Design and Electrochemical Study of Platinum-Based Nanomaterials for Sensitive Detection of Nitric Oxide in Biomedical Applications
Abstract
:1. Introduction
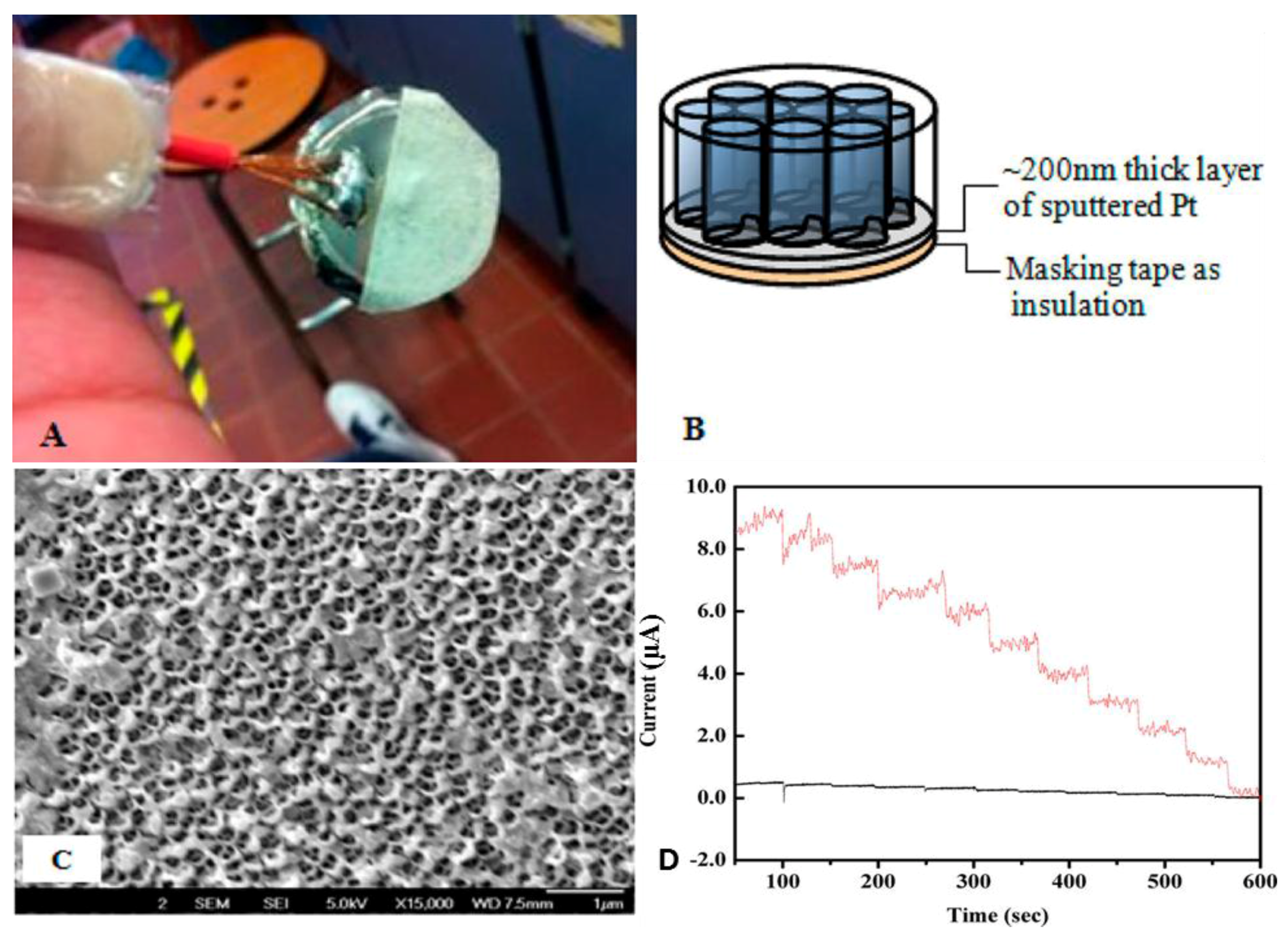
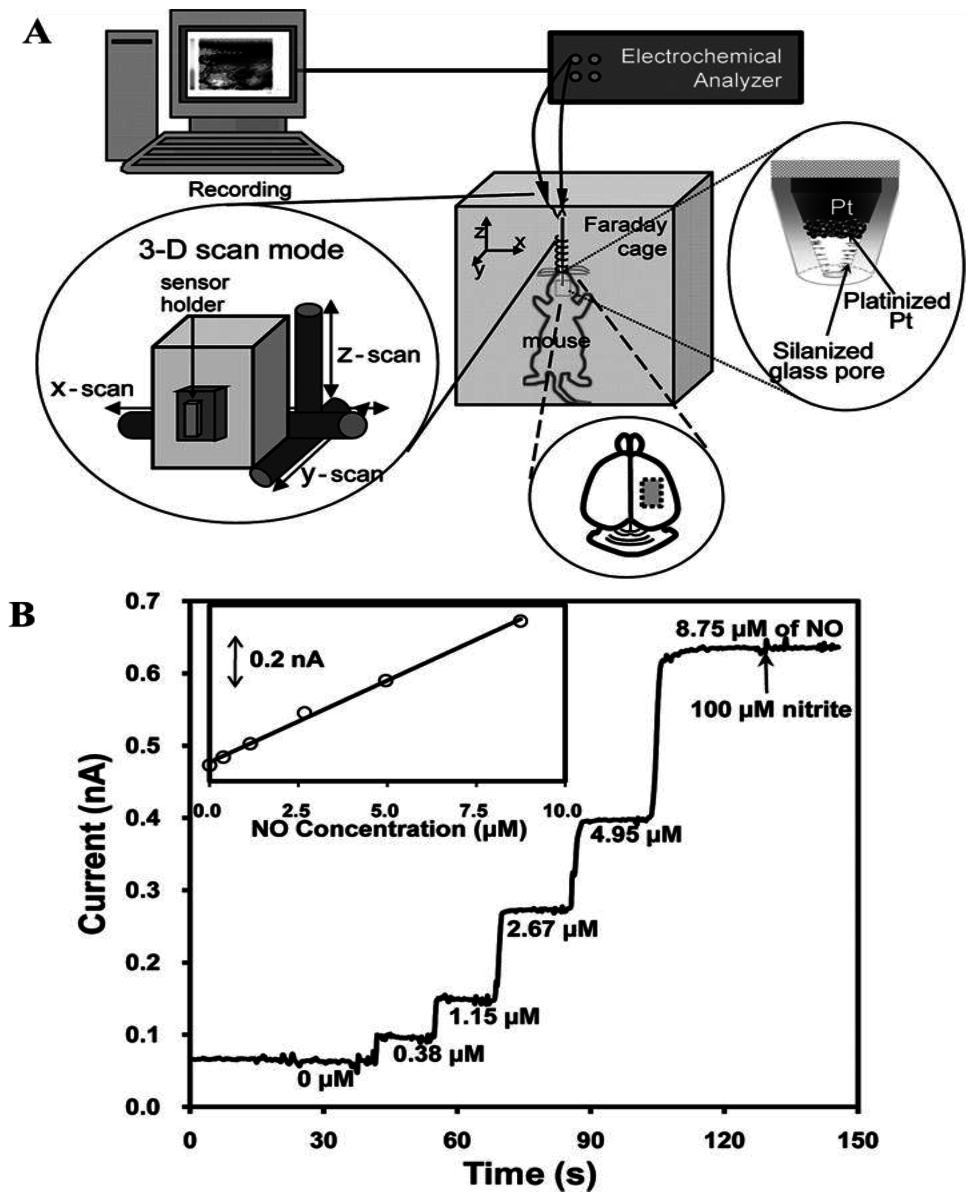
2. Nanoporous Platinum
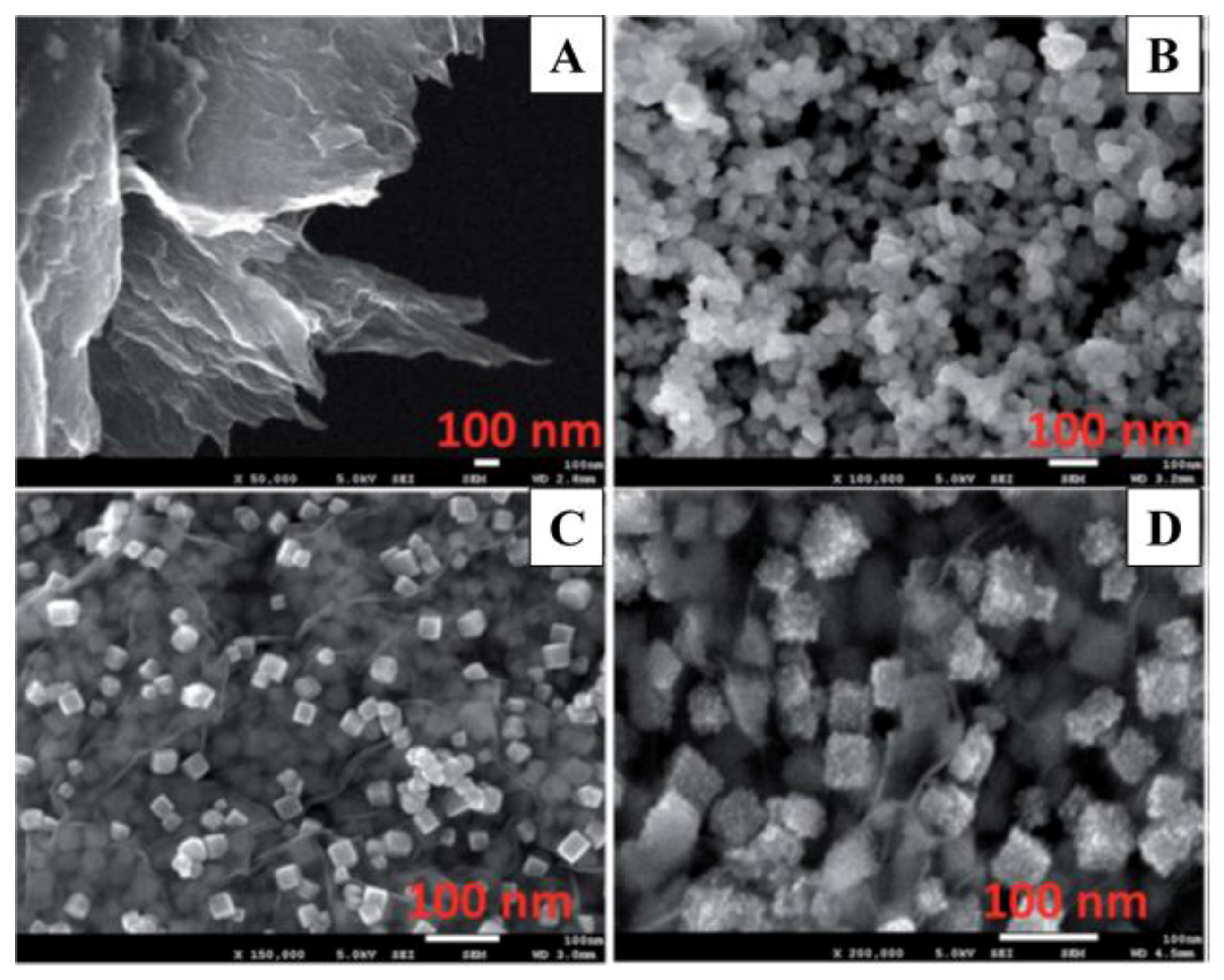
3. Platinum Nanoparticles
4. Platinum–Gold Nanoparticle/Graphene Nanocomposites
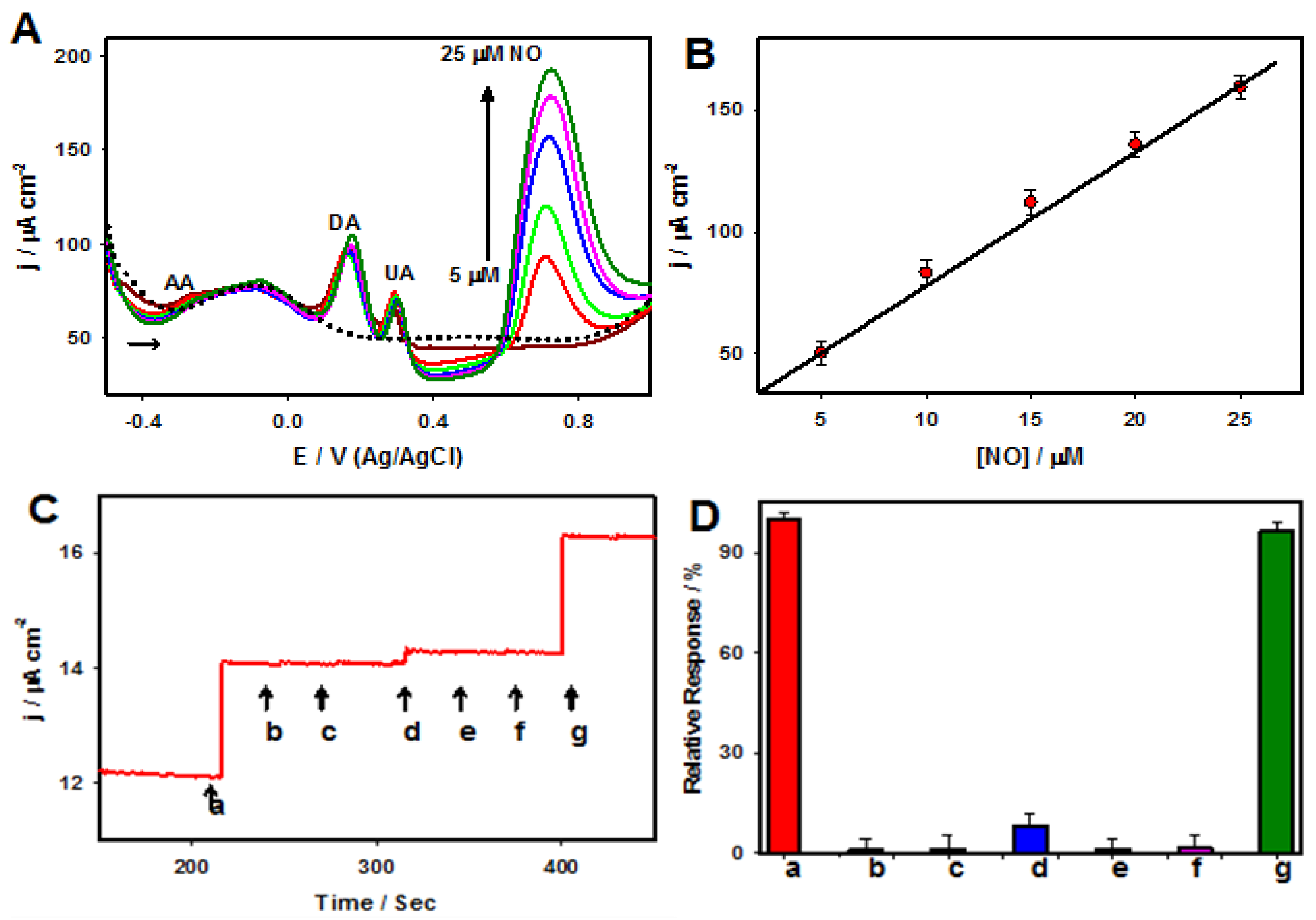
5. Platinum–Tungsten Nanoparticle/Graphene-Ionic Liquid Nanocomposites
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eroglu, E.; Gottschalk, B.; Charoensin, S.; Blass, S.; Bischof, H.; Rost, R.; Madreiter-Sokolowski, C.T.; Pelzmann, B.; Bernhart, E.; Sattler, W.; et al. Development of Novel FP-Based Probes for Live-Cell Imaging of Nitric Oxide Dynamics. Nat. Commun. 2016, 7, 10623. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.Y.; Kehr, G.; Daniliuc, C.G.; Liu, L.; Grimme, S.; Erker, G. Coupling of Carbon Monoxide with Nitrogen Monoxide at a Frustrated Lewis Pair Template. Angew. Chem. Int. Ed. 2016, 55, 9216–9219. [Google Scholar] [CrossRef] [PubMed]
- Baldim, V.; Ismail, A.; Taladriz-Blanco, P.; Griveau, S.; de Oliveira, M.G.; Bedioui, F. Amperometric Quantification of S-Nitrosoglutathione Using Gold Nanoparticles: A Step toward Determination of S-Nitrosothiols in Plasma. Anal. Chem. 2016, 88, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, T.; Jung, H.S.; Jang, J.H.; Kim, T.W.; Kang, C.; Kim, J.S. Chemical Sensing of Neurotransmitters. Chem. Soc. Rev. 2014, 43, 4684–4713. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Li, C.; Li, X.; Li, T.; Cui, H.; Lv, S. Ag7Au6 Cluster as a Potential Gas Sensor for CO, HCN, and NO Detection. J. Phys. Chem. C 2015, 119, 7534–7540. [Google Scholar] [CrossRef]
- Chang, C.W.; Maduraiveeran, G.; Xu, J.C.; Hunter, G.W.; Dutta, P.K. Design, Fabrication, and Testing of MEMS-Based Miniaturized Potentiometric Nitric Oxide Sensors. Sens. Actuators B Chem. 2014, 204, 183–189. [Google Scholar] [CrossRef]
- Sun, C.H.; Maduraiveeran, G.; Dutta, P. Nitric Oxide Sensors Using Combination of p- and n-type Semiconducting Oxides and Its Application for Detecting NO in Human Breath. Sens. Actuators B Chem. 2013, 186, 117–125. [Google Scholar] [CrossRef]
