Cytotoxic Induction and Photoacoustic Imaging of Breast Cancer Cells Using Astaxanthin-Reduced Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
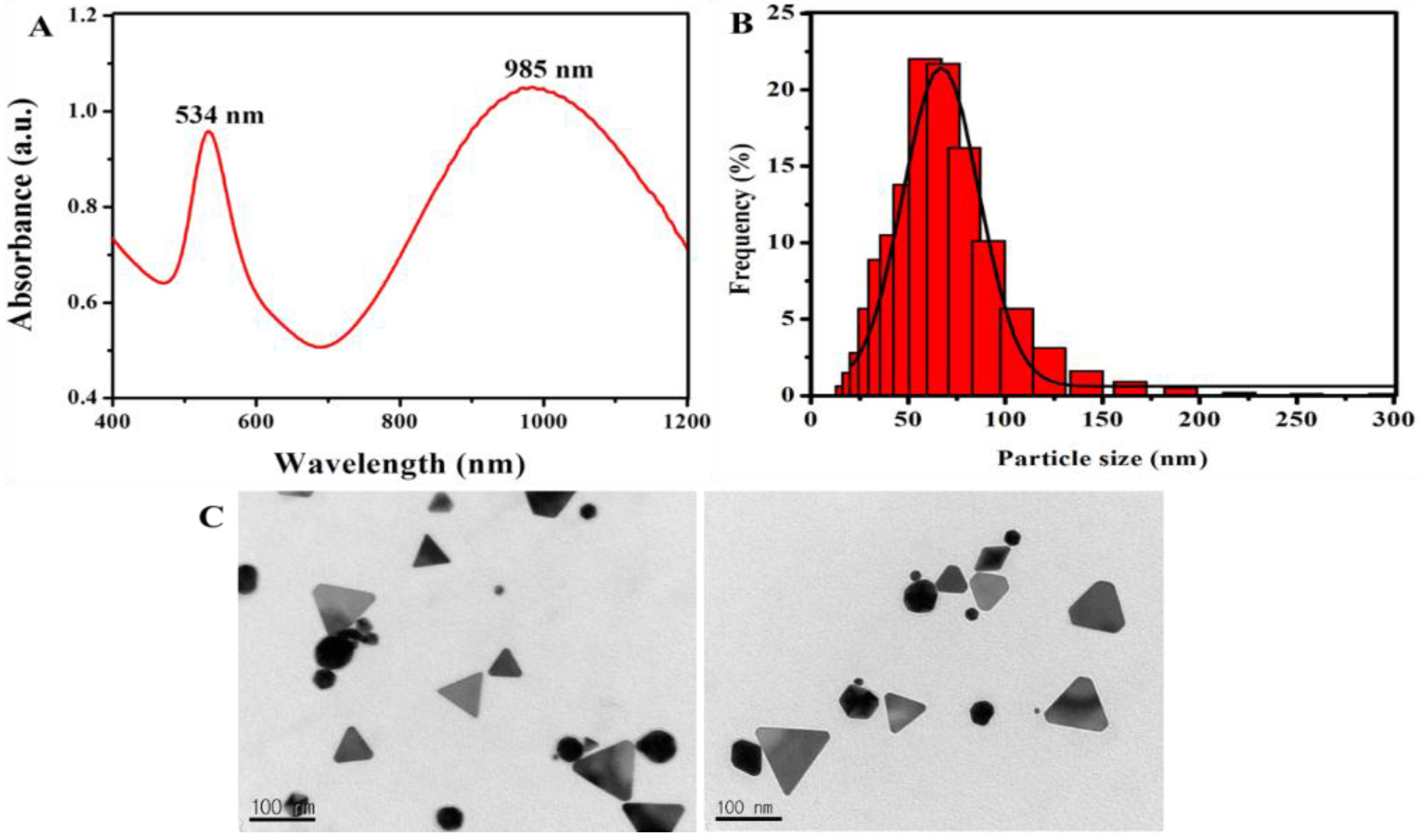
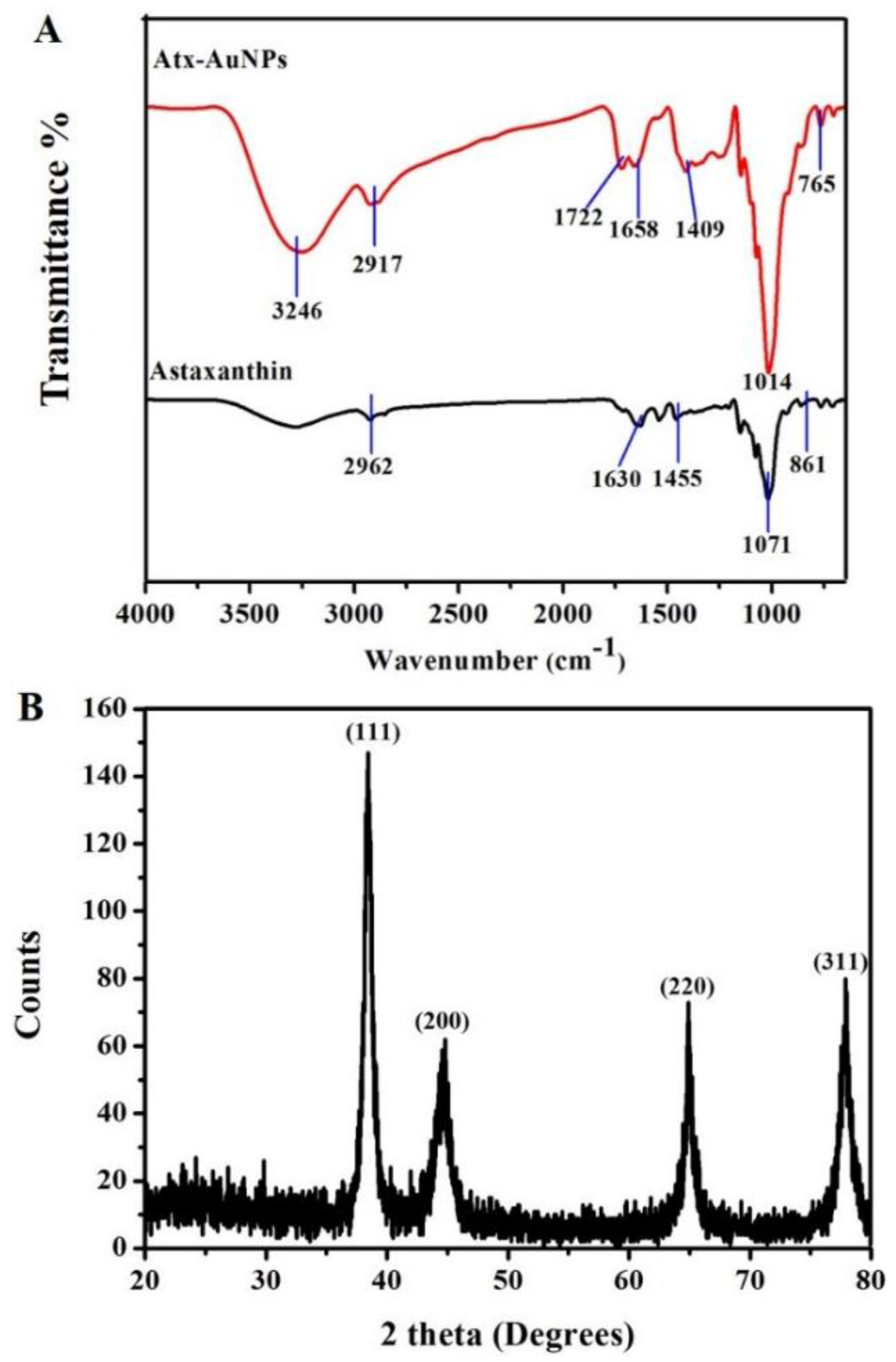
2.1. Synthesis and Characterization of Gold Nanoparticles
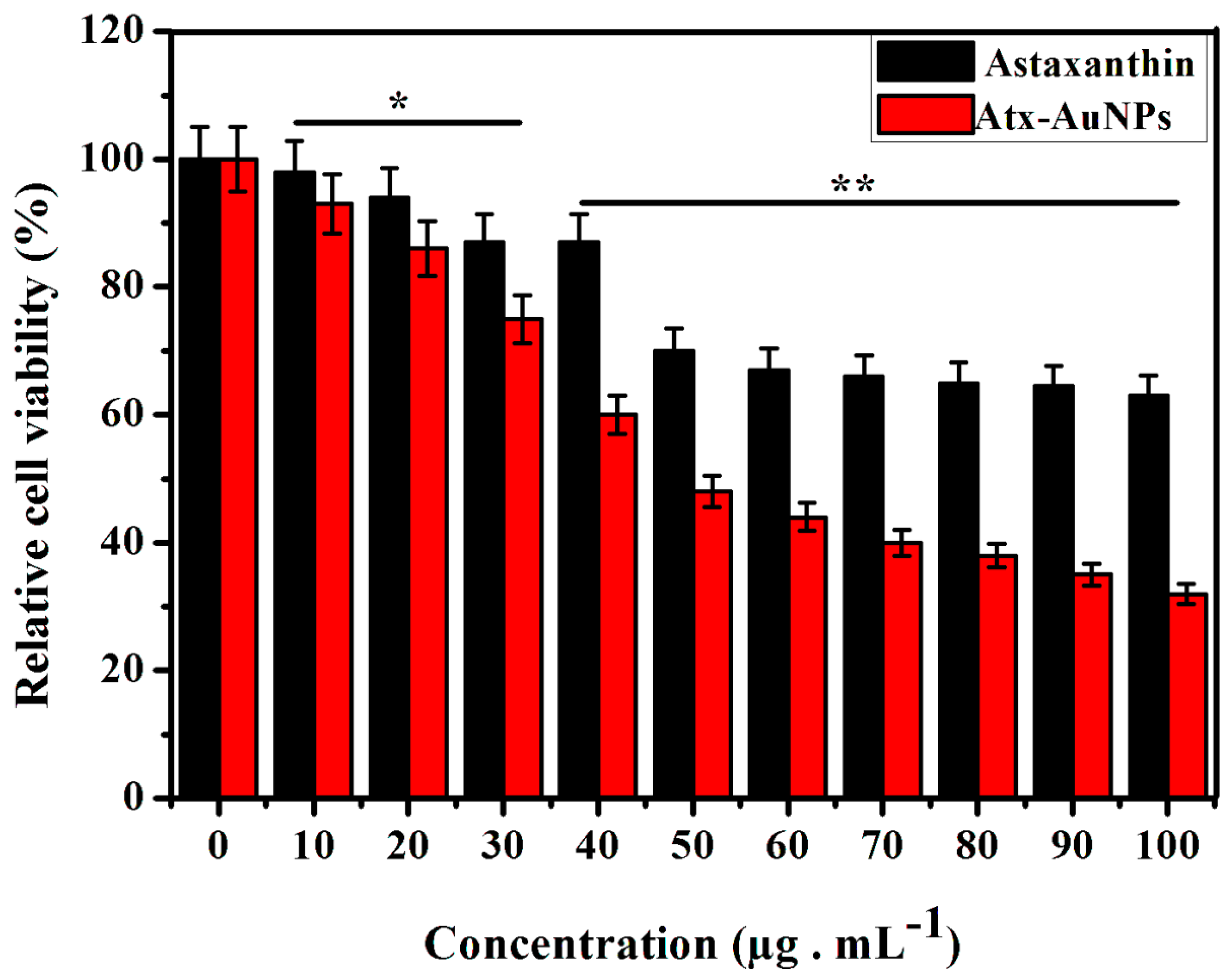
2.2. Assessment of Cytotoxic Effect of Atx-AuNPs
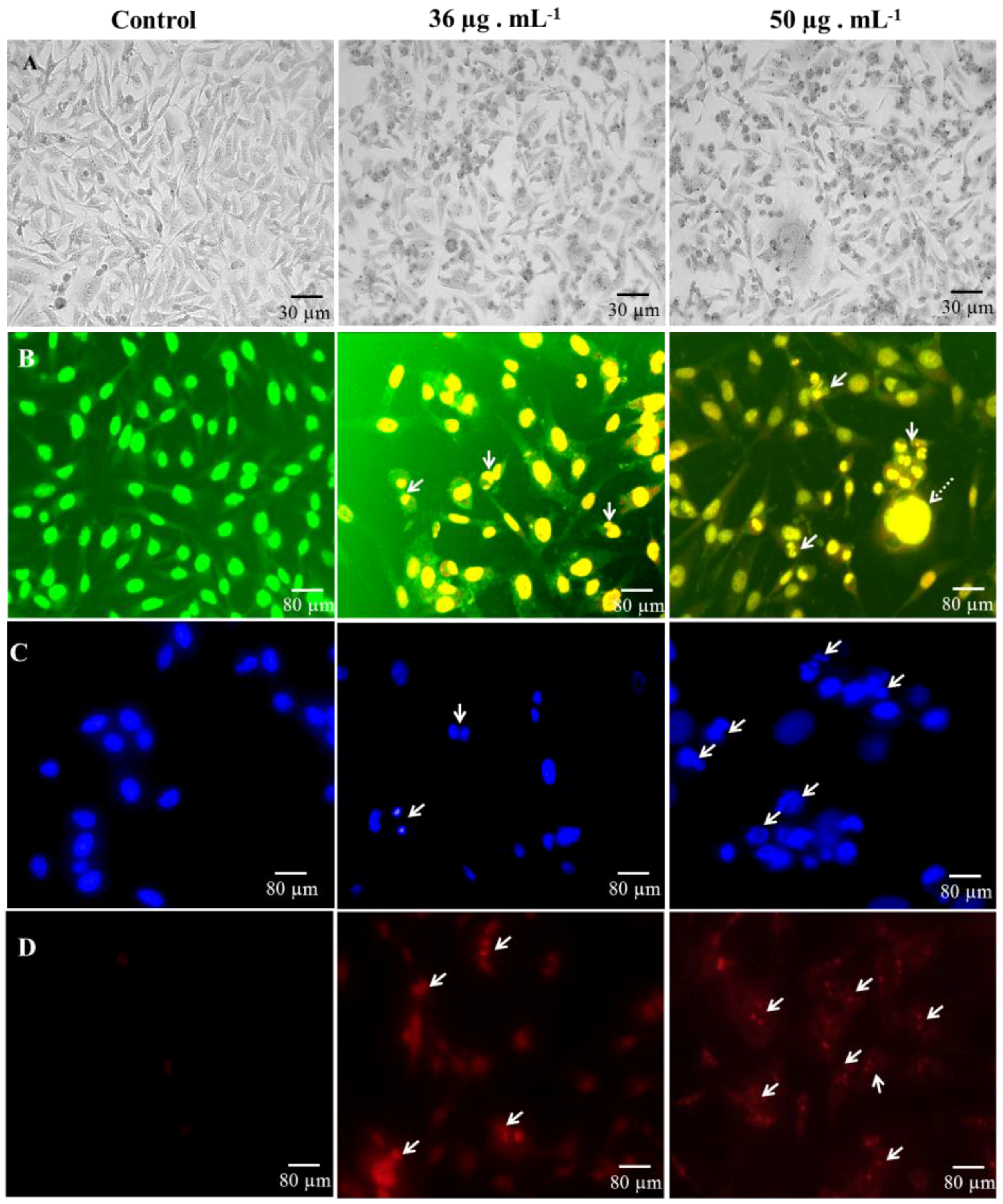
2.3. Microscopic Analysis of Cell Death
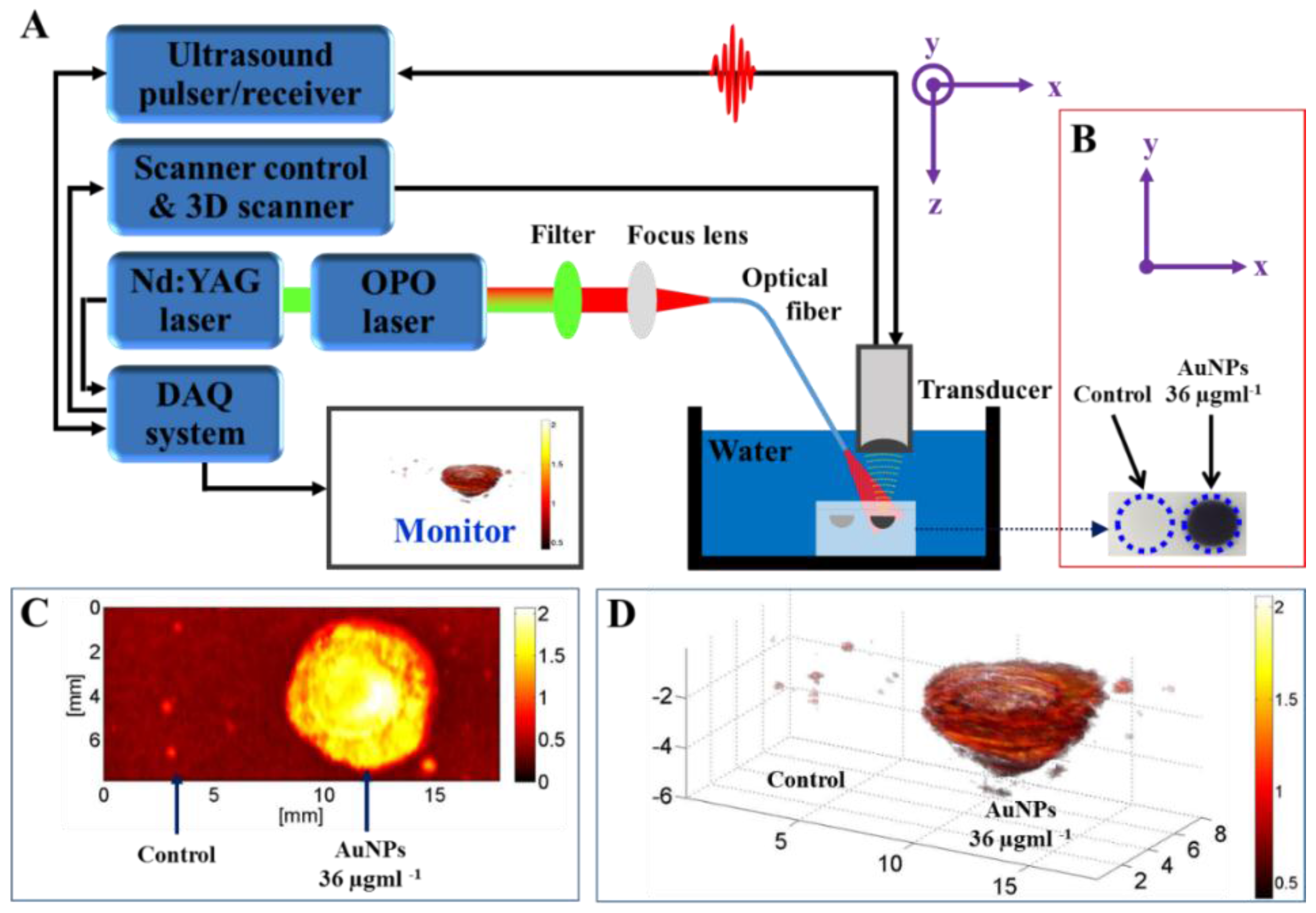
2.4. Photoacoustic Image
3. Materials and Methods
3.1. Reagents
3.2. Formation of AuNPs
3.3. Characterization of Gold Nanoparticles
3.4. Cell Culture
3.5. Antiproliferative Assay
3.6. AO-EB Staining
3.7. Hoechst 33342 Staining
3.8. Propidium Iodide Staining
3.9. Tissue-Mimicking Phantom Experiment
3.10. Photoacoustic Tomography
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Atx-AuNPs | Astaxanthin-synthesized gold nanoparticles |
| AuNPs | Gold nanoparticles |
| MDA-MB-231 | Human breast cancer cell line |
| NIR | Near infrared region |
| SPR | Surface plasmon resonance |
| PAT | Photoacoustic tomography |
