Porphyrin-Based Nanostructures for Photocatalytic Applications
Abstract
:1. Introduction
2. Design and Photocatalytic Applications
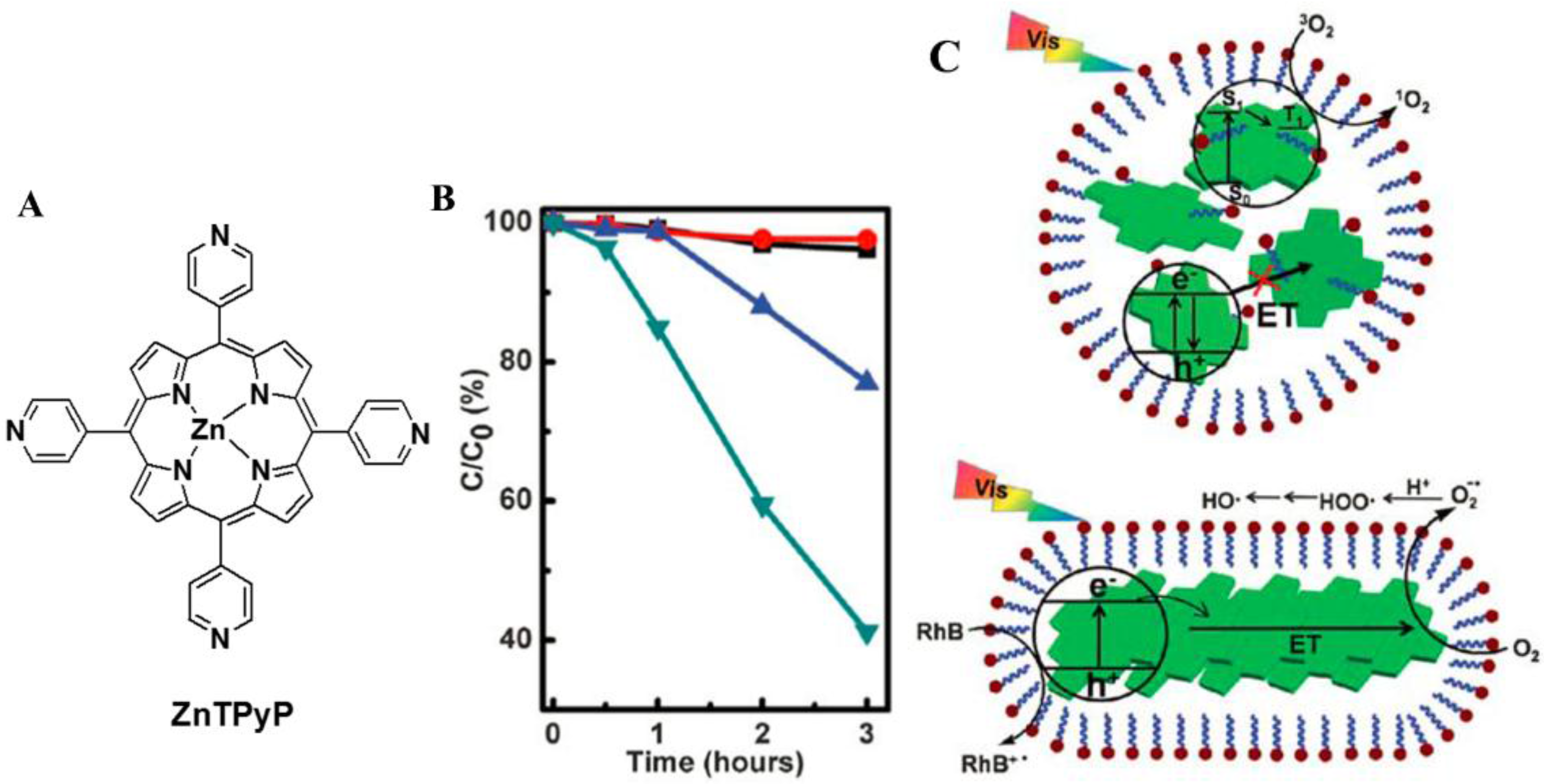
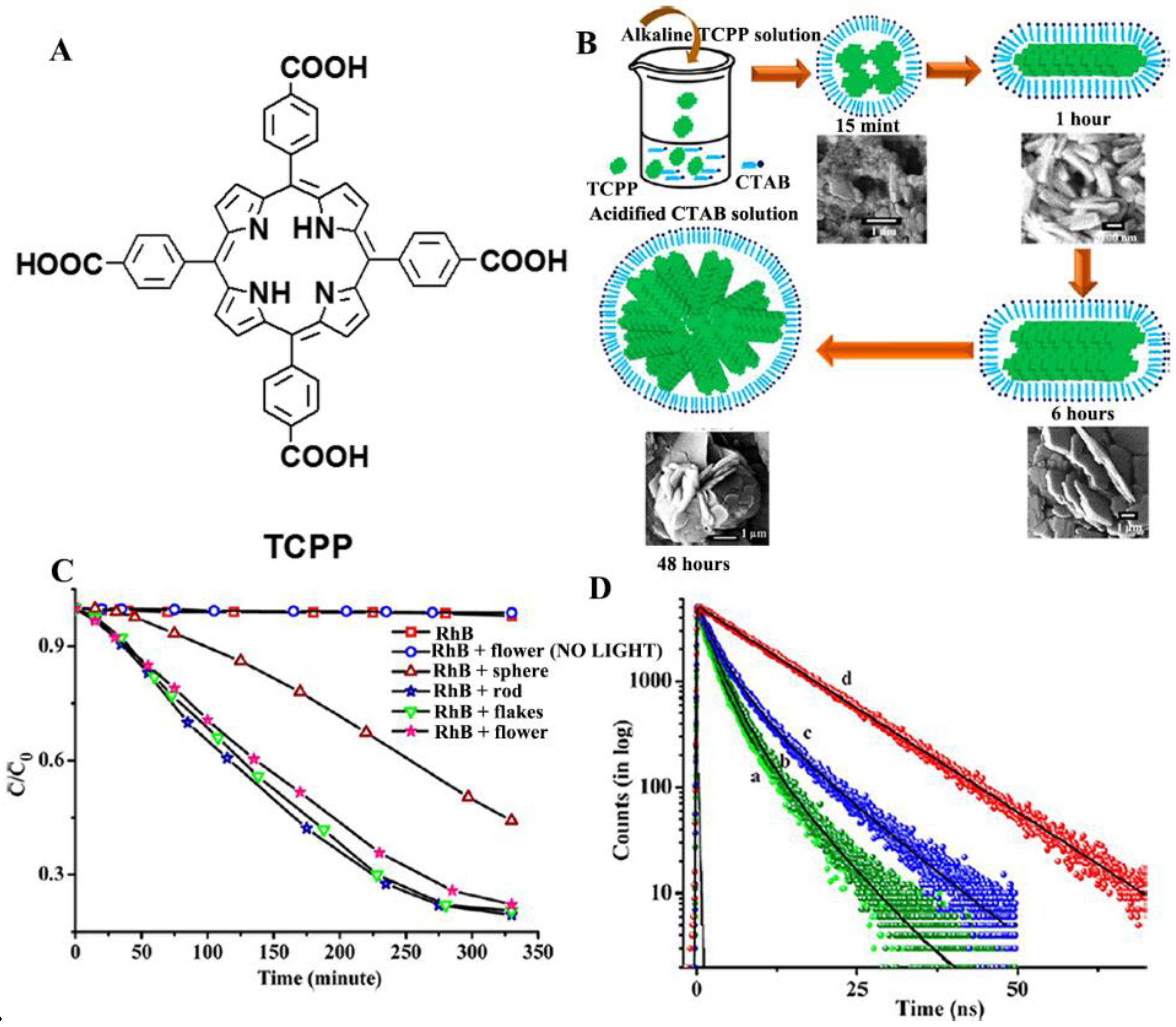
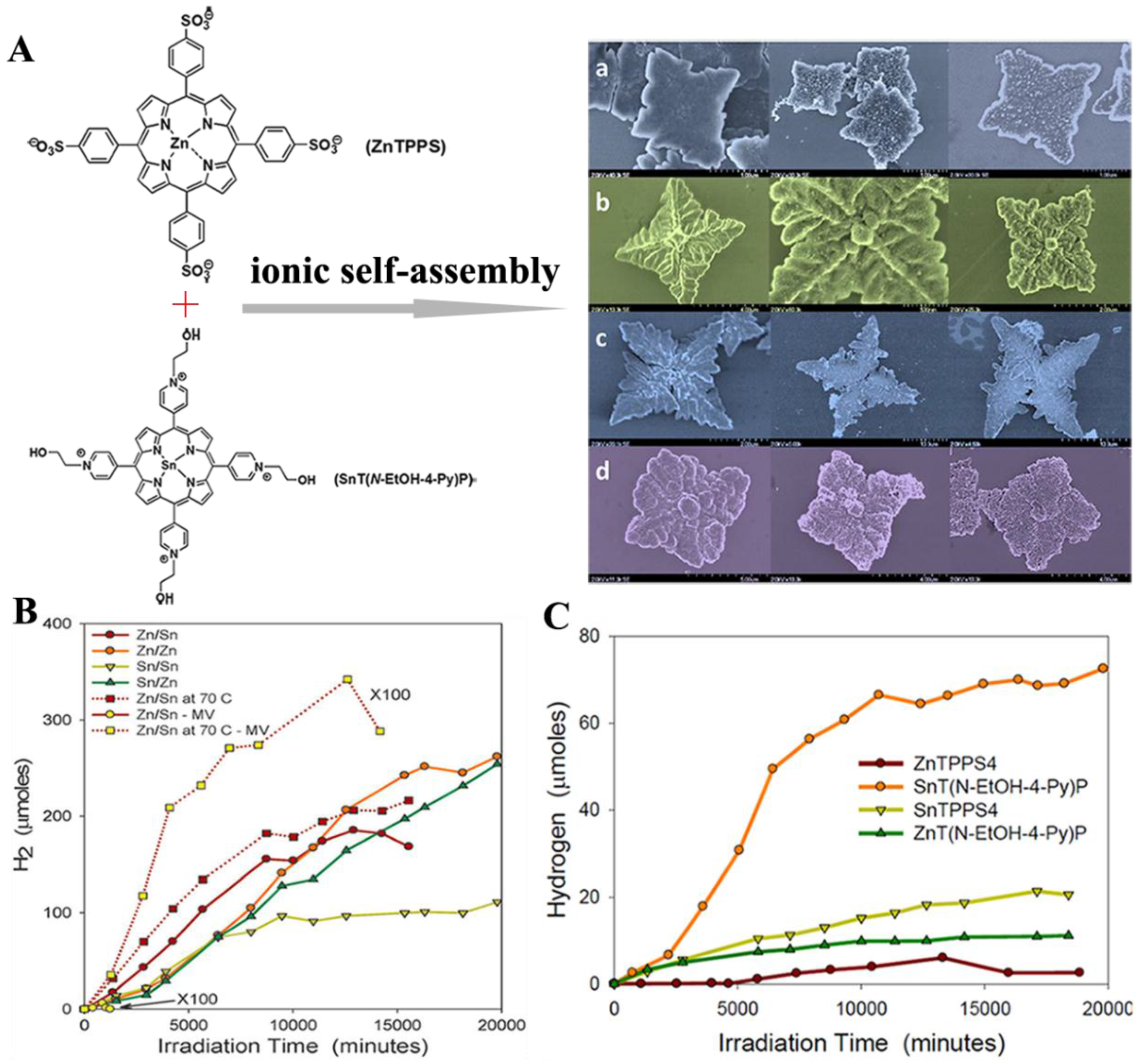
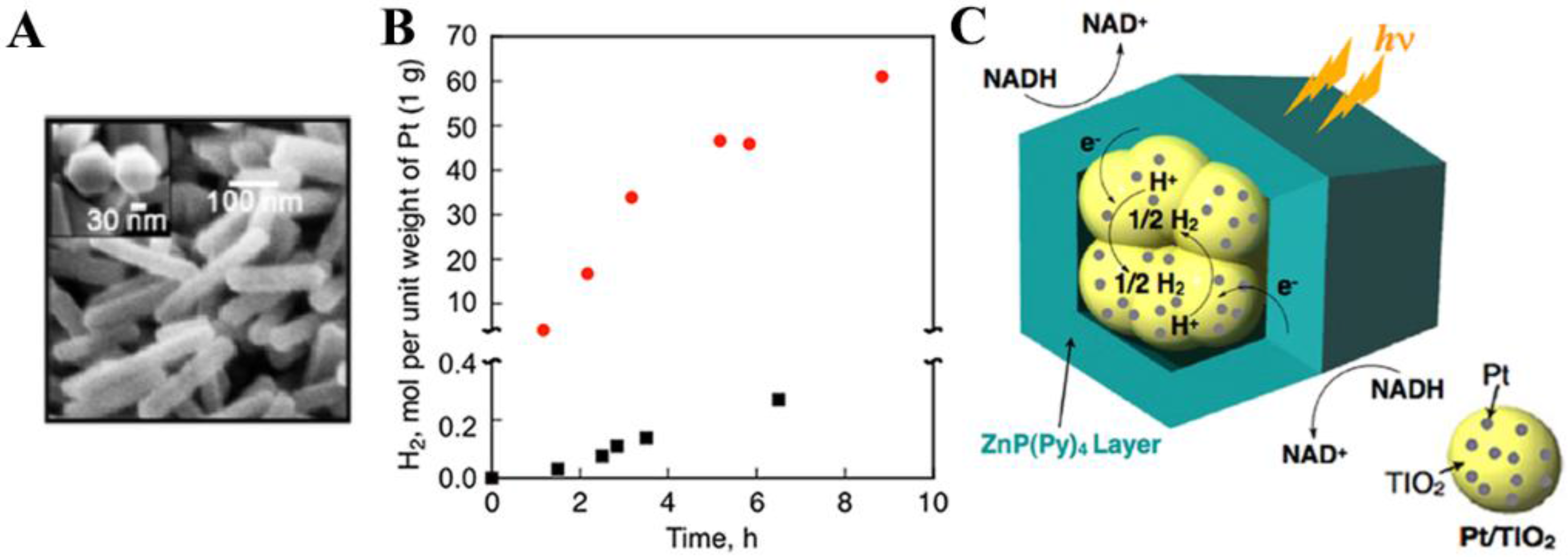
2.1. Isolated Porphyrin Nanostructures for Photocatalysis
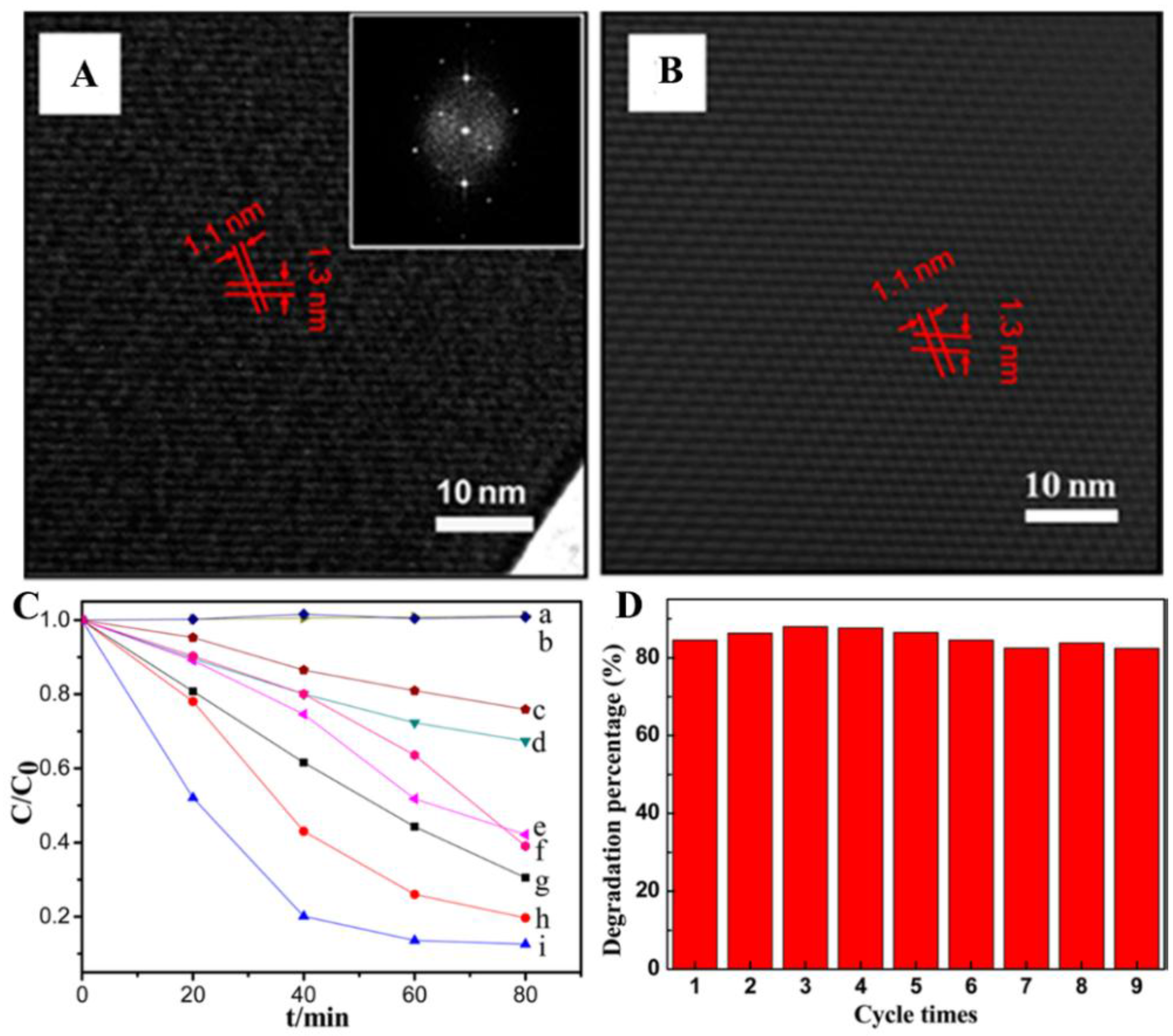
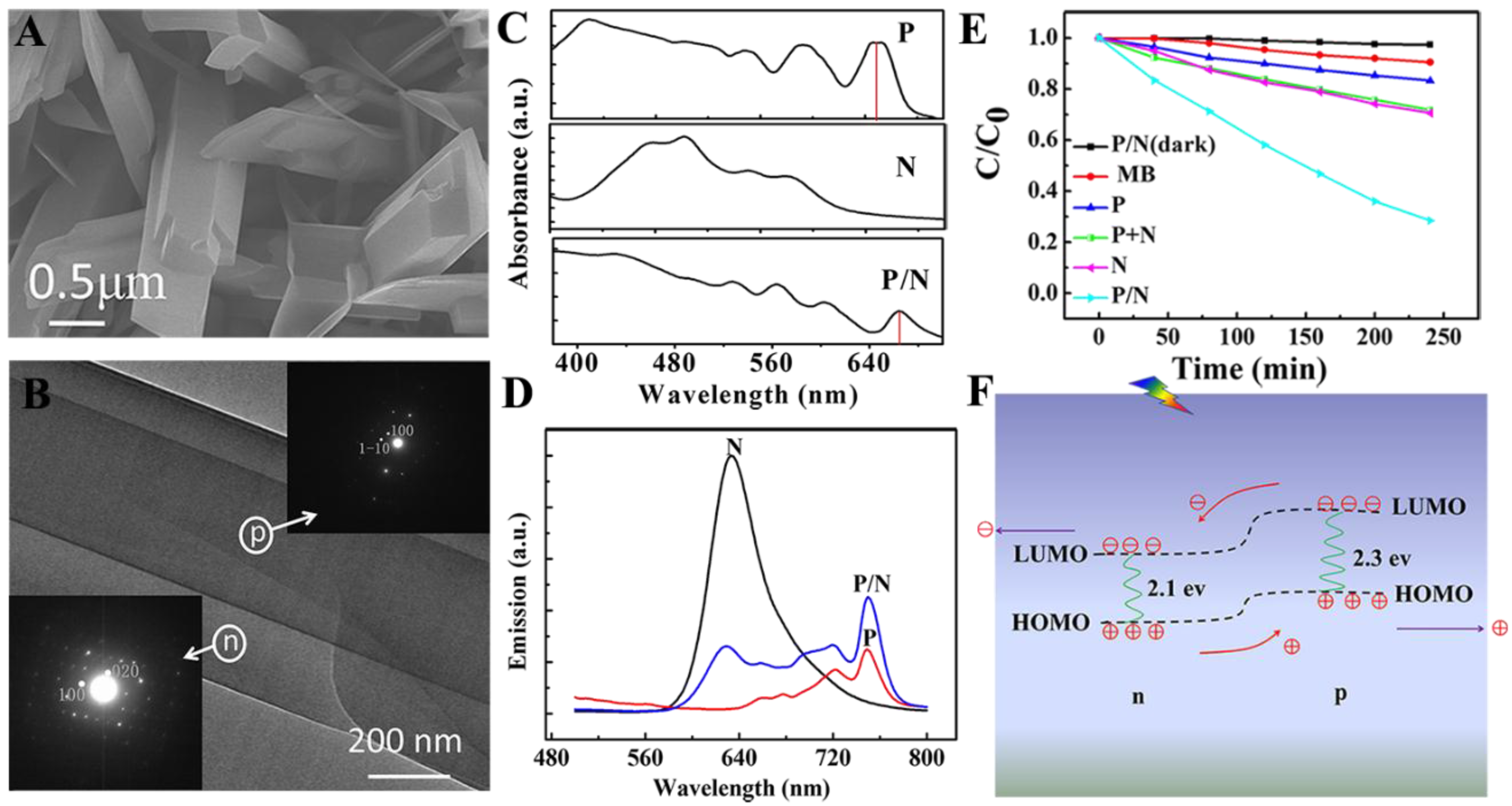
2.2. Organic p/n Nano-Heterojunction for Photocatalysis
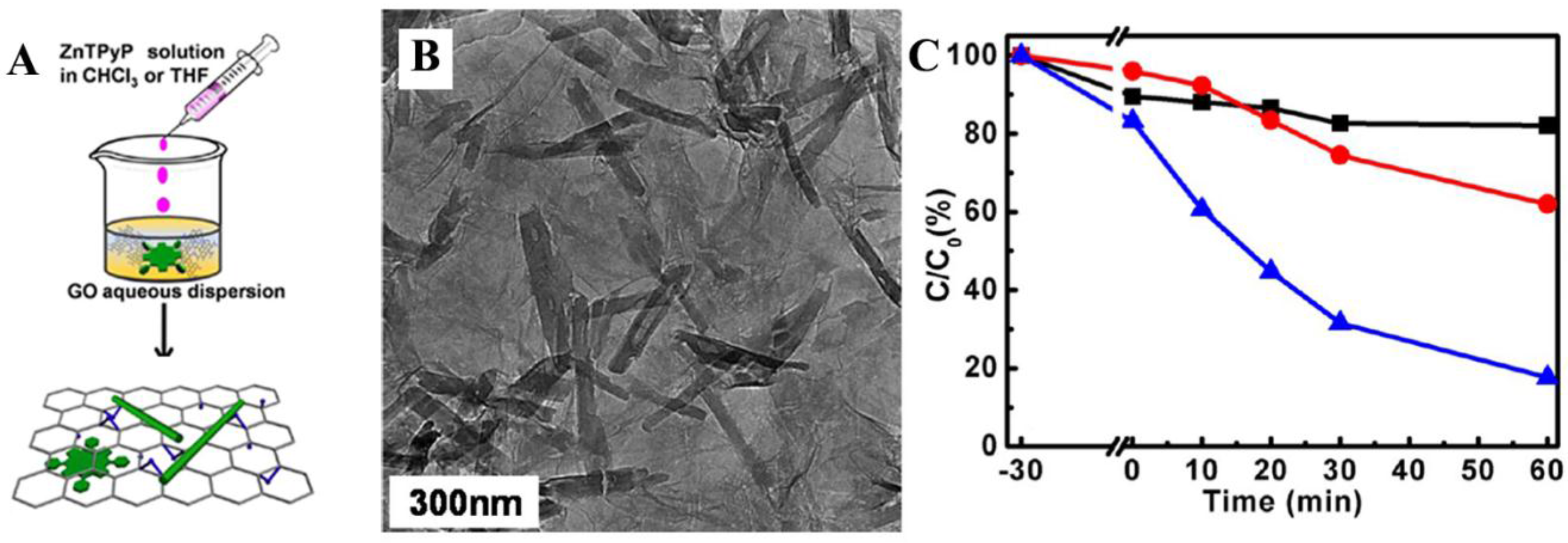
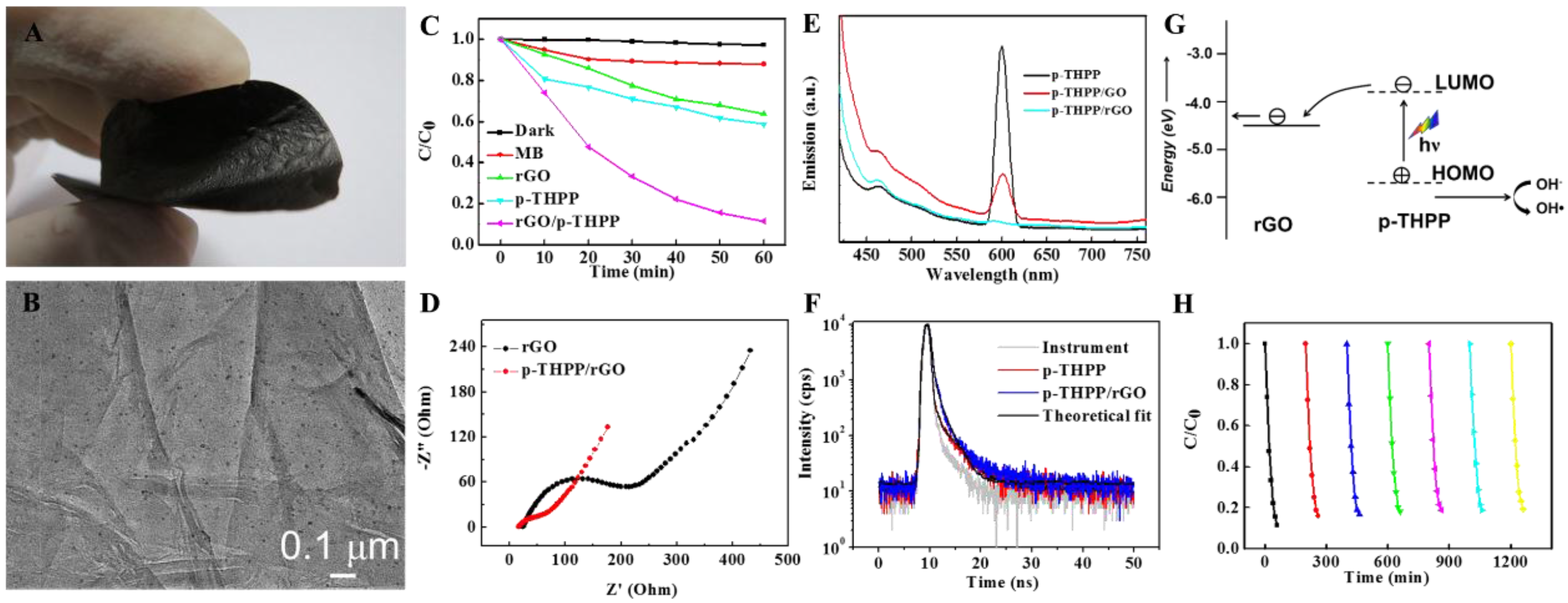
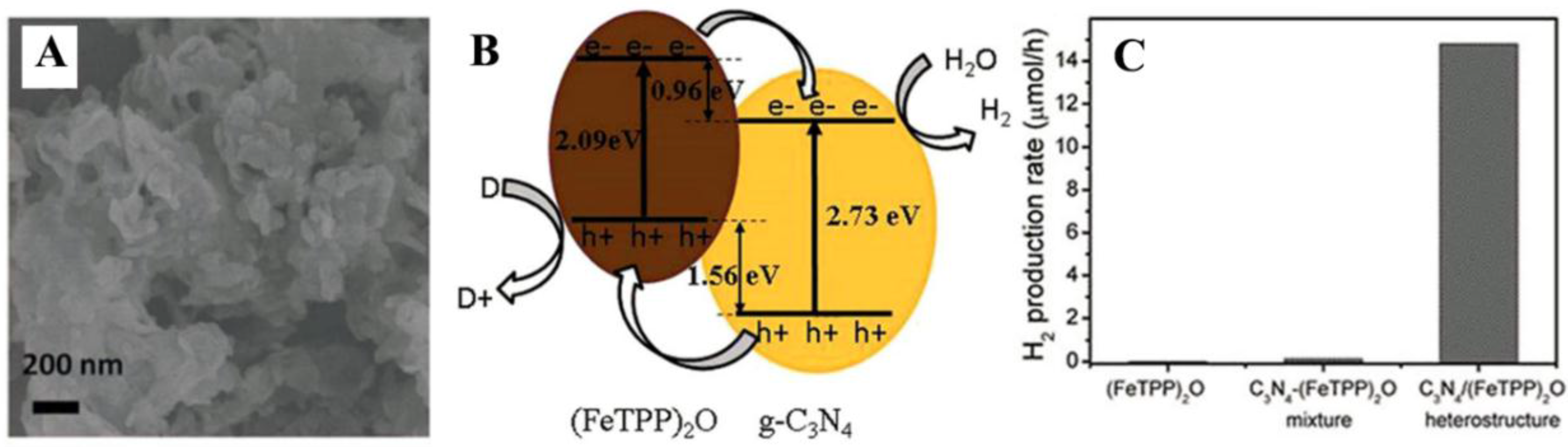
2.3. Organic/Inorganic Nano-Heterojunction for Photocatalysis
2.4. Others
3. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiao, H.W.; Mujumdar, A.; Che, L. Pollution: Uncouple from economy boom. Nature 2015, 517. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.B. Powering the planet with solar fuel. Nat. Chem. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V.; Tvrdy, K.; Baker, D.R.; Radich, J.G. Beyond photovoltaics: Semiconductor nanoarchitectures for liquid-junction solar cells. Chem. Rev. 2010, 110, 6664–6688. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Photolysis-decomposition of water at the surface of an irradiated semiconductor. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 2011, 112, 1710–1750. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295–295. [Google Scholar] [CrossRef] [PubMed]
- Esswein, A.J.; Nocera, D.G. Hydrogen production by molecular photocatalysis. Chem. Rev. 2007, 107, 4022–4047. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Z.; Zhou, Q.; Meng, B.; Meng, X.; Qiu, J. Highly efficient low-temperature plasma-assisted modification of TiO2 nanosheets with exposed {001} facets for enhanced visible-light photocatalytic activity. Chem-Eur. J. 2014, 20, 14763–14770. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, C.H.; Kim, D.Y.; Locquet, J.-P.; Seo, J.W. Preparation and photocatalytic activity of potassium-incorporated titanium oxide nanostructures produced by the wet corrosion process using various titanium alloys. Nanomaterials 2015, 5, 1397–1417. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Liao, Q.; Yao, J. Construction and optoelectronic properties of organic one-dimensional nanostructures. Acc. Chem. Res. 2009, 43, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Xiao, D.; Yao, J. Low-dimensional nanomaterials based on small organic molecules: Preparation and optoelectronic properties. Adv.Mater. 2008, 20, 2859–2876. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, Y.; Jie, J.; Zhang, X.; Xing, Y.; Wu, H.; Zou, B.; Zhang, X.; Zhang, X. Aligned ultralong nanowire arrays and their application in flexible photodetector devices. J. Mater. Chem. 2012, 22, 14357–14362. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, W.; Zhang, X.; Zhang, X.; Zhang, X.; Xing, Y.; Jie, J. In situ integration of squaraine-nanowire-array-based schottky-type photodetectors with enhanced switching performance. ACS. Appl. Mater. Inter. 2013, 5, 12288–12294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, G.; Yu, J.C. Design, fabrication, and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir 2009, 26, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, Q.; Yu, J.; Zhang, Y.; Liu, S. Nanostructured 2D diporphyrin honeycomb film: Photoelectrochemistry, photodegradation and antibacterial activity. ACS. Appl. Mater. Inter. 2015, 7, 11783–11791. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, B.; Zhang, M.; Mu, J.; Shao, C.; Liu, Y. Zinc phthalocyanine hierarchical nanostructure with hollow interior space: Solvent-thermal synthesis and high visible photocatalytic property. J. Colloid Interface Sci. 2010, 348, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Sun, C.; Pang, X.; Sheng, H.; Li, Y.; Ji, H.; Song, W.; Chen, C.; Ma, W.; Zhao, J. Inverse kinetic solvent isotope effect in TiO2 photocatalytic dehalogenation of non-adsorbable aromatic halides: A proton-induced pathway. Angew. Chem. Int. Ed. 2015, 54, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T. Porphyrin-based supramolecular nanoarchitectures for solar energy conversion. J. Phys. Chem. Lett. 2013, 4, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Che, Y.; Moore, J.S. One-dimensional self-assembly of planar π-conjugated molecules: Adaptable building blocks for organic nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jacobs, D.L.; Xu, J.; Li, Y.; Wang, C.; Zang, L. 1D nanofiber composites of perylene diimides for visible-light-driven hydrogen evolution from water. RSC Adv. 2014, 4, 48486–48491. [Google Scholar] [CrossRef]
