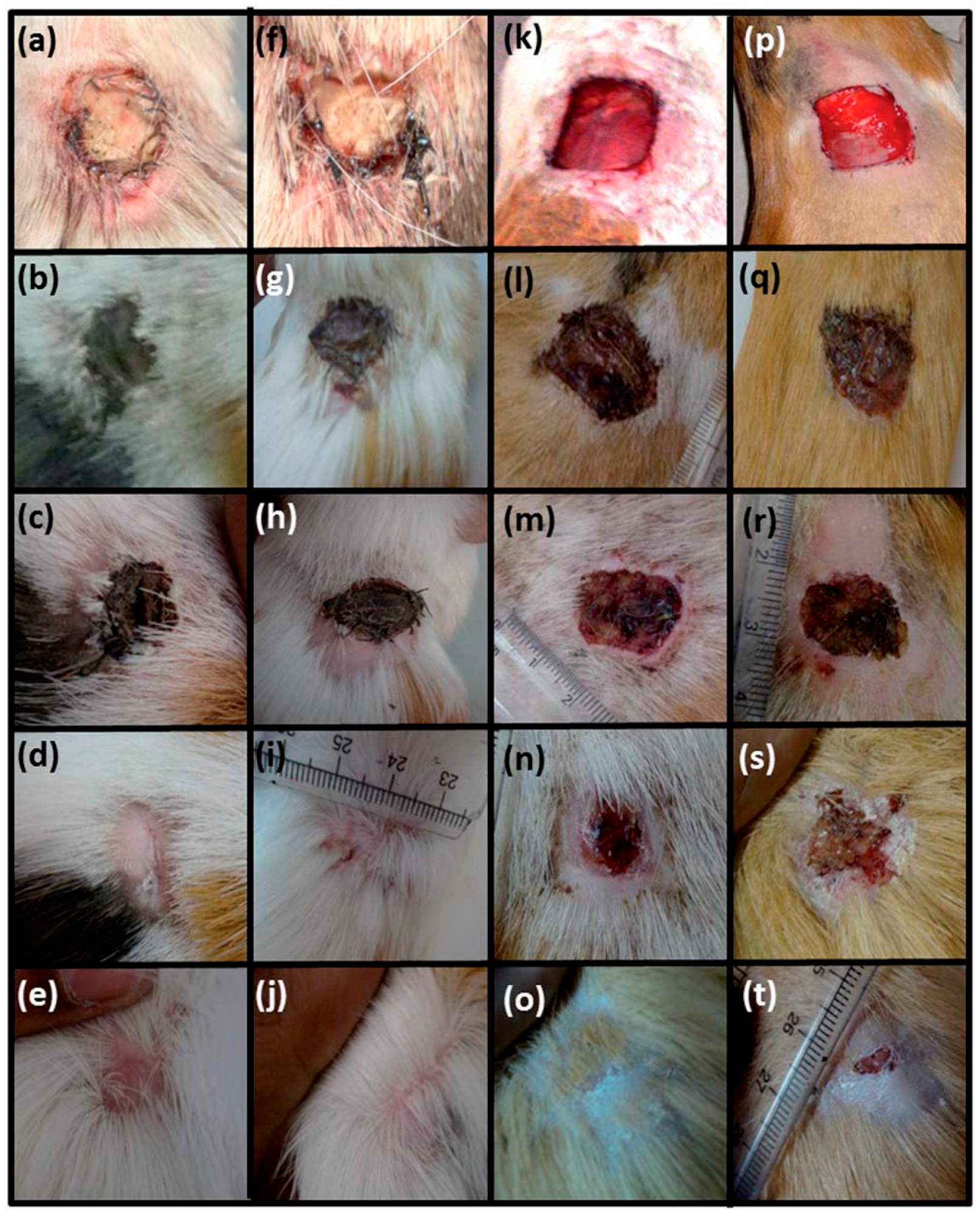
4.3. Wound Healing Applications
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, Si32–Si34. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Liu, X.M.; Yeung, K.W.K.; Liu, C.S.; Yang, X.J. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C 2011, 31, 11. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Cauda, V.; Onida, B.; Vitale-Brovarone, C. Mesoporous glass coating on bone tissue engineering scaffolds to improve bioactivity. J. Tissue Eng. Regen. M 2014, 8, 231. [Google Scholar]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Liu, X.H.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.L.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stabil. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
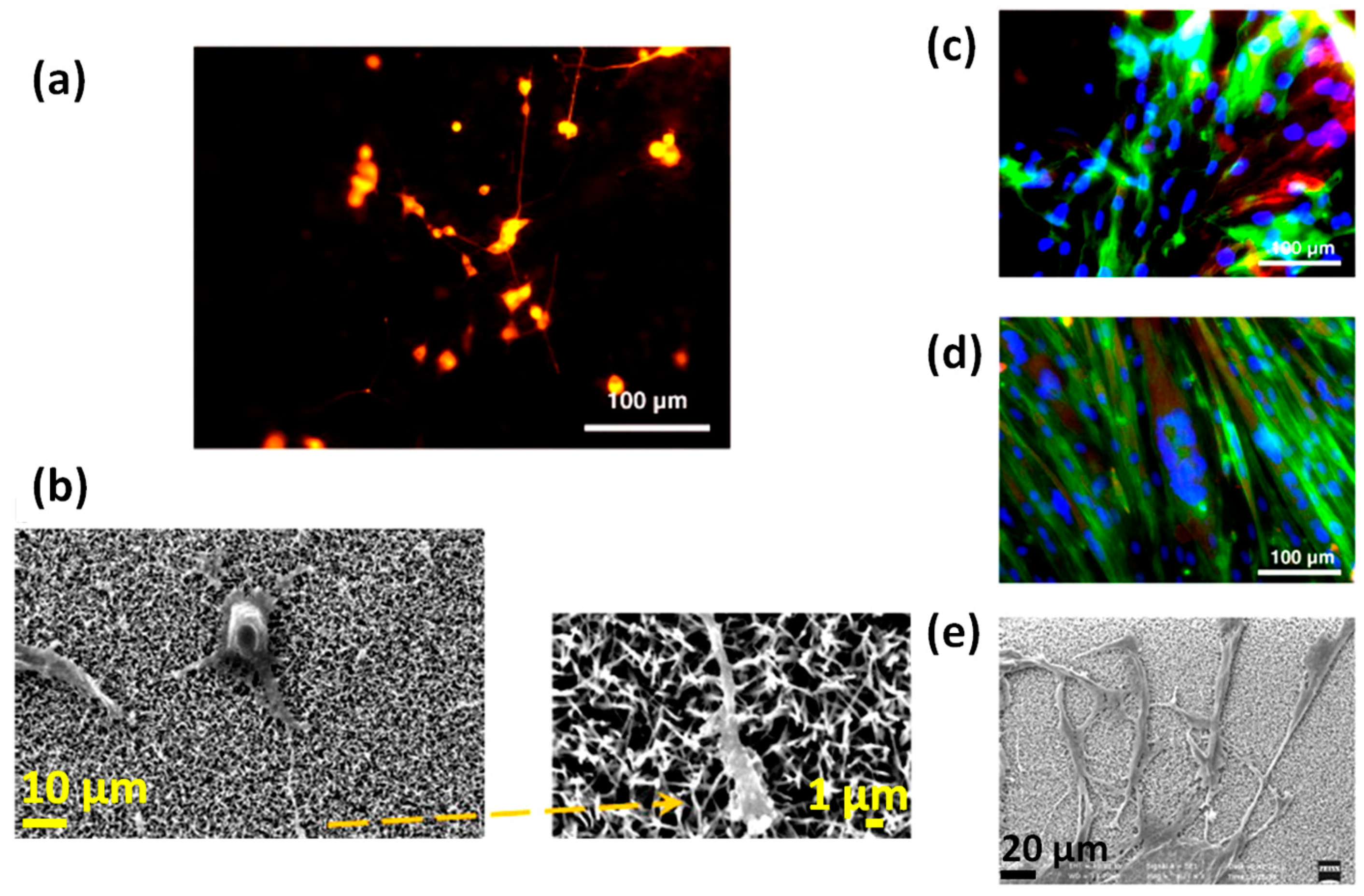
- Ciofani, G.; Genchi, G.G.; Mattoli, V. ZnO nanowire arrays as substrates for cell proliferation and differentiation. Mat. Sci. Eng. C-Mater. 2012, 32, 341–347. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, B.S.; Hicks, B.; Chancellor, T.F.; Chu, B.H.; Wang, H.T.; Keselowsky, B.G.; Ren, F.; Lele, T.P. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 2008, 29, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kim, Y.J.; Yeom, J.; Jeon, J.H.; Yi, G.C.; Je, J.H.; Hahn, S.K. The Topographic Effect of Zinc Oxide Nanoflowers on Osteoblast Growth and Osseointegration. Adv. Mater. 2010, 22, 4857–4861. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Feng, P.; Wei, P.P.; Shuai, C.J.; Peng, S.P. Characterization of Mechanical and Biological Properties of 3-D Scaffolds Reinforced with Zinc Oxide for Bone Tissue Engineering. PLoS ONE 2014, 9, e87755. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.; Bose, S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013, 9, 9137–9148. [Google Scholar] [CrossRef] [PubMed]
- Fielding, G.A.; Bandyopadhyay, A.; Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 2012, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Barui, A.K.; Veeriah, V.; Mukherjee, S.; Manna, J.; Patel, A.K.; Patra, S.; Pal, K.; Murali, S.; Rana, R.K.; Chatterjee, S.; et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale 2012, 4, 7861–7869. [Google Scholar] [CrossRef] [PubMed]
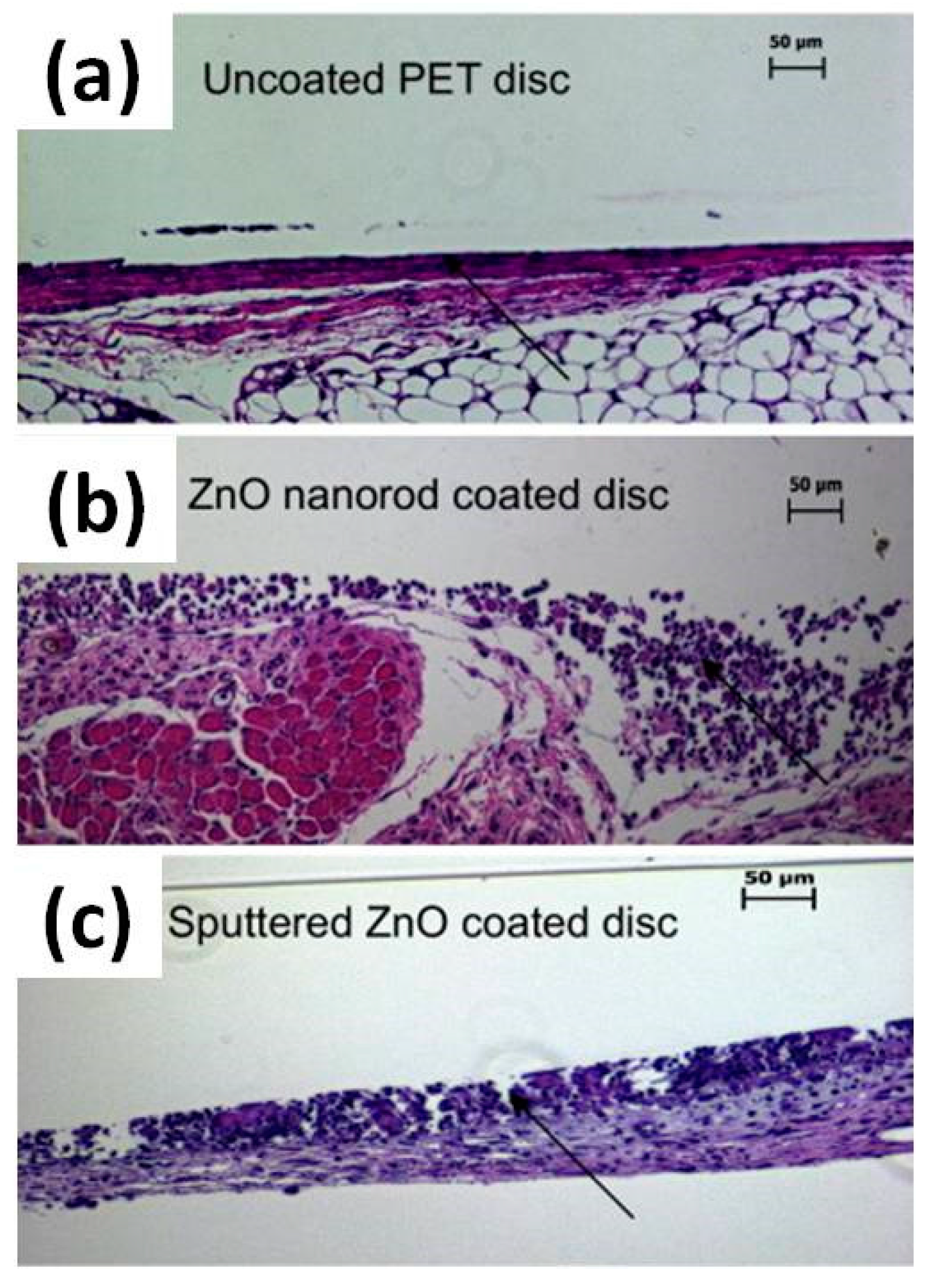
- Grenho, L.; Salgado, C.L.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Antibacterial activity and biocompatibility of three-dimensional nanostructured porous granules of hydroxyapatite and zinc oxide nanoparticles-an in vitro and in vivo study. Nanotechnology 2015, 26, 315101. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone membranes incorporated with ZnO nanoparticles as skin substitutes with enhanced fibroblast proliferation and wound healing. RSC Adv. 2014, 4, 24777–24785. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 4, 51528–51536. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and Microporous Chitosan Hydrogel/Nano ZnO Composite Bandages for Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rao, K.S.R.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Zhu, P.; Weng, Z.Y.; Li, X.; Liu, X.M.; Wu, S.L.; Yeung, K.W.K.; Wang, X.B.; Cui, Z.D.; Yang, X.J.; Chu, P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys.-Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Cauda, V.; Stassi, S.; Lamberti, A.; Morello, M.; Pirri, C.F.; Canavese, G. Leveraging ZnO morphologies in piezoelectric composites for mechanical energy harvesting. Nano Energy 2015, 18, 212–221. [Google Scholar] [CrossRef]
- Laurenti, M.; Verna, A.; Chiolerio, A. Evidence of Negative Capacitance in Piezoelectric ZnO Thin Films Sputtered on Interdigital Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 24470–24479. [Google Scholar] [CrossRef] [PubMed]
- Gazia, R.; Chiodoni, A.; Bianco, S.; Lamberti, A.; Quaglio, M.; Sacco, A.; Tresso, E.; Mandracci, P.; Pirri, C.F. An easy method for the room-temperature growth of spongelike nanostructured Zn films as initial step for the fabrication of nanostructured ZnO. Thin Solid Films 2012, 524, 107–112. [Google Scholar] [CrossRef]
- Pacholski, C.; Kornowski, A.; Weller, H. Self-assembly of ZnO: From nanodots, to nanorods. Angew. Chem. Int. Ed. 2002, 41, 1188–1191. [Google Scholar] [CrossRef]
- Laurenti, M.; Verna, A.; Fontana, M.; Quaglio, M.; Porro, S. Selective growth of ZnO nanowires on substrates patterned by photolithography and inkjet printing. Appl. Phys. A 2014, 117, 901–907. [Google Scholar] [CrossRef]
- Greene, L.E.; Yuhas, B.D.; Law, M.; Zitoun, D.; Yang, P.D. Solution-grown zinc oxide nanowires. Inorg. Chem. 2006, 45, 7535–7543. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellah, M.; Moghimi, N.; Zhang, L.; Thomas, J.P.; McGillivray, D.; Srivastava, S.; Leung, K.T. Plasmonic gold nanoparticles for ZnO-nanotube photoanodes in dye-sensitized solar cell application. Nanoscale 2016, 8, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of semiconducting oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.L.; Wang, Z.L. Controlled synthesis and manipulation of ZnO nanorings and nanobows. Appl. Phys. Lett. 2005, 86, 43106. [Google Scholar] [CrossRef]
- Shi, R.X.; Yang, P.; Wang, J.R.; Zhang, A.Y.; Zhu, Y.N.; Cao, Y.Q.; Ma, Q. Growth of flower-like ZnO via surfactant-free hydrothermal synthesis on ITO substrate at low temperature. Crystengcomm 2012, 14, 5996–6003. [Google Scholar] [CrossRef]
- Chen, P.; Gu, L.; Xue, X.D.; Song, Y.Y.; Zhu, L.W.; Cao, X.B. Facile synthesis of highly uniform ZnO multipods as the supports of Au and Ag nanoparticles. Mater. Chem. Phys. 2010, 122, 41–48. [Google Scholar] [CrossRef]