References
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Cabuzu, D.; Cirja, A.; Puiu, R.; Grumezescu, A.M. Biomedical applications of gold nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Iwakuma, N.; Sharma, P.; Moudgil, B.; Wu, C.; McNeill, J.; Jiang, H.; Grobmyer, S. Gold nanoparticles as a contrast agent for in vivo tumor imaging with photoacoustic tomography. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.J.; Vali, D.N.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by piper longum fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (chionoecetes opilio) and shrimp (pandalus borealis) processing discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-D.; Zhang, J.-J.; Wang, M.-R.; Liu, W.-B.; Gu, X.-B.; Lv, C.-J. Astaxanthin induces mitochondria-mediated apoptosis in rat hepatocellular carcinoma CBRH-7919 cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as contrast agents for in vivo bioimaging: Current status and future perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Dai, X.; Lu, Y.; Yu, E.; Brahmbatt, N.; Carter, N.; Tchouwou, C.; Singh, A.K.; Jones, Y.; Yu, H. Enhancing targeted tumor treatment by near IR light-activatable photodynamic-photothermal synergistic therapy. Mol. Pharm. 2014, 11, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, X.-S.; Wang, H.-D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.-L.; Wang, X.-L. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Zheng, J.; Gao, G.; Kong, Y.F.; Zhi, X.; Wang, K.; Zhang, X.Q.; Cui, D.X. Biosynthesis of gold nanoparticles using chloroplasts. Int. J. Nanomed. 2011, 6, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Klekotko, M.; Matczyszyn, K.; Siednienko, J.; Olesiak-Banska, J.; Pawlik, K.; Samoc, M. Bio-mediated synthesis, characterization and cytotoxicity of gold nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 29014–29019. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Nanoshells from probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Aromal, S.A.; Philip, D. Benincasa hispida seed mediated green synthesis of gold nanoparticles and its optical nonlinearity. Phys. E 2012, 44, 1329–1334. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Muthukumaran, P.; Ramachandran, R.; Balakumaran, M.; Kalaichelvan, P. Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 2014, 4, 42–49. [Google Scholar] [CrossRef]
- Gangula, A.; Podila, R.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef] [PubMed]
- Schröfel, A.; Kratošová, G.; Bohunická, M.; Dobročka, E.; Vávra, I. Biosynthesis of gold nanoparticles using diatoms—Silica-gold and EPS-gold bionanocomposite formation. J. Nanopart. Res. 2011, 13, 3207–3216. [Google Scholar] [CrossRef]
- Lü, X.; Song, Y.; Zhu, A.; Wu, F.; Song, Y. Synthesis of gold nanoparticles using cefopernazone as a stabilizing reagent and its application. Int. J. Electrochem. Sci 2012, 7, 11236–11245. [Google Scholar]
- Venkatpurwar, V.; Shiras, A.; Pokharkar, V. Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: In vitro cytotoxicity study. Int. J. Pharm. 2011, 409, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J. Cytotoxic effects of gold nanoparticles: A multiparametric study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- Chueh, P.J.; Liang, R.-Y.; Lee, Y.-H.; Zeng, Z.-M.; Chuang, S.-M. Differential cytotoxic effects of gold nanoparticles in different mammalian cell lines. J. Hazard. Mater. 2014, 264, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- El-Kassas, H.Y.; El-Sheekh, M.M. Cytotoxic activity of biosynthesized gold nanoparticles with an extract of the red seaweed corallina officinalis on the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. APJCP 2013, 15, 4311–4317. [Google Scholar] [CrossRef]
- Purschke, M.; Rubio, N.; Held, K.D.; Redmond, R.W. Phototoxicity of Hoechst 33342 in time-lapse fluorescence microscopy. Photochem. Photobiol. Sci. 2010, 9, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Zamzami, N.; Kroemer, G. Apoptosis: Condensed matter in cell death. Nature 1999, 401, 127–128. [Google Scholar] [PubMed]
- Bui, N.Q.; Hlaing, K.K.; Nguyen, V.P.; Nguyen, T.H.; Oh, Y.-O.; Fan, X.F.; Lee, Y.W.; Nam, S.Y.; Kang, H.W.; Oh, J. Intravascular ultrasonic–photoacoustic (IVUP) endoscope with 2.2-mm diameter catheter for medical imaging. Comput. Med. Imaging Graph. 2015, 45, 57–62. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharathiraja, S.; Manivasagan, P.; Quang Bui, N.; Oh, Y.