- Kim, F.S.; Ren, G.; Jenekhe, S.A. One-dimensional nanostructures of π-conjugated molecular systems: Assembly, properties, and applications from photovoltaics, sensors, and nanophotonics to nanoelectronics. Chem. Mater. 2010, 23, 682–732. [Google Scholar] [CrossRef]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 7261–7277. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ly, J.; Lei, B.; Fan, W.; Zhang, D.; Han, J.; Meyyappan, M.; Thompson, M.; Zhou, C. Data storage studies on nanowire transistors with self-assembled porphyrin molecules. J. Phys. Chem. B 2004, 108, 9646–9649. [Google Scholar] [CrossRef]
- Ng, K.K.; Lovell, J.F.; Vedadi, A.; Hajian, T.; Zheng, G. Self-assembled porphyrin nanodiscs with structure-dependent activation for phototherapy and photodiagnostic applications. ACS Nano 2013, 7, 3484–3490. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.D.; Smith, D.E.; Bond-Watts, B.; Johnston, D.E.; Hone, J.; Johnson, A.T.; de Paula, J.C.; Smith, W.F. Photoconductivity of self-assembled porphyrin nanorods. Nano Lett. 2004, 4, 1261–1265. [Google Scholar] [CrossRef]
- Aratani, N.; Osuka, A. Monodisperse giant porphyrin arrays. Macromol. Rapid Commun. 2001, 22, 725–740. [Google Scholar] [CrossRef]
- Haycock, R.A.; Yartsev, A.; Michelsen, U.; Sundström, V.; Hunter, C.A. Self-assembly of pentameric porphyrin light-harvesting antennae complexes. Angew. Chem. Int. Ed. 2000, 39, 3616–3619. [Google Scholar] [CrossRef]
- Hasobe, T. Photo-and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef] [PubMed]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hupp, J.T.; Nguyen, S.T. Growth of narrowly dispersed porphyrin nanowires and their hierarchical assembly into macroscopic columns. J. Am. Chem. Soc. 2008, 130, 9632–9633. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T.; Nguyen, S.T. Amphiphilic porphyrin nanocrystals: Morphology tuning and hierarchical assembly. Adv. Mater. 2008, 20, 3543–3549. [Google Scholar] [CrossRef]
- Hu, J.S.; Guo, Y.G.; Liang, H.P.; Wan, L.J.; Jiang, L. Three-dimensional self-organization of supramolecular self-assembled porphyrin hollow hexagonal nanoprisms. J. Am. Chem. Soc. 2005, 127, 17090–17095. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Medforth, C.J.; Shelnutt, J.A. Self-assembly and self-metallization of porphyrin nanosheets. J. Am. Chem. Soc. 2007, 129, 2440–2441. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, P.; Liu, M. Porphyrin assemblies via a surfactant-assisted method: From nanospheres to nanofibers with tunable length. Langmuir 2012, 28, 15482–15490. [Google Scholar] [CrossRef] [PubMed]