- Calestani, D.; Zha, M.; Mosca, R.; Zappettini, A.; Carotta, M.C.; Di Natale, V.; Zanotti, L. Growth of ZnO tetrapods for nanostructure-based gas sensors. Sens. Actuators B Chem. 2010, 144, 472–478. [Google Scholar] [CrossRef]
- Ottone, C.; Bejtka, K.; Chiodoni, A.; Farias, V.; Roppolo, I.; Canavese, G.; Stassi, S.; Cauda, V. Comprehensive study of the templating effect on the ZnO nanostructure formation within porous hard membranes. New J. Chem. 2014, 38, 2058–2065. [Google Scholar] [CrossRef]
- Ottone, C.; Rivera, V.F.; Fontana, M.; Bejtka, K.; Onida, B.; Cauda, V. Ultralong and Mesoporous ZnO and gamma-Al2O3 Oriented Nanowires Obtained by Template-assisted Hydrothermal Approach. J. Mater. Sci. Technol. 2014, 30, 1167–1173. [Google Scholar] [CrossRef]
- Dumontel, B.; Canta, M.; Engelke, H.; Chiodoni, A.; Racca, L.; Ancona, A.; Limongi, T.; Canavese, G.; Cauda, V. Enhanced Biostability and Cellular Uptake of Zinc Oxide Nanocrystals Shielded with Phospholipid Bilayer. J. Mater. Chem. B 2017, in press. [Google Scholar] [CrossRef]
- Baruwati, B.; Kumar, D.K.; Manorama, S.V. Hydrothermal synthesis of highly crystalline ZnO nanoparticles: A competitive sensor for LPG and EtOH. Sens. Actuators B Chem. 2006, 119, 676–682. [Google Scholar] [CrossRef]
- Kandjani, A.E.; Tabriz, M.F.; Pourabbas, B. Sonochemical synthesis of ZnO nanoparticles: The effect of temperature and sonication power. Mater. Res. Bull. 2008, 43, 645–654. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Podrezova, L.V.; Cauda, V.; Stassi, S.; Cicero, G.; Abdullin, K.A.; Alpysbaeva, B.E. Properties of ZnO nanorods grown by hydrothermal synthesis on conductive layers. Cryst. Res. Technol. 2014, 49, 599–605. [Google Scholar] [CrossRef]
- Podrezova, L.V.; Porro, S.; Cauda, V.; Fontana, M.; Cicero, G. Comparison between ZnO nanowires grown by chemical vapor deposition and hydrothermal synthesis. Appl. Phys. A 2013, 113, 623–632. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V.; Gazia, R.; Fontana, M.; Rivera, V.F.; Bianco, S.; Canavese, G. Wettability Control on ZnO Nanowires Driven by Seed Layer Properties. Eur. J. Inorg. Chem. 2013, 2520–2527. [Google Scholar] [CrossRef]
- Laurenti, M.; Verna, A.; Fontana, M.; Stassi, S.; Canavese, G.; Marasso, S.L.; Cauda, V. How Micropatterning and Surface Functionalization Affect the Wetting Behavior of ZnO Nanostructured Surfaces. Adv. Mater. Interfaces 2016, 3, 1600110. [Google Scholar] [CrossRef]
- Stassi, S.; Chiado, A.; Cauda, V.; Palmara, G.; Canavese, G.; Laurenti, M.; Ricciardi, C. Functionalized ZnO nanowires for microcantilever biosensors with enhanced binding capability. Anal. Bioanal. Chem. 2017, 409, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.F.; Chen, Q.W.; Xu, D.S. Hierarchical ZnO nanostructures obtained by electrodeposition. J. Phys. Chem. C 2007, 111, 11560–11565. [Google Scholar] [CrossRef]