- Coneski, P.N.; Schoenfisch, M.H. Nitric Oxide Release: Part III. Measurement and Reporting. Chem. Soc. Rev. 2012, 41, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Beall, C.M.; Laskowski, D.; Erzurum, S.C. Nitric Oxide in Adaptation to Altitude. Free Radic. Biol. Med. 2012, 52, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical Nitric Oxide Sensors for Physiological Measurements. Chem. Soc. Rev. 2010, 39, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric Oxide and Macrophage Function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Schleicher, U.; Schroll, A.; Sonnweber, T.; Theurl, I.; Ludwiczek, S.; Talasz, H.; Brandacher, G.; Moser, P.L.; Muckenthaler, M.U.; et al. Nitric Oxide-Mediated Regulation of Ferroportin-1 Controls Macrophage Iron Homeostasis and Immune Function in Salmonella Infection. J. Exp. Med. 2013, 210, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Rahat, M.A.; Hemmerlein, B. Macrophage-Tumor Cell Interactions Regulate the Function of Nitric Oxide. Front. Physiol. 2013, 4, 144. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Schuhmann, W. Detection of Nitric Oxide Release from Single Cells by using Constant-Distance-Mode Scanning Electrochemical Microscopy. Angew. Chem. Int. Ed. 2006, 45, 7451–7454. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Zeng, Z.; Cohen, Y.; Greeley, J.; Gewirth, A.A. Electrochemical Surface Stress Development during CO and NO Oxidation on Pt. J. Phys. Chem. C 2016, 120, 8674–8683. [Google Scholar] [CrossRef]
- Hunter, R.A.; Storm, W.L.; Coneski, P.N.; Schoenfisch, M.H. Inaccuracies of Nitric Oxide Measurement Methods in Biological Media. Anal. Chem. 2013, 85, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chatterjee, S. Nanomaterials Based Electrochemical Sensors for Biomedical Applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Bedioui, F.; Griveau, S. Electrochemical Detection of Nitric Oxide: Assessement of Twenty Years of Strategies. Electroanalysis 2013, 25, 587–600. [Google Scholar] [CrossRef]
- Dang, X.; Hu, H.; Wang, S.; Hu, S. Nanomaterials-based electrochemical sensors for nitric oxide. Microchim. Acta 2014, 182, 455–467. [Google Scholar] [CrossRef]
- Govindhan, M.; Adhikari, B.-R.; Chen, A. Nanomaterials-Based Electrochemical Detection of Chemical Contaminants. RSC Adv. 2014, 4, 63741–63760. [Google Scholar] [CrossRef]
- Koh, W.C.; Chandra, P.; Kim, D.M.; Shim, Y.B. Electropolymerized Self-Assembled Layer on Gold Nanoparticles: Detection of Inducible Nitric Oxide Synthase in Neuronal Cell Culture. Anal. Chem. 2011, 83, 6177–6183. [Google Scholar] [CrossRef] [PubMed]