- Atula, S. Sonication-assisted supramolecular nanorods of meso-diaryl-substituted porphyrins. Chem. Commun. 2008, 724–726. [Google Scholar]
- Yoon, S.M.; Hwang, I.C.; Kim, K.S.; Choi, H.C. Synthesis of single-crystal tetra (4-pyridyl) porphyrin rectangular nanotubes in the vapor phase. Angew. Chem. Int. Ed. 2009, 48, 2506–2509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanotubes by ionic self-assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Self-metallization of photocatalytic porphyrin nanotubes. J. Am. Chem. Soc. 2004, 126, 16720–16721. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Porta, F. Open network architectures from the self-assembly of AgNO3 and 5, 10, 15, 20-tetra (4-pyridyl) porphyrin (H2TPyP) building blocks: The exceptional self-penetrating topology of the 3D network of [Ag8(ZnIITPyP)7(H2O)2](NO3)8. Angew. Chem. 2003, 115, 331–336. [Google Scholar] [CrossRef]
- Ohmura, T.; Usuki, A.; Fukumori, K.; Ohta, T.; Ito, M.; Tatsumi, K. New porphyrin-based metal-organic framework with high porosity: 2-D infinite 22.2-Å square-grid coordination network. Inorg. Chem. 2006, 45, 7988–7990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lybarger, L.E.; Wang, W.; Medforth, C.J.; Miller, J.E.; Shelnutt, J.A. Monodisperse porphyrin nanospheres synthesized by coordination polymerization. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, P.; Ma, W.; Liu, M. Morphology-dependent supramolecular photocatalytic performance of porphyrin nanoassemblies: From molecule to artificial supramolecular nanoantenna. J. Mater. Chem. 2012, 22, 20243–20249. [Google Scholar] [CrossRef]
- Jang, J.H.; Jeon, K.S.; Oh, S.; Kim, H.J.; Asahi, T.; Masuhara, H.; Yoon, M. Synthesis of Sn-porphyrin-intercalated trititanate nanofibers: Optoelectronic properties and photocatalytic activities. Chem. Mater. 2007, 19, 1984–1991. [Google Scholar] [CrossRef]