- Xu, L.F.; Guo, Y.; Liao, Q.; Zhang, J.P.; Xu, D.S. Morphological control of ZnO nanostructures by electrodeposition. J. Phys. Chem. B 2005, 109, 13519–13522. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Zapien, J.A.; Yao, Y.; Meng, X.M.; Lee, C.S.; Fan, S.S.; Lifshitz, Y.; Lee, S.T. High-density, ordered ultraviolet light-emitting ZnO nanowire arrays. Adv. Mater. 2003, 15, 838–841. [Google Scholar] [CrossRef]
- Stassi, S.; Cauda, V.; Ottone, C.; Chiodoni, A.; Pirri, C.F.; Canavese, G. Flexible piezoelectric energy nanogenerator based on ZnO nanotubes hosted in a polycarbonate membrane. Nano Energy 2015, 13, 474–481. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.W.; Yi, G.C. Metalorganic vapor-phase epitaxial growth of vertically well-aligned ZnO nanorods. Appl. Phys. Lett. 2002, 80, 4232–4234. [Google Scholar] [CrossRef]
- Chiou, W.T.; Wu, W.Y.; Ting, J.M. Growth of single crystal ZnO nanowires using sputter deposition. Diam. Relat. Mater. 2003, 12, 1841–1844. [Google Scholar] [CrossRef]
- Laurenti, M.; Garino, N.; Porro, S.; Fontana, M.; Gerbaldi, C. Zinc oxide nanostructures by chemical vapour deposition as anodes for Li-ion batteries. J. Alloy. Compd. 2015, 640, 321–326. [Google Scholar] [CrossRef]
- Chang, P.C.; Fan, Z.Y.; Wang, D.W.; Tseng, W.Y.; Chiou, W.A.; Hong, J.; Lu, J.G. ZnO nanowires synthesized by vapor trapping CVD method. Chem. Mater. 2004, 16, 5133–5137. [Google Scholar] [CrossRef]
- Valerini, D.; Caricato, A.P.; Lomascolo, M.; Romano, F.; Taurino, A.; Tunno, T.; Martino, M. Zinc oxide nanostructures grown by pulsed laser deposition. Appl. Phys. A 2008, 93, 729–733. [Google Scholar] [CrossRef]
- Rahm, A.; Lorenz, M.; Nobis, T.; Zimmermann, G.; Grundmann, M.; Fuhrmann, B.; Syrowatka, F. Pulsed-laser deposition and characterization of ZnO nanowires with regular lateral arrangement. Appl. Phys. A 2007, 88, 31–34. [Google Scholar] [CrossRef]
- Hartanto, A.B.; Ning, X.; Nakata, Y.; Okada, T. Growth mechanism of ZnO nanorods from nanoparticles formed in a laser ablation plume. Appl. Phys. A 2004, 78, 299–301. [Google Scholar] [CrossRef]
- Choopun, S.; Hongsith, N.; Tanunchai, S.; Chairuangsri, T.; Krua-in, C.; Singkarat, S.; Vilaithonga, T.; Mangkorntong, P.; Mangkorntong, N. Single-crystalline ZnO nanobelts by RF sputtering. J. Cryst. Growth 2005, 282, 365–369. [Google Scholar] [CrossRef]
- Chen, M.T.; Ting, J.M. Sputter deposition of ZnO nanorods/thin-film structures on Si. Thin Solid Films 2006, 494, 250–254. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Canavese, G.; Cauda, V. Surface Engineering of Nanostructured ZnO Surfaces. Adv. Mater. Interfaces 2017, 4, 1600758. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoc, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 41301. [Google Scholar] [CrossRef]
