Innovative Self-Cleaning and Biocompatible Polyester Textiles Nano-Decorated with Fe–N-Doped Titanium Dioxide
Abstract
:1. Introduction
2. Results
2.1. Characterization of Photocatalysts
2.2. SEM/EDX Analysis of the Treated Fabrics
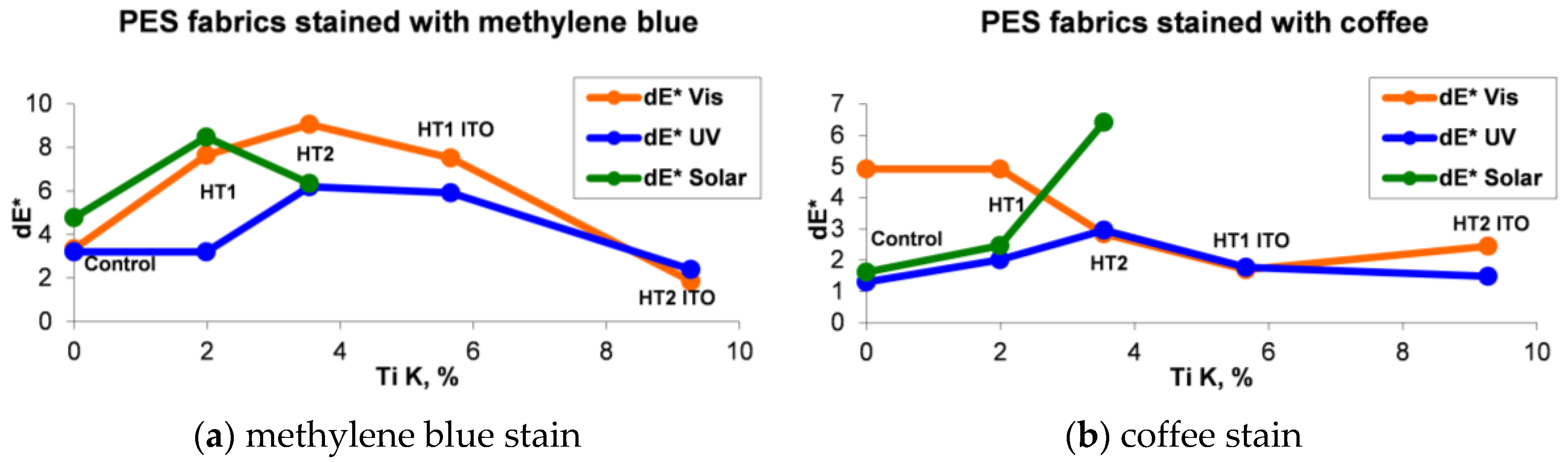
2.3. Evaluation of the Photocatalytic Efficiency
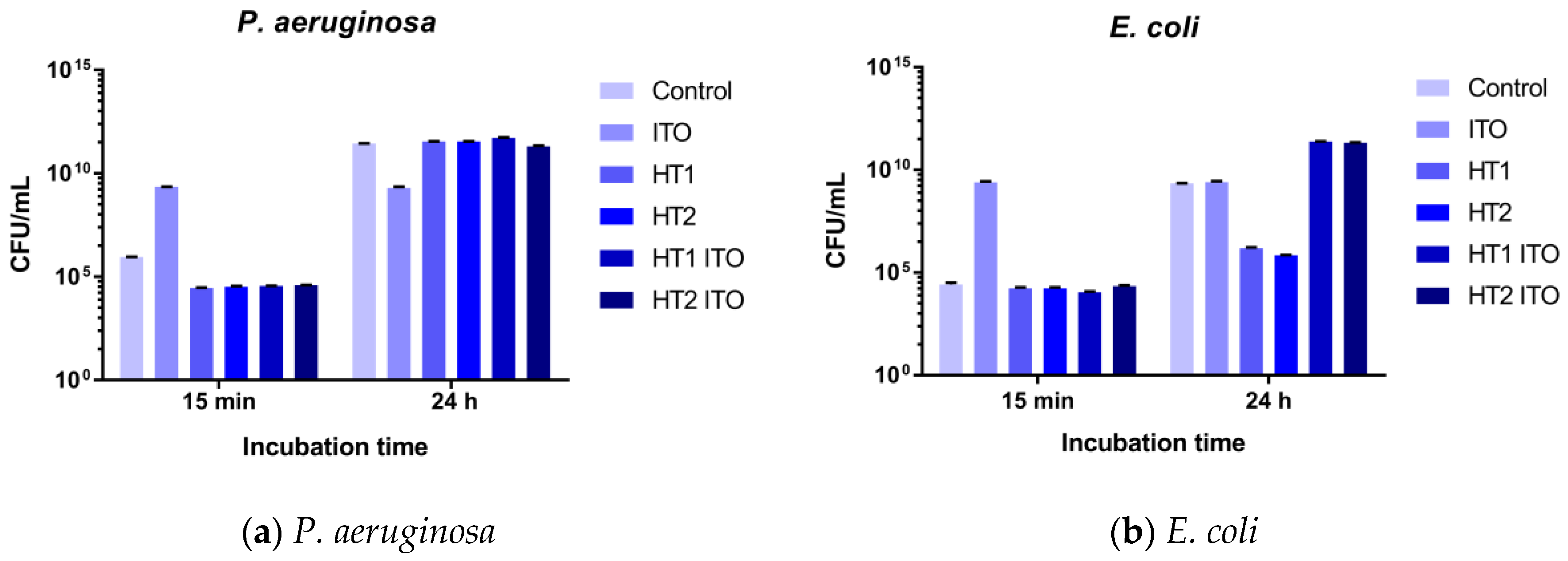
2.4. Antibacterial Efficiency
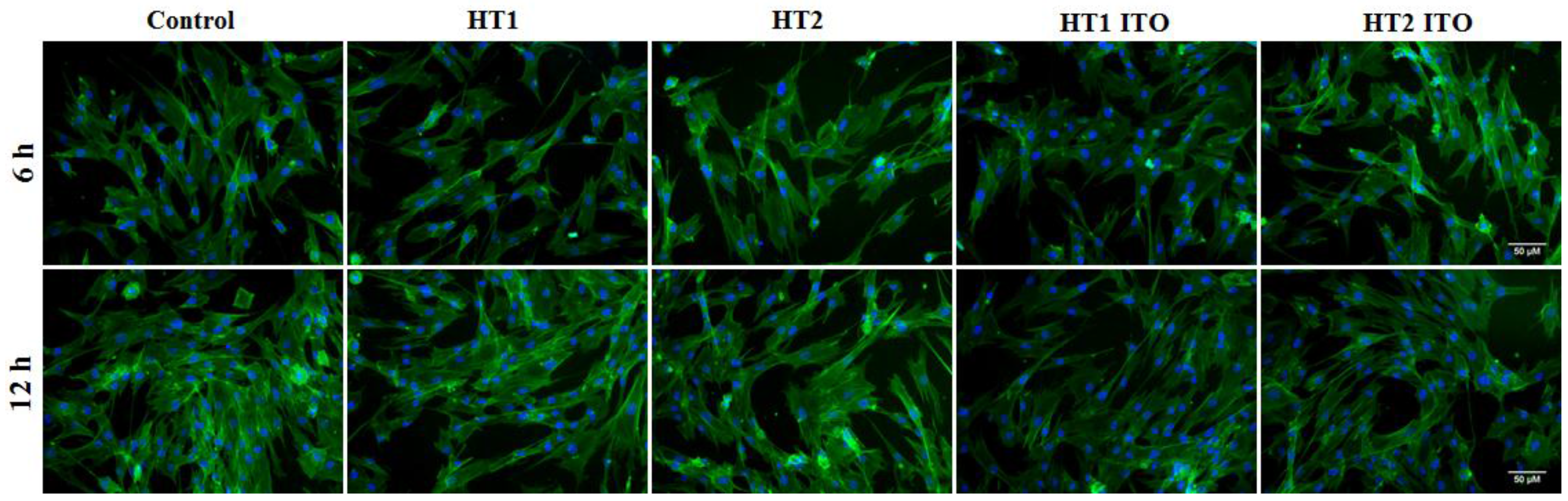
2.5. Biocompatibility Results
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of Photocatalysts
4.2. Antimicrobial Activity Assay
4.3. In Vitro Biocompatibility Assessment
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nommiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-basedthin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Tateda, K. Antibiotic-resistant bacteria and new directions of antimicrobial chemotherapy. Rinsho Byori 2012, 60, 443–448. [Google Scholar] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Tryba, B. Increase of the photocatalytic activity of TiO2 by carbon and iron modifications. Int. J. Photoenergy 2008, 2008, 721824. [Google Scholar] [CrossRef]