- Jayabal, S.; Viswanathan, P.; Ramaraj, R. Reduced Graphene Oxide–Gold Nanorod Composite Material Stabilized in Silicate Sol–Gel Matrix for Nitric Oxide Sensor. RSC Adv. 2014, 4, 33541–33548. [Google Scholar] [CrossRef]
- Zan, X.; Fang, Z.; Wu, J.; Xiao, F.; Huo, F.; Duan, H. Freestanding Graphene Paper Decorated with 2D-Assembly of Au@Pt Nanoparticles as Flexible Biosensors to Monitor Live Cell Secretion of Nitric Oxide. Biosens. Bioelectron. 2013, 49, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Punckt, C.; Pope, M.A.; Gelperin, A.; Aksay, I.A. Electrochemical Sensing of Nitric Oxide with Functionalized Graphene Electrodes. ACS Appl. Mater. Interfaces 2013, 5, 12624–12630. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.L.; Guo, C.X.; Leong, K.C.; Kim, D.-H.; Li, C.M.; Chen, P. Gold Nanoparticles Decorated Reduced Graphene Oxide for Detecting the Presence and Cellular Release of Nitric Oxide. Electrochim. Acta 2013, 111, 441–446. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au Nanoparticles-3D Graphene Hydrogel Nanocomposite to Boost Synergistically In Situ Detection Sensitivity toward Cell-Released Nitric Oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wei, Z. Recent Advancements in Pt and Pt-Free Catalysts for Oxygen Reduction Reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Chen, T.; Alia, S.; Pivovar, B.S.; Xu, W.L. Single-Molecule Nanocatalysis Shows In Situ Deactivation of Pt/C Electrocatalysts during the Hydrogen-Oxidation Reaction. Angew. Chem. Int. Ed. 2016, 55, 3086–3090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Yu, G.L.; Tian, K.L.; Xu, C.X. A Highly Sensitive and Stable Electrochemical Sensor for Simultaneous Detection towards Ascorbic acid, Dopamine, and Uric acid based on the Hierarchical Nanoporous PtTi Alloy. Biosens. Bioelectron. 2016, 82, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, L.Z.; Hong, B.; Ren, J.L.; Zhang, M.S.; Que, Q.M.; Ji, J.Y. Nonenzymatic Detection of Glucose using Three-Dimensional PtNi Nanoclusters Electrodeposited on the Multiwalled Carbon Nanotubes. Sens. Actuators B Chem. 2016, 231, 800–810. [Google Scholar] [CrossRef]
- Griveau, S.; Bedioui, F. Overview of Significant Examples of Electrochemical Sensor Arrays Designed for Detection of Nitric Oxide and Relevant Species in a Biological Environment. Anal. Bioanal. Chem. 2013, 405, 3475–3488. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C. Electrocatalysis and Photoelectrochemistry Based on Functional Nanomaterials. Can. J. Chem. 2014, 92, 581–597. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.F.; Zhang, X.P.; Xie, G.C.; Su, Z.Q.; Wei, G. Nanoporous Carbon Nanofibers Decorated with Platinum Nanoparticles for Non-Enzymatic Electrochemical Sensing of H2O2. Nanomaterials 2015, 5, 1891–1905. [Google Scholar] [CrossRef]