-O.; Lim, I.G.; Park, S.; Oh, J. Cytotoxic Induction and Photoacoustic Imaging of Breast Cancer Cells Using Astaxanthin-Reduced Gold Nanoparticles. Nanomaterials 2016, 6, 78. https://doi.org/10.3390/nano6040078
Bharathiraja S, Manivasagan P, Quang Bui N, Oh Y-O, Lim IG, Park S, Oh J. Cytotoxic Induction and Photoacoustic Imaging of Breast Cancer Cells Using Astaxanthin-Reduced Gold Nanoparticles. Nanomaterials. 2016; 6(4):78. https://doi.org/10.3390/nano6040078
Chicago/Turabian StyleBharathiraja, Subramaniyan, Panchanathan Manivasagan, Nhat Quang Bui, Yun-Ok Oh, In Gweon Lim, Suhyun Park, and Junghwan Oh. 2016. "Cytotoxic Induction and Photoacoustic Imaging of Breast Cancer Cells Using Astaxanthin-Reduced Gold Nanoparticles" Nanomaterials 6, no. 4: 78. https://doi.org/10.3390/nano6040078
APA StyleBharathiraja, S., Manivasagan, P., Quang Bui, N., Oh, Y.-O., Lim, I. G., Park, S., & Oh, J. (2016). Cytotoxic Induction and Photoacoustic Imaging of Breast Cancer Cells Using Astaxanthin-Reduced Gold Nanoparticles. Nanomaterials, 6(4), 78. https://doi.org/10.3390/nano6040078