- Tian, Z.R.R.; Voigt, J.A.; Liu, J.; McKenzie, B.; McDermott, M.J.; Rodriguez, M.A.; Konishi, H.; Xu, H.F. Complex and oriented ZnO nanostructures. Nat. Mater. 2003, 2, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingettt, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Joseph, A.; Jose, A.J.; Narayana, B. Enhancement of corrosion protection of mild steel by chitosan/ZnO nanoparticle composite membranes. Prog. Org. Coat. 2015, 84, 28–34. [Google Scholar] [CrossRef]
- Sun, M.M.; Chen, Z.Y.; Bu, Y.Y.; Yu, J.Q.; Hou, B.R. Effect of ZnO on the corrosion of zinc, Q235 carbon steel and 304 stainless steel under white light illumination. Corros. Sci. 2014, 82, 77–84. [Google Scholar] [CrossRef]
- Chiolerio, A.; Roppolo, I.; Cauda, V.; Crepaldi, M.; Bocchini, S.; Bejtka, K.; Verna, A.; Pirri, C.F. Ultraviolet mem-sensors: Flexible anisotropic composites featuring giant photocurrent enhancement. Nano Res. 2015, 8, 1956–1963. [Google Scholar] [CrossRef]
- Norris, B.J.; Anderson, J.; Wager, J.F.; Keszler, D.A. Spin-coated zinc oxide transparent transistors. J. Phys. D Appl. Phys. 2003, 36, L105–L107. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Zhao, Q.X.; Yang, L.L.; Lorenz, M.; Cao, B.Q.; Perez, J.Z.; Czekalla, C.; Zimmermann, G.; Grundmann, M.; et al. Zinc oxide nanorod based photonic devices: Recent progress in growth, light emitting diodes and lasers. Nanotechnology 2009, 20, 332001. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Canavese, G.; Sacco, A.; Fontana, M.; Bejtka, K.; Castellino, M.; Pirri, C.F.; Cauda, V. Nanobranched ZnO Structure: P-Type Doping Induces Piezoelectric Voltage Generation and Ferroelectric-Photovoltaic Effect. Adv. Mater. 2015, 27, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Canavese, G.; Stassi, S.; Fontana, M.; Castellino, M.; Pirri, C.F.; Cauda, V. A porous nanobranched structure: An effective way to improve piezoelectricity in sputtered ZnO thin films. RSC Adv. 2016, 6, 76996. [Google Scholar] [CrossRef]
- Laurenti, M.; Stassi, S.; Lorenzoni, M.; Fontana, M.; Canavese, G.; Cauda, V.; Pirri, C.F. Evaluation of the piezoelectric properties and voltage generation of flexible zinc oxide thin films. Nanotechnology 2015, 26, 215704. [Google Scholar] [CrossRef] [PubMed]
- Rivera, V.F.; Auras, F.; Motto, P.; Stassi, S.; Canavese, G.; Celasco, E.; Bein, T.; Onida, B.; Cauda, V. Length-Dependent Charge Generation from Vertical Arrays of High-Aspect-Ratio ZnO Nanowires. Chem.-Eur. J. 2013, 19, 14665–14674. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.; Pugliese, D.; Garino, N.; Sacco, A.; Bianco, S.; Bella, F.; Lamberti, A.; Gerbaldi, C. Multi-functional energy conversion and storage electrodes using flower-like Zinc oxide nanostructures. Energy 2014, 65, 639–646. [Google Scholar] [CrossRef]