- Chen, A.C.; Holt-Hindle, P. Platinum-Based Nanostructured Materials: Synthesis, Properties, and Applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef] [PubMed]
- Rick, J.; Tsai, M.C.; Hwang, B.J. Biosensors Incorporating Bimetallic Nanoparticles. Nanomaterials 2016, 6, 5. [Google Scholar] [CrossRef]
- Leus, K.; Dendooven, J.; Tahir, N.; Ramachandran, R.K.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Goeman, J.L.; Van der Eycken, J.; Detavernier, C.; et al. Atomic Layer Deposition of Pt Nanoparticles within the Cages of MIL-101: A Mild and Recyclable Hydrogenation Catalyst. Nanomaterials 2016, 6, 45. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, H.Y.; Wang, X.D.; Liu, L.; Jiang, Y.; Wang, L.; Zhuang, G.L.; Chu, Y.Q.; Li, X.N.; Wang, J.G. Pt@Au Nanorods Uniformly Decorated on Pyridyne Cycloaddition Graphene as a Highly Effective Electrocatalyst for Oxygen Reduction. ACS Appl. Mater. Interfaces 2014, 6, 13448–13454. [Google Scholar] [CrossRef] [PubMed]
- Alammari, W.; Govindhan, M.; Chen, A. Modification of TiO2 Nanotubes with PtRu/Graphene Nanocomposites for Enhanced Oxygen Reduction Reaction. ChemElectroChem 2015, 2, 2041–2047. [Google Scholar] [CrossRef]
- Irshad, M.; Iqbal, N.; Mujahid, A.; Afzal, A.; Hussain, T.; Sharif, A.; Ahmad, E.; Athar, M.M. Molecularly Imprinted Nanomaterials for Sensor Applications. Nanomaterials 2013, 3, 615–637. [Google Scholar] [CrossRef]
- Liu, Z.; Forsyth, H.; Khaper, N.; Chen, A. Sensitive Electrochemical Detection of Nitric Oxide based on AuPt and Reduced Graphene Oxide Nanocomposites. Analyst 2016, 141, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, W.; Cao, D. Metal (Pd,Pt)-decorated Carbon Nanotubes for CO and NO Sensing. Sens. Actuators B Chem. 2011, 159, 171–177. [Google Scholar] [CrossRef]
- Shahid, M.M.; Rameshkumar, P.; Pandikumar, A.; Lim, H.N.; Ng, Y.H.; Huang, N.M. An Electrochemical Sensing Platform based on a Reduced Graphene Oxide–Cobalt Oxide Nanocube@Platinum Nanocomposite for Nitric Oxide Detection. J. Mater. Chem. A 2015, 3, 14458–14468. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Han, S.; Wang, C.; Li, J.P.; Xu, G.B. Single-Walled Carbon Nanohorns for Energy Applications. Nanomaterials 2015, 5, 1732–1755. [Google Scholar] [CrossRef]