- Guo, P.; Chen, P.; Liu, M. One-dimensional porphyrin nanoassemblies assisted via graphene oxide: Sheetlike functional surfactant and enhanced photocatalytic behaviors. ACS Appl. Mater. Inter. 2013, 5, 5336–5345. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, H.; Li, X.Q.; Würthner, F.; Lochbrunner, S. One-dimensional exciton diffusion in perylene bisimide aggregates. J. Phys. Chem. A 2010, 115, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Nayak, S.K.; Mallampalli, S.; Patra, A. Surfactant-assisted porphyrin based hierarchical nano/micro assemblies and their efficient photocatalytic behavior. ACS Appl. Mater. Inter. 2013, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Fu, Y.; Li, H.; Hu, W. Single-crystalline, size, and orientation controllable nanowires and ultralong microwires of organic semiconductor with strong photoswitching property. J. Am. Chem. Soc. 2008, 130, 3937–3941. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Tao, J.; Pan, Z.; Puretzky, A.A.; Ivanov, I.N.; Pennycook, S.J.; Geohegan, D.B. Single-crystal organic nanowires of copper-tetracyanoquinodimethane: Synthesis, patterning, characterization, and device applications. Angew. Chem. 2007, 119, 2704–2708. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, J.; Zhang, R.; Wei, W.; Wang, H.; Lü, X.; Bai, F.; Wu, H.; Haddad, R.; Fan, H. Morphology-controlled self-assembly and synthesis of photocatalytic nanocrystals. Nano Lett. 2014, 14, 7175–7179. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, Z.; Zhang, R.; Bai, F.; Wu, H.; Haddad, R.; Fan, H. Interfacial self-assembly driven formation of hierarchically structured nanocrystals with photocatalytic activity. ACS Nano 2014, 8, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liow, C.; Bisht, A.; Liu, X.; Sum, T.C.; Chen, X.; Li, S. Engineering interfacial photo-induced charge transfer based on nanobamboo array architecture for efficient solar-to-chemical energy conversion. Adv. Mater. 2015, 27, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.H.; Jiang, L.; Zhang, C.; Zhao, Y.S.; Hu, W.; Yao, J. Coaxial organic p-n heterojunction nanowire arrays: One-step synthesis and photoelectric properties. Adv. Mater. 2012, 24, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sakai, R.; Abe, T.; Iyoda, T.; Norimatsu, T.; Nagai, K. Photoelectrochemical and photocatalytic properties of biphasic organic p-and n-type semiconductor nanoparticles fabricated by a reprecipitation process. ACS Appl. Mater. Inter. 