- Lamberti, A.; Sacco, A.; Laurenti, M.; Fontana, M.; Pirri, C.F.; Bianco, S. Sponge-like ZnO nanostructures by low temperature water vapor-oxidation method as dye-sensitized solar cell photoanodes. J. Alloy. Compd. 2014, 615, S487–S490. [Google Scholar] [CrossRef]
- Pugliese, D.; Bella, F.; Cauda, V.; Lamberti, A.; Sacco, A.; Tresso, E.; Bianco, S. A Chemometric Approach for the Sensitization Procedure of ZnO Flowerlike Microstructures for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 11288–11295. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 Core-Shell Nanostructures for Enhanced Photoelectrochemical Water Splitting under Solar Light Illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Hidalgo, D.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Cauda, V.; Tresso, E.; Saracco, G. Comparison of photocatalytic and transport properties of TiO2 and ZnO nanostructures for solar-driven water splitting. Phys. Chem. Chem. Phys. 2015, 17, 7775–7786. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, B.; Cauda, V.; Bonanno, A.; Sanginario, A.; Bejtka, K.; Bella, F.; Fontana, M.; Demarchi, D. One-Dimensional ZnO/Gold Junction for Simultaneous and Versatile Multisensing Measurements. Sci. Rep. 2016, 6, 29763. [Google Scholar] [CrossRef] [PubMed]
- Sanginario, A.; Cauda, V.; Bonanno, A.; Bejtka, K.; Sapienza, S.; Demarchi, D. An electronic platform for real-time detection of bovine serum albumin by means of amine-functionalized zinc oxide microwires. RSC Adv. 2016, 6, 891–897. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. A 2006, 78, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, R.S.; Yu, M.; Bai, F.; Li, C.; Wang, Z.L. Cellular Level Biocompatibility and Biosafety of ZnO Nanowires. J. Phys. Chem. C 2008, 112, 20114–20117. [Google Scholar] [CrossRef]
- Foroutan, T.; Mousavi, S. The effects of zinc oxide nanoparticles on differentiation of human mesenchymal stem cells to osteoblast. Nanomed. J. 2014, 1, 6. [Google Scholar] [CrossRef]
- Gopikrishnan, R.; Zhang, K.; Ravichandran, P.; Biradar, S.; Ramesh, V.; Goornavar, V.; Jeffers, R.B.; Pradhan, A.; Hall, J.C.; Baluchamy, S.; et al. Epitaxial growth of the zinc oxide nanorods, their characterization and in vitro biocompatibility studies. J. Mater. Sci. Mater. Med. 2011, 22, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Castellino, M.; Perrone, D.; Asvarov, A.; Canavese, G.; Chiolerio, A. Lead-free piezoelectrics: V3+ to V5+ ion conversion promoting the performances of V-doped Zinc Oxide. Sci. Rep. 2017, 7, 41957. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kim, S.W. Energy harvesting based on semiconducting piezoelectric ZnO nanostructures. Nano Energy 2012, 1, 342–355. [Google Scholar] [CrossRef]
- Wang, Z.L. Self-Powered Nanosensors and Nanosystems. Adv. Mater. 2012, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Murillo, G.; Blanquer, A.; Vargas-Estevez, C.; Barrios, L.; Ibanez, E.; Nogues, C.; Esteve, J. Electromechanical Nanogenerator-Cell Interaction Modulates Cell Activity. Adv. Mater. 2017, 29, 1605048. [Google Scholar] [CrossRef] [PubMed]