- Xian, Y.; Liu, M.; Cai, Q.; Li, H.; Lu, J.; Jin, L. Preparation of Microporous Aluminium Anodic Oxide Film Modified Pt Nano Array Electrode and Application in Direct Measurement of Nitric Oxide Release from Myocardial Cells. Analyst 2001, 126, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.M.; Xu, G.Q.; Ang, S.G. Amperometric Nitric Oxide Sensor Based on Nanoporous Platinum Phthalocyanine Modified Electrodes. Anal. Chem. 2013, 85, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, X. Electrodeposition of Pt–Fe(III) nanoparticle on glassy carbon electrode for electrochemical nitric oxide sensor. Electrochim. Acta 2005, 50, 2887–2891. [Google Scholar] [CrossRef]
- Walcarius, A.; Minteer, S.D.; Wang, J.; Lin, Y.H.; Merkoci, A. Nanomaterials for Bio-Functionalized Electrodes: Recent Trends. J. Mater. Chem. B 2013, 1, 4878–4908. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Govindhan, M.; Chen, A. Carbon Nanomaterials Based Electrochemical Sensors/Biosensors for the Sensitive Detection of Pharmaceutical and Biological Compounds. Sensors 2015, 15, 22490–22508. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene Based Metal and Metal Oxide Nanocomposites: Synthesis, Properties and Their Applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef]
- Zhang, J.T.; Li, C.M. Nanoporous Metals: Fabrication Strategies and Advanced Electrochemical Applications in Catalysis, Sensing and Energy Systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef] [PubMed]
- Pailleret, A.; Oni, J.; Reiter, S.; Isik, S.; Etienne, M.; Bedioui, F.; Schuhmann, W. In Situ Formation and Scanning Electrochemical Microscopy Assisted Positioning of NO-Sensors above Human Umbilical Vein Endothelial Cells for the Detection of Nitric Oxide Release. Electrochem. Commun. 2003, 5, 847–852. [Google Scholar] [CrossRef]
- Park, S.S.; Tatum, C.E.; Lee, Y. Dual Electrochemical Microsensor for Simultaneous Measurements of Nitric Oxide and Oxygen: Fabrication and Characterization. Electrochem. Commun. 2009, 11, 2040–2043. [Google Scholar] [CrossRef]
- Kato, D.; Kunitake, M.; Nishizawa, M.; Matsue, T.; Mizutani, F. Amperometric Nitric Oxide Microsensor Using Two-Dimensional Cross-Linked Langmuir–Blodgett Films of Polysiloxane Copolymer. Sens. Actuators B Chem. 2005, 108, 384–388. [Google Scholar] [CrossRef]