2011, 3, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Arunachalam, P.; Abe, T.; Iyoda, T.; Nagai, K. Photocatalytic decomposition of N-methyl-2-pyrrolidone, aldehydes, and thiol by biphase and p/n junction-like organic semiconductor composite nanoparticles responsive to nearly full spectrum of visible light. J. Photoch. Photobio. A 2012, 244, 18–23. [Google Scholar] [CrossRef]
- Abe, T.; Taira, N.; Tanno, Y.; Kikuchi, Y.; Nagai, K. Decomposition of hydrazine by an organic fullerene-phthalocyanine p-n bilayer photocatalysis system over the entire visible-light region. Chem. Commun. 2014, 50, 1950–1952. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Zhang, X.; Ou, X.; Zhang, X. One-step growth of organic single-crystal p-n nano-heterojunctions with enhanced visible-light photocatalytic activity. Chem. Commun. 2013, 49, 9200–9202. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Martin, K.E.; Shelnutt, J.Y.T.; Evans, L.; Busani, T.; Miller, J.E.; Medforth, C.J.; Shelnutt, J.A. Morphological families of self-assembled porphyrin structures and their photosensitization of hydrogen generation. Chem. Commun. 2011, 47, 6069–6071. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; Tian, Y.; Busani, T.; Medforth, C.J.; Franco, R.; van Swol, F.; Shelnutt, J.A. Charge effects on the structure and composition of porphyrin binary ionic solids: ZnTPPS/SnTMePyP nanomaterials. Chem. Mater. 2013, 25, 441–447. [Google Scholar] [CrossRef]
- Kotiaho, A.; Lahtinen, R.M.; Tkachenko, N.V.; Efimov, A.; Kira, A.; Imahori, H.; Lemmetyinen, H. Gold nanoparticle enhanced charge transfer in thin film assemblies of porphyrin-fullerene dyads. Langmuir 2007, 23, 13117–13125. [Google Scholar] [CrossRef] [PubMed]
- Robatjazi, H.; Bahauddin, S.M.; Doiron, C.; Thomann, I. Direct plasmon-driven photoelectrocatalysis. Nano Lett. 2015, 15, 6155–6161. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Li, Y.; Song, L.; Xiong, Y. Coupling solar energy into reactions: Materials design for surface plasmon-mediated catalysis. Small 2015, 11, 3873–3889. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Nakada, M.; Terasaki, N.; Yamada, S. Photocurrent enhancement in a porphyrin-gold nanoparticle nanostructure assisted by localized plasmon excitation. Chem. Commun. 2006, 395–397. [Google Scholar] [CrossRef]