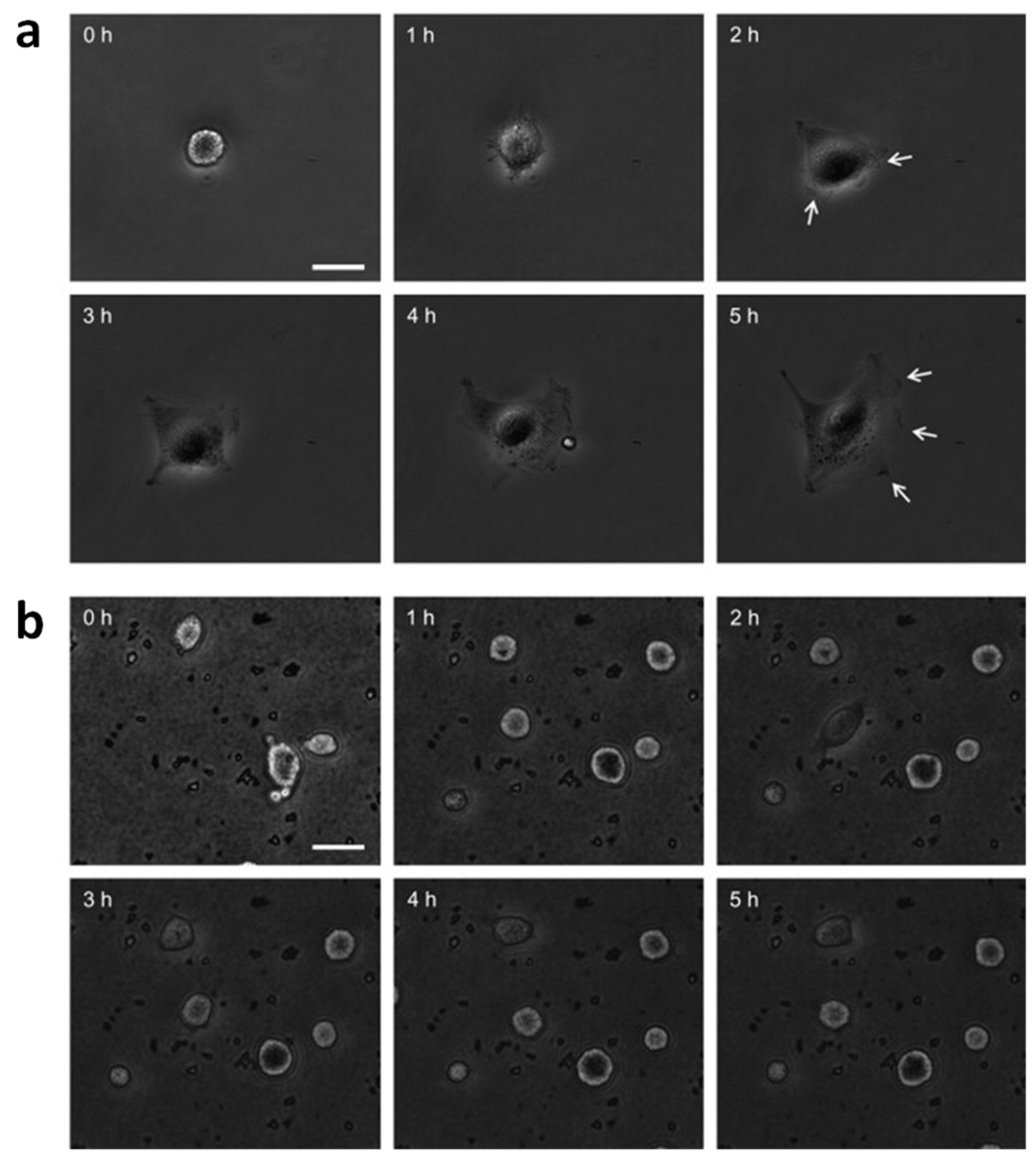
- Zaveri, T.D.; Dolgova, N.V.; Chu, B.H.; Lee, J.Y.; Wong, J.E.; Lele, T.P.; Ren, F.; Keselowsky, B.G. Contributions of surface topography and cytotoxicity to the macrophage response to zinc oxide nanorods. Biomaterials 2010, 31, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Sun, Y.Y.; Cao, Y.; Yu, X.H.; Ji, X.M.; Yang, L. Porous zinc oxide films: Controlled synthesis, cytotoxicity and photocatalytic activity. Chem. Eng. J. 2011, 178, 8–14. [Google Scholar] [CrossRef]
- Petrochenko, P.E.; Zhang, Q.; Bayati, R.; Skoog, S.A.; Phillips, K.S.; Kumar, G.; Narayan, R.J.; Goering, P.L. Cytotoxic evaluation of nanostructured zinc oxide (ZnO) thin films and leachates. Toxicol. In Vitro 2014, 28, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, M.; Ohtsuki, C.; Inada, H.; Tanihara, M.; Miyazaki, T. Effect of ZnO addition on bioactive CaO-SiO2-P2O5-CaF2 glass-ceramics containing apatite and wollastonite. Acta Biomater. 2006, 2, 467–471. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.M.; Ali, A.F. Fabrication and characterization of ZnO modified bioactive glass nanoparticles. Ceram. Int. 2012, 38, 1195–1204. [Google Scholar] [CrossRef]
- Bini, M.; Grandi, S.; Capsoni, D.; Mustarelli, P.; Saino, E.; Visai, L. SiO2-P2O5-CaO Glasses and Glass-Ceramics with and without ZnO: Relationships among Composition, Microstructure, and Bioactivity. J. Phys. Chem. C 2009, 113, 8821–8828. [Google Scholar] [CrossRef]
- Li, H.C.; Wang, D.G.; Chen, C.Z. Effect of zinc oxide and zirconia on structure, degradability and in vitro bioactivity of wollastonite. Ceram. Int. 2015, 41, 10160–10169. [Google Scholar] [CrossRef]
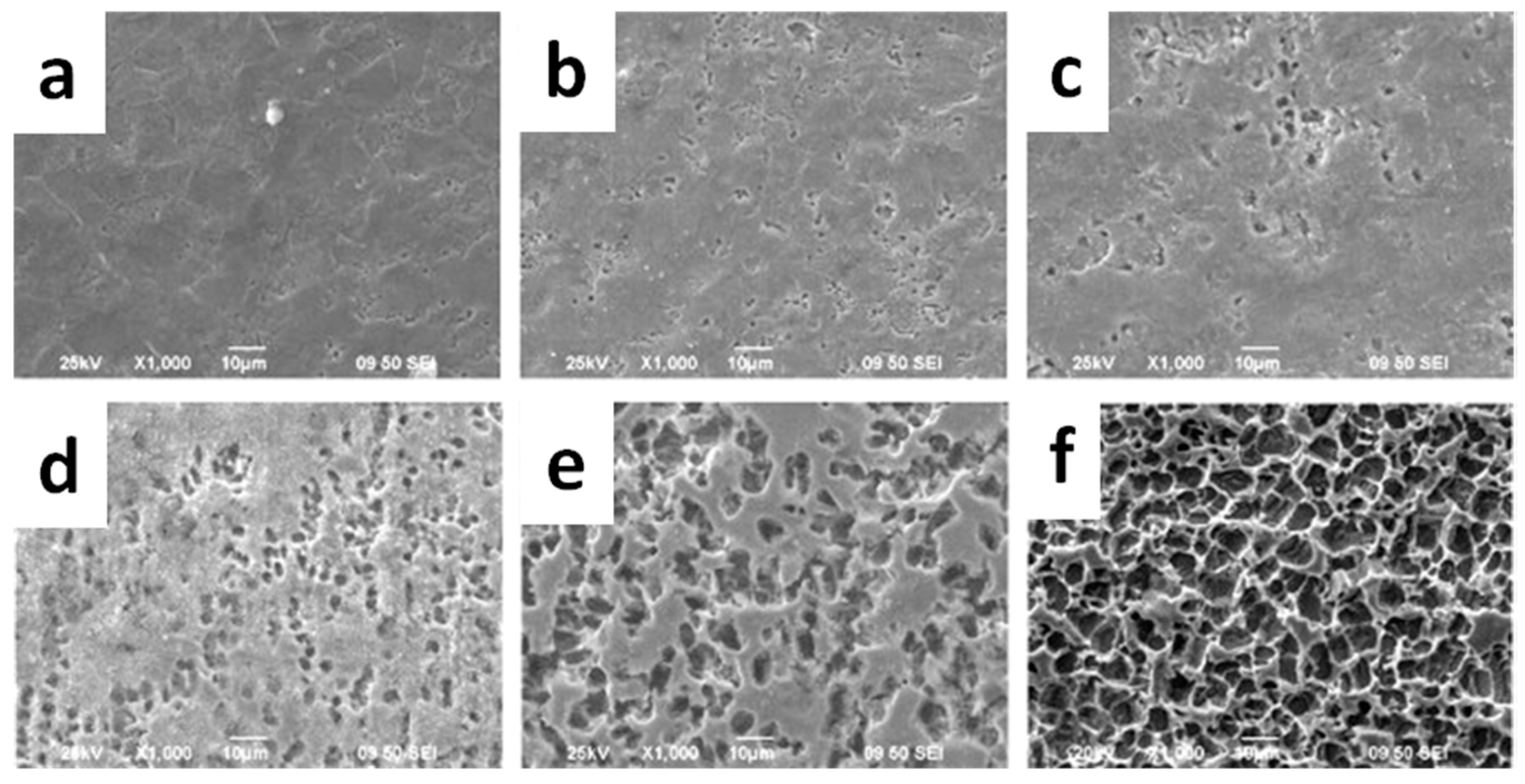
- Augustine, R.; Kalarikkal, N.; Thomas, S. Effect of zinc oxide nanoparticles on the in vitro degradation of electrospun polycaprolactone membranes in simulated body fluid. Int. J. Polym. Mater. Polym. 2016, 65, 28–37. [Google Scholar] [CrossRef]
- Buzarovska, A. Preparation and characterization of poly(epsilon-caprolactone)/ZnO foams for tissue engineering applications. J. Mater. Sci. 2017, 52, 12067–12078. [Google Scholar] [CrossRef]
- Guo, W.H.; Zhao, F.J.; Wang, Y.D.; Tang, J.Y.; Chen, X.F. Characterization of the mechanical behaviors and bioactivity of tetrapod ZnO whiskers reinforced bioactive glass/gelatin composite scaffolds. J. Mech. Behav. Biomed. 2017, 68, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Akinc, M. Si, Zn-modified tricalcium phosphates: A phase composition and crystal structure study. Key Eng. Mater. 2005, 284, 83–86. [Google Scholar] [CrossRef]