- Isik, S.; Etienne, M.; Oni, J.; Blochl, A.; Reiter, S.; Schuhmann, W. Dual Microelectrodes for Distance Control and Detection of Nitric Oxide from Endothelial Cells by Means of Scanning Electrochemical Microscope. Anal. Chem. 2004, 76, 6389–6394. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.A.; Arundell, M.; Parker, K.H.; Yeoman, M.S.; O’Hare, D. Detection of Nitric Oxide Release from Single Neurons in the Pond Snail, Lymnaea Stagnalis. Anal. Chem. 2006, 78, 7643–7648. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lee, Y. Amperometric Nitric Oxide Microsensor Based on Nanopore-Platinized Platinum: The Application for Imaging NO Concentrations. Anal. Chem. 2009, 81, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Do, H.; Jhon, G.J.; Suh, M.; Lee, Y. Electrochemical Nanosensor for Real-Time Direct Imaging of Nitric Oxide in Living Brain. Anal. Chem. 2011, 83, 8314–8319. [Google Scholar] [CrossRef] [PubMed]
- Hamad, B.; El-Bayyari, Z.; Marashdeh, A. Investigation of the Stability of Platinum Clusters and the Adsorption of Nitrogen Monoxide: First Principles Calculations. Chem. Phys. 2014, 443, 26–32. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Z.; Zhao, G.-C.; Wei, X.-W. Electrodeposited Platinum Nanoparticles on the Multi-Walled Carbon Nanotubes and Its Electrocatalytic for Nitric Oxide. Int. J. Electrochem. Sci. 2008, 3, 746–754. [Google Scholar]
- Zheng, D.; Liu, X.; Zhou, D.; Hu, S. Sensing of Nitric Oxide Using a Glassy Carbon Electrode Modified with an Electrocatalytic Film Composed of Dihexadecyl Hydrogen Phosphate, Platinum Nanoparticles, and Acetylene Black. Microchim. Acta 2011, 176, 49–55. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.W.; Tao, F. Shape Control of Bimetallic Nanocatalysts Through Well-Designed Colloidal Chemistry Approaches. Chem. Soc. Rev. 2012, 41, 8050–8065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Liu, J.J.; Liu, C.G.; Wang, F.; Song, Y. Amorphous CuPt Alloy Nanotubes Induced by Na2S2O3 as Efficient Catalysts for the Methanol Oxidation Reaction. ACS Catal. 2016, 6, 4127–4134. [Google Scholar] [CrossRef]
- Shang, H.S.; Pan, K.C.; Zhang, L.; Zhang, B.; Xiang, X. Enhanced Activity of Supported Ni Catalysts Promoted by Pt for Rapid Reduction of Aromatic Nitro Compounds. Nanomaterials 2016, 6, 103. [Google Scholar] [CrossRef]