- Matsumoto, R.; Yamada, S.; Yonemura, H. Effects of gold nanoparticles on photocurrents of zinc-porphyrin-viologen linked compound-gold nanoparticle composite films. Mol. Cryst. Liq. Cryst. 2014, 598, 86–91. [Google Scholar] [CrossRef]
- Draxl, C.; Nabok, D.; Hannewald, K. Organic/inorganic hybrid materials: Challenges for ab initio methodology. Acc. Chem. Res. 2014, 47, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Parasuraman, P.S.; Tsai, H.C.; Jhu, J.J.; Imae, T. Synthesis and characterization of porphyrin-TiO2 core-shell nanoparticles as visible light photocatalyst. RSC. Adv. 2014, 4, 6540–6544. [Google Scholar] [CrossRef]
- Hasobe, T.; Sakai, H.; Mase, K.; Ohkubo, K.; Fukuzumi, S. Remarkable enhancement of photocatalytic hydrogen evolution efficiency utilizing an internal cavity of supramolecular porphyrin hexagonal nanocylinders under visible-light irradiation. J. Phys. Chem. C 2013, 117, 4441–4449. [Google Scholar] [CrossRef]
- Du, A.; Ng, Y.H.; Bell, N.J.; Zhu, Z.; Amal, R.; Smith, S.C. Hybrid graphene/titania nanocomposite: Interface charge transfer, hole doping, and sensitization for visible light response. J. Phys. Chem. Lett. 2011, 2, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Sanvito, S.; Li, Z.; Wang, D.; Jiao, Y.; Liao, T.; Sun, Q.; Ng, Y.H.; Zhu, Z.; Amal, R. Hybrid graphene and graphitic carbon nitride nanocomposite: Gap opening, electron-hole puddle, interfacial charge transfer, and enhanced visible light response. J. Am. Chem. Soc. 2012, 134, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wang, Z.; Yoshizawa, N.; Hatori, H.; Hirotsu, T. Lamellar carbon nanosheets function as templates for two-dimensional deposition of tubular titanate. Chem. Commun. 2008, 4348–4350. [Google Scholar] [CrossRef] [PubMed]
- Adán-Más, A.; Wei, D. Photoelectrochemical properties of graphene and its derivatives. Nanomaterials 2013, 3, 325–356. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Z.; Xiao, B.; Lu, Y.; Du, Y.; Yang, P.; Wang, X. Surfactant assistance in improvement of photocatalytic hydrogen production with the porphyrin noncovalently functionalized graphene nanocomposite. ACS Appl. Mater. Inter. 2013, 5, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, D.; Mays, J.W.; Zhang, X.P.; Bratcher, M.S. Carbon nanotubes with covalently linked porphyrin antennae: Photoinduced electron transfer. J. Am. Chem. Soc. 2005, 127, 6916–6917. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Sandanayaka, A.S.; Hasobe, T.; Economopoulos, S.P.; Sarantopoulou, E.; Tagmatarchis, N. Graphene oxide with covalently linked porphyrin antennae: Synthesis, characterization and photophysical properties. J. Mater. Chem. 2011, 21, 109–117. [Google Scholar] [CrossRef]
- Sun, J.; Meng, D.; Jiang, S.; Wu, G.; Yan, S.; Geng, J.; Huang, Y. Multiple-bilayered RGO–porphyrin films: From preparation to application in photoelectrochemical cells. J. Mater. Chem. 2012, 22, 18879–18886. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Graphene-based nanoassemblies for energy conversion. J. Phys. Chem. Lett. 2011, 2, 242–251. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J. Graphene-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2013, 4, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, Q.H.; Yang, Y.; Lv, W.; Wen, Y.; Hou, P.X.; Wang, M.; Cheng, H.M. Self-assembled free-standing graphite oxide membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar] [CrossRef]
- Li, D.; Mueller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z.H.; Yue, M.; Kang, F. Integrating porphyrin nanoparticles into a 2D graphene matrix for free-standing nanohybrid films with enhanced visible-light photocatalytic activity. Nanoscale 2014, 6, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yu, J. g-C3N4-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Wang, D.; Pan, J.; Li, H.; Liu, J.; Wang, Y.; Kang, L.; Yao, J. A pure organic heterostructure of μ-oxo dimeric iron (iii) porphyrin and graphitic-C3N4 for solar H2 roduction from water. J. Mater. Chem. A 2016, 4, 290–296. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. https://doi.org/10.3390/nano6030051
Chen Y, Li A, Huang Z-H, Wang L-N, Kang F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials. 2016; 6(3):51. https://doi.org/10.3390/nano6030051
Chicago/Turabian StyleChen, Yingzhi, Aoxiang Li, Zheng-Hong Huang, Lu-Ning Wang, and Feiyu Kang. 2016. "Porphyrin-Based Nanostructures for Photocatalytic Applications" Nanomaterials 6, no. 3: 51. https://doi.org/10.3390/nano6030051
APA StyleChen, Y., Li, A., Huang, Z.-H., Wang, L.-N., & Kang, F. (2016). Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials, 6(3), 51. https://doi.org/10.3390/nano6030051