- Hashizume, M.; Yamaguchi, M. Stimulatory Effect of Beta-Alanyl-L-Histidinato Zinc on Cell-Proliferation Is Dependent on Protein-Synthesis in Osteoblastic Mc3t3-E1 Cells. Mol. Cell. Biochem. 1993, 122, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salcedo, S.; Shruti, S.; Salinas, A.J.; Malavasi, G.; Menabue, L.; Vallet-Regi, M. In vitro antibacterial capacity and cytocompatibility of SiO2-CaO-P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. B 2014, 2, 4836–4847. [Google Scholar] [CrossRef]
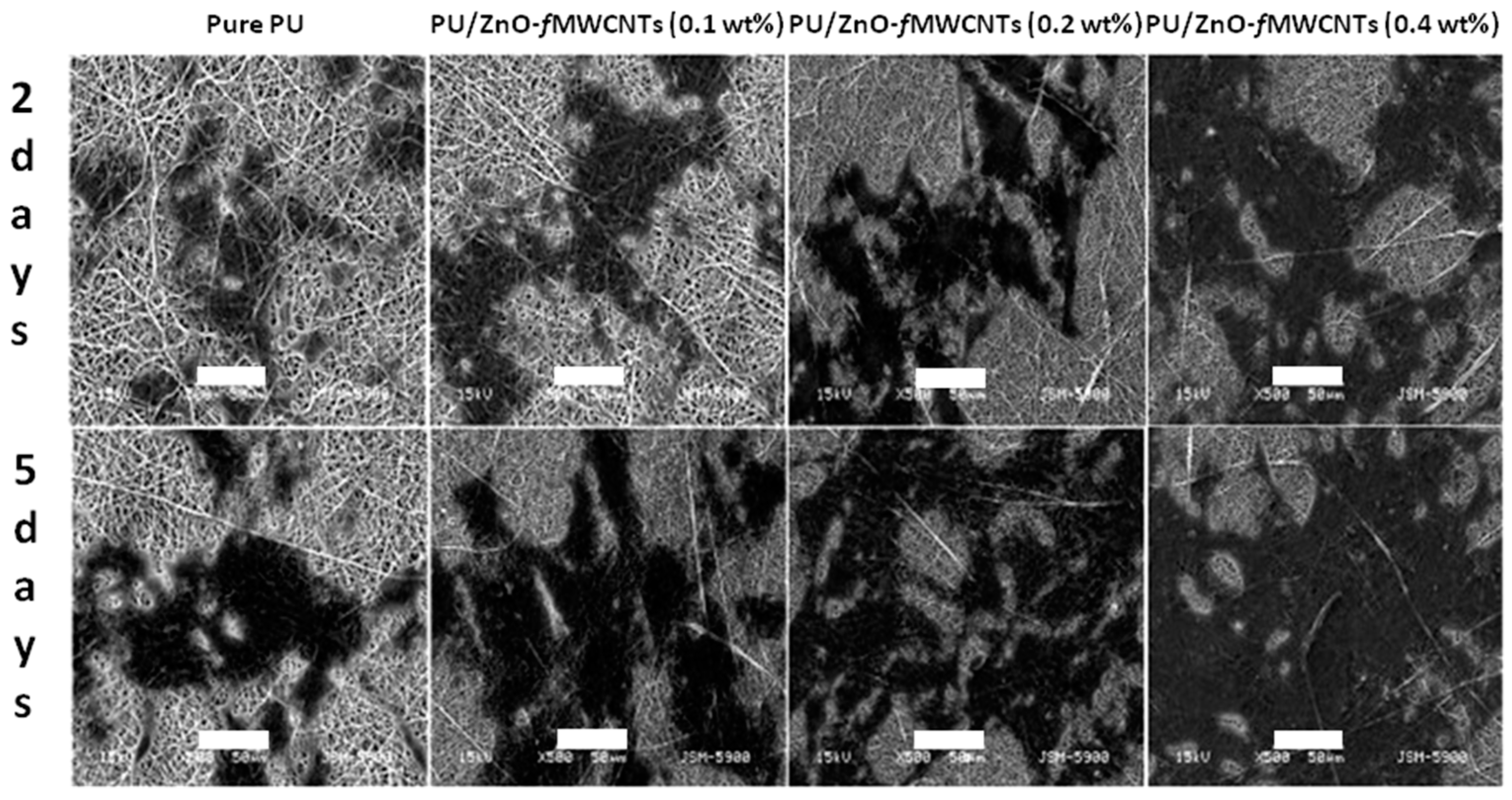
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.I.; Ko, S.W.; Kim, H.J.; Park, C.H.; Kim, C.S. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. Des. 2017, 133, 69–81. [Google Scholar] [CrossRef]
- Bhowmick, A.; Pramanik, N.; Manna, P.J.; Mitra, T.; Selvaraj, T.K.R.; Gnanamani, A.; Das, M.; Kundu, P.P. Development of porous and antimicrobial CTS-PEG-HAP-ZnO nano-composites for bone tissue engineering. RSC Adv. 2015, 5, 99385–99393. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, X.; Cai, H.; Chen, Z.Q.; Wang, T.; Jia, L.L.; Wang, J.; Wan, Q.B.; Pei, X.B. Osteogenic activity and antibacterial effect of zinc oxide/carboxylated graphene oxide nanocomposites: Preparation and in vitro evaluation. Colloid Surf. B 2016, 147, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Munchow, E.A.; Albuquerque, M.T.P.; Zero, B.; Kamocki, K.; Piva, E.; Gregory, R.L.; Bottino, M.C. Development and characterization of novel ZnO-loaded electrospun membranes for periodontal regeneration. Dent. Mater. 2015, 31, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Amna, T.; Hassan, M.S.; Sheikh, F.A.; Lee, H.K.; Seo, K.S.; Yoon, D.; Hwang, I.H. Zinc oxide-doped poly(urethane) spider web nanofibrous scaffold via one-step electrospinning: A novel matrix for tissue engineering. Appl. Microbiol. Biot. 2013, 97, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Tokuda, H.; Yasuda, E.; Noda, T.; Ohta, T.; Takal, S.; Kozawa, O. Up-regulation by zinc of FGF-2-induced VEGF release through enhancing p44/p42 MAP kinase activation in osteoblasts. Life Sci. 2006, 80, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
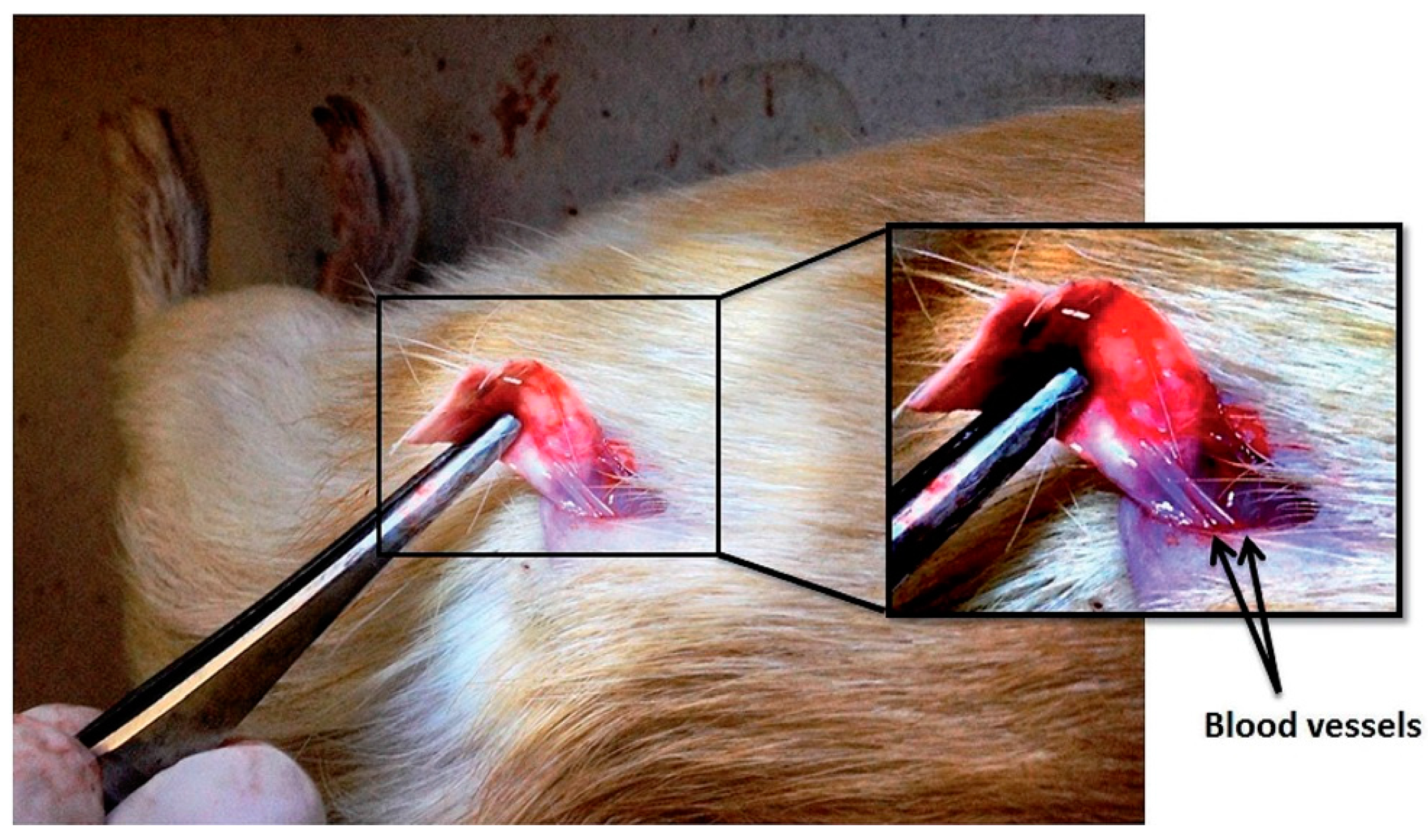
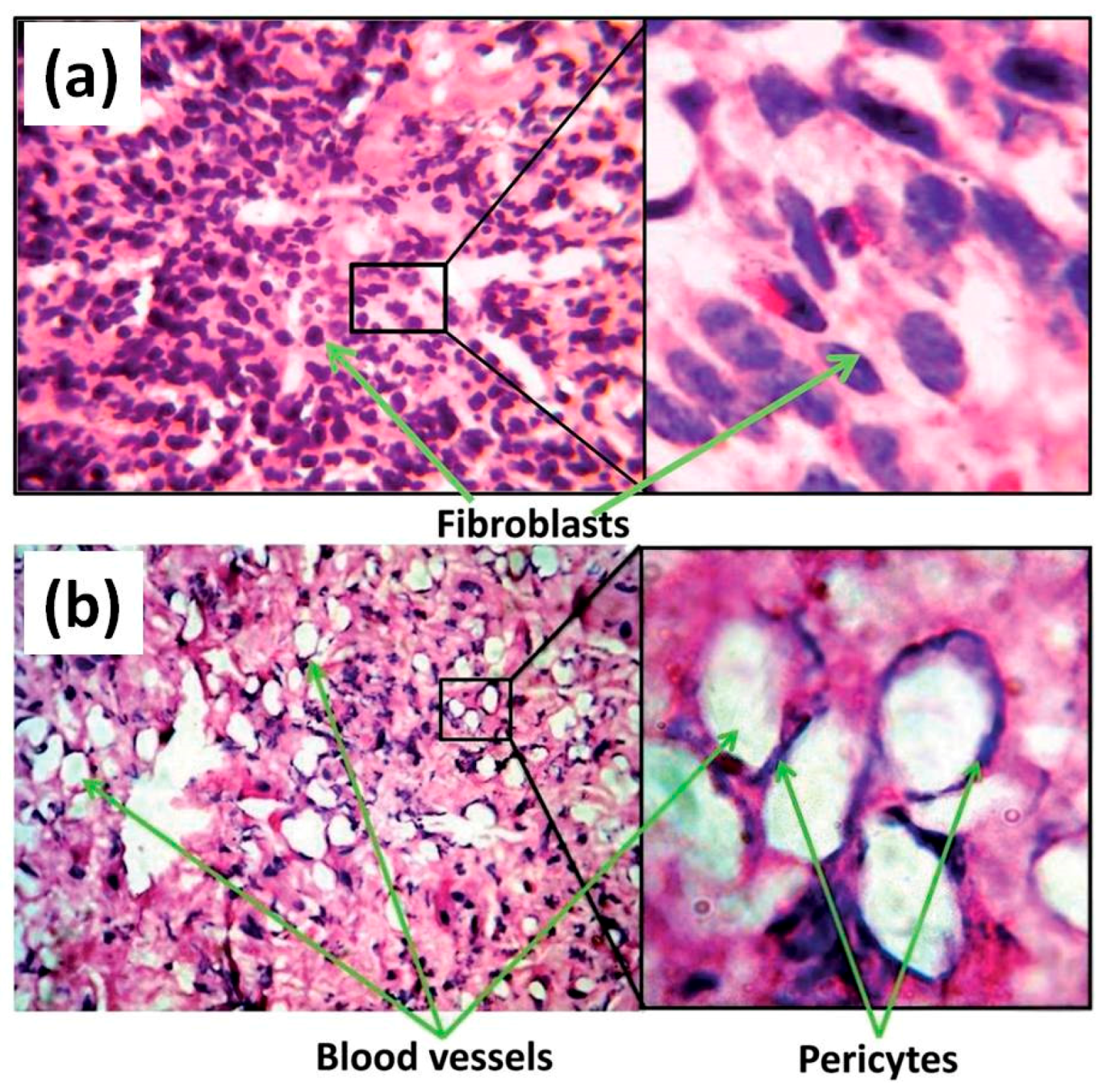
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurenti, M.; Cauda, V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials 2017, 7, 374. https://doi.org/10.3390/nano7110374
Laurenti M, Cauda V. ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials. 2017; 7(11):374. https://doi.org/10.3390/nano7110374
Chicago/Turabian StyleLaurenti, Marco, and Valentina Cauda. 2017. "ZnO Nanostructures for Tissue Engineering Applications" Nanomaterials 7, no. 11: 374. https://doi.org/10.3390/nano7110374
APA StyleLaurenti, M., & Cauda, V. (2017). ZnO Nanostructures for Tissue Engineering Applications. Nanomaterials, 7(11), 374. https://doi.org/10.3390/nano7110374