- Castegnaro, M.V.; Alexandre, J.; Baibich, I.M.; Alves, M.C.M.; Morais, J. Green Synthesis of Pt and Ag Nanoparticles and their use Towards Nitric Oxide Abatement. Mater. Res. Express 2014, 1, 044001. [Google Scholar] [CrossRef]
- Govindhan, M.; Mao, B.; Chen, A. Novel Cobalt Quantum Dot/Graphene Nanocomposites as Highly Efficient Electrocatalysts for Water Splitting. Nanoscale 2016, 8, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Govindhan, M.; Amiri, M.; Chen, A. Au Nanoparticle/Graphene Nanocomposite as a Platform for the Sensitive Detection of NADH in Human Urine. Biosens. Bioelectron. 2015, 66, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Govindhan, M.; Chen, A. Simultaneous Synthesis of Gold Nanoparticle/Graphene Nanocomposite for Enhanced Oxygen Reduction Reaction. J. Power Sources 2015, 274, 928–936. [Google Scholar] [CrossRef]
- Adams, B.D.; Asmussen, R.M.; Ostrom, C.K.; Chen, A.C. Synthesis and Comparative Study of Nanoporous Palladium-Based Bimetallic Catalysts for Formic Acid Oxidation. J. Phys. Chem. C 2014, 118, 29903–29910. [Google Scholar] [CrossRef]
- Yates, J.T. Chemisorption of Nitric Oxide on Tungsten. J. Chem. Phys. 1966, 45, 1623. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Kannan, P.; Jonsson-Niedziolka, M.; Niedziolka-Jonsson, J. Tungsten Carbide Nanotubes Supported Platinum Nanoparticles as a Potential Sensing Platform for Oxalic Acid. Anal. Chem. 2014, 86, 7849–7857. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Z.; Shi, J.L.; Chen, H.R.; Zhang, L.X.; Guo, L.M.; Gao, J.H.; Li, J.B. Platinum/mesoporous WO3 as a Carbon-Free Electrocatalyst with Enhanced Electrochemical Activity for Methanol Oxidation. J. Phys. Chem. B 2008, 112, 12024–12031. [Google Scholar] [CrossRef] [PubMed]
- Govindhan, M.; Chen, A. Enhanced Sensing of Nitric Oxide using a Glassy Carbon Electrode Modified with a Nanocomposite Consisting of Platinum-Tungsten Nanoparticles, Reduced Graphene Oxide and an Ionic Liquid. Microchim. Acta 2016, 183, 2879–2887. [Google Scholar] [CrossRef]
- Jensen, G.C.; Zheng, Z.; Meyerhoff, M.E. Amperometric Nitric Oxide Sensors with Enhanced Selectivity over Carbon Monoxide via Platinum Oxide Formation under Alkaline Conditions. Anal. Chem. 2013, 85, 10057–10061. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Oh, B.K.; Meyerhoff, M.E. Improved Planar Amperometric Nitric Oxide Sensor based on Platinized Platinum Anode. 1. Experimental Results and Theory When Applied for Monitoring NO Release from Diazeniumdiolate-Doped Polymeric Films. Anal. Chem. 2004, 76, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.A.; Privett, B.J.; Henley, W.H.; Breed, E.R.; Liang, Z.; Mittal, R.; Yoseph, B.P.; McDunn, J.E.; Burd, E.M.; Coopersmith, C.M.; et al. Microfluidic Amperometric Sensor for Analysis of Nitric Oxide in Whole Blood. Anal. Chem. 2013, 85, 6066–6072. [Google Scholar] [CrossRef] [PubMed]
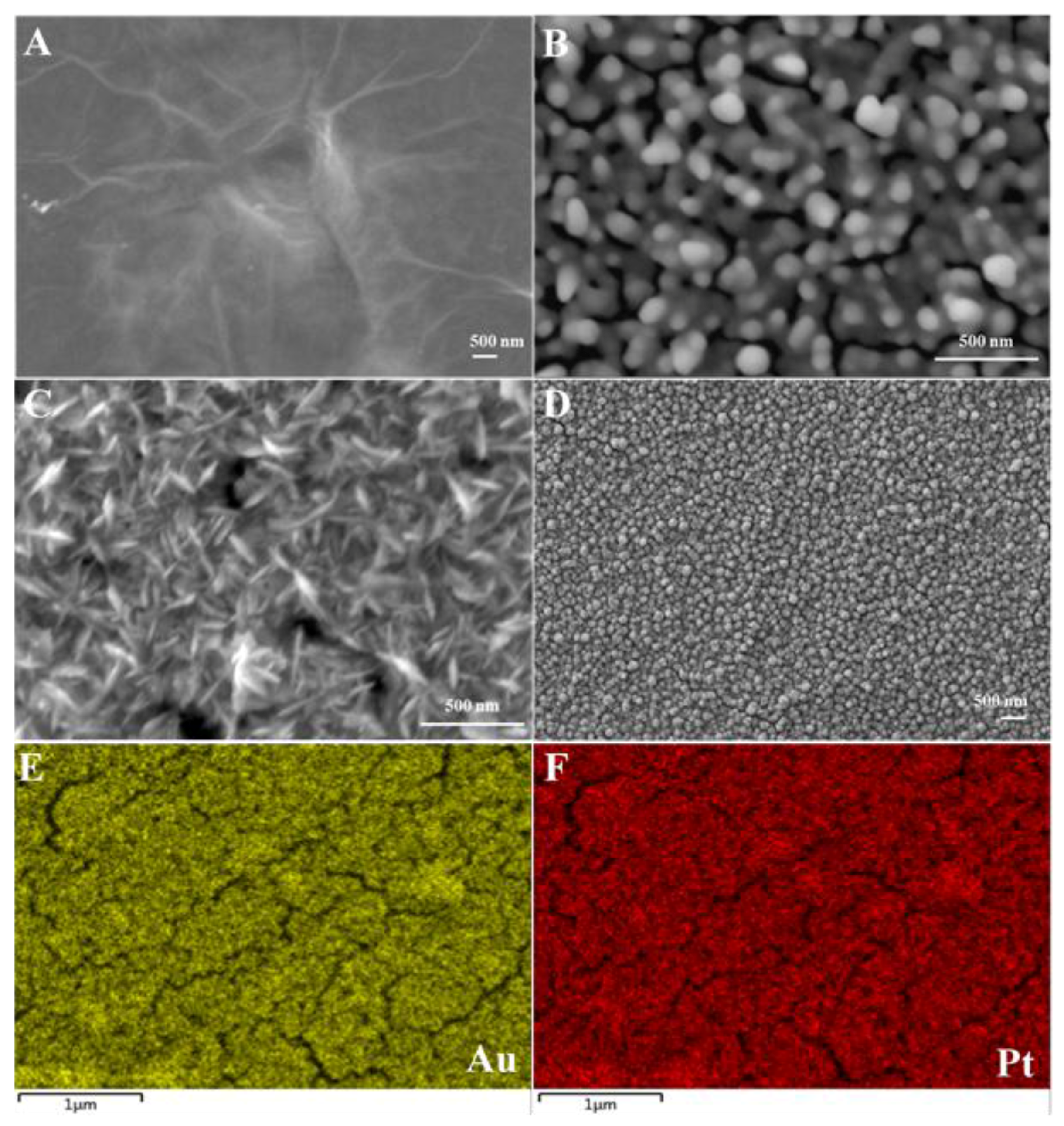

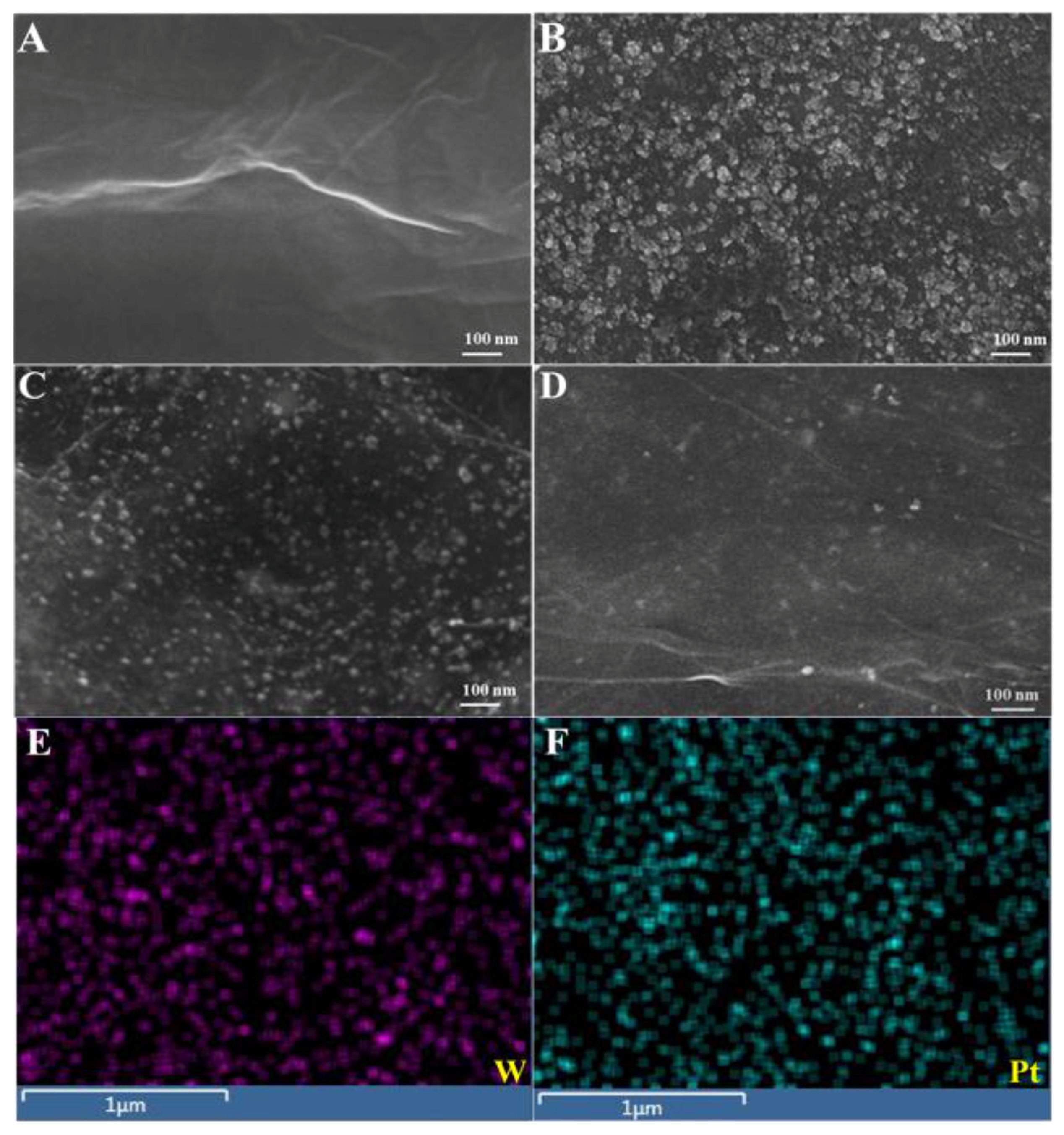
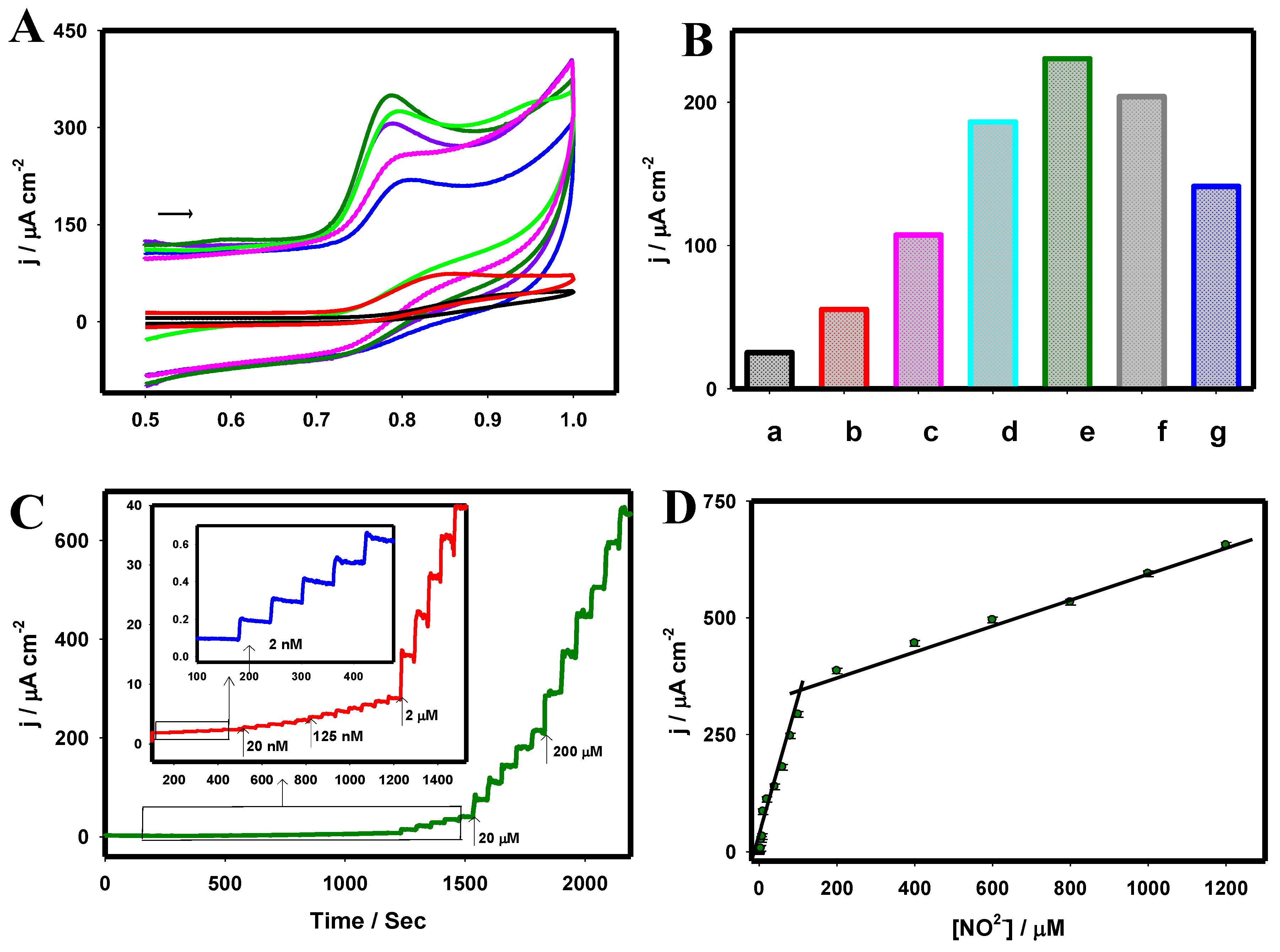
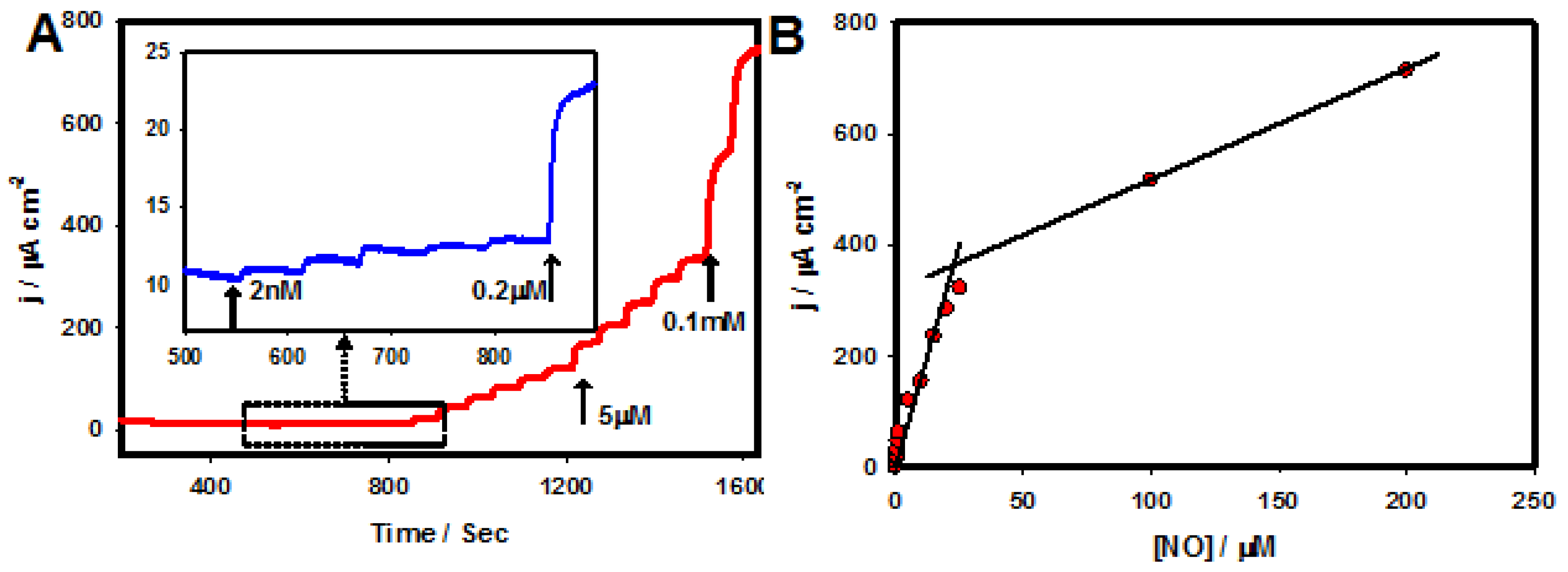
| Electrode Material | Techniques | Detection Limit | Linear Range | Reference |
|---|---|---|---|---|
| Nanoporous Pt | Amperometry | 32 nM | 0.20–1.80 µM | [56] |
| Nanoporous Pt | Amperometry | 10 nM | 0.0–8.75 µM | [57] |
| Nanoporous Pt | Amperometry | 10 nM | 0.10–1.0 μM | [45] |
| /MTAPc | ||||
| PtO | Amperometry | 1 nM | 0.50–4.0 μM | [73] |
| Pt/AAO | DPV | 10 nM | 0.0–3.40 µM | [44] |
| Pt | Amperometry | 10 nM | - | [74] |
| Pt | Amperometry | 0.50 μM | 20–100 μM | [75] |
| Pt/MWNT | Amperometry | 0.10 µM | 0.40 µM–0.10 mM | [59] |
| Pt/AB | Amperometry | 50 nM | 0.18–120.0 μM | [60] |
| Pt/Co3O4-rGO | Amperometry | 1.73 µM | 10–650 μM | [42] |
| Pt-Fe(III) | DPV | 18 nM | 84 nM–0.78 mM | [46] |
| PtAu/rGO | Amperometry | 3.69 nM | 0.02–50 μM | [40] |
| DPV | 2.88 nM | 0.02–10 μM | ||
| PtW/rGO-IL | Amperometry | 0.13 nM | 2 nM–1.20 mM | [72] |
| DPV | 42.49 nM | 0.50 µM–1.0 mM |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindhan, M.; Liu, Z.; Chen, A. Design and Electrochemical Study of Platinum-Based Nanomaterials for Sensitive Detection of Nitric Oxide in Biomedical Applications. Nanomaterials 2016, 6, 211. https://doi.org/10.3390/nano6110211
Govindhan M, Liu Z, Chen A. Design and Electrochemical Study of Platinum-Based Nanomaterials for Sensitive Detection of Nitric Oxide in Biomedical Applications. Nanomaterials. 2016; 6(11):211. https://doi.org/10.3390/nano6110211
Chicago/Turabian StyleGovindhan, Maduraiveeran, Zhonggang Liu, and Aicheng Chen. 2016. "Design and Electrochemical Study of Platinum-Based Nanomaterials for Sensitive Detection of Nitric Oxide in Biomedical Applications" Nanomaterials 6, no. 11: 211. https://doi.org/10.3390/nano6110211
APA StyleGovindhan, M., Liu, Z., & Chen, A. (2016). Design and Electrochemical Study of Platinum-Based Nanomaterials for Sensitive Detection of Nitric Oxide in Biomedical Applications. Nanomaterials, 6(11), 211. https://doi.org/10.3390/nano6110211