- Li, G.; Nie, X.; Chen, J.; Jiang, Q.; An, T.; Wong, P.K.; Zhang, H.; Zhao, H.; Yamashita, H. Enhanced visible-light-driven photocatalytic inactivation of Escherichia coli using g-C3N4/TiO2 hybrid photocatalyst synthesized using a hydrothermal-calcination approach. Water Res. 2015, 15, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Diamandescu, L.; Vasiliu, F.; Mihaila, D.T.; Feder, M.; Vlaicu, A.M.; Teodorescu, C.M.; Macovei, D.; Enculescu, I.; Parvulescu, V.; Vasile, E. Structural and photocatalytic properties of iron and europium doped TiO2 nanoparticles obtained under hydrothermal conditions. Mater. Chem. Phys. 2008, 112, 146–153. [Google Scholar] [CrossRef]
- Banerjee, A.N. The design, fabrication, and photocatalytic utility of nanostructured semiconductors: Focus on TiO2-based nanostructures. Nanotechnol. Sci. Appl. 2011, 4, 35–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, X.; Lu, D.; Yang, J. Facile synthesis and enhanced visible light photocatalytic activity of N and Zr co-doped TiO2 nanostructures from nanotubular titanic acid precursors. Nanoscale Res. Lett. 2013, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Glampedaki, P.; Calvimontes, A.; Dutschk, V.; Warmoeskerken, M.M. Polyester textile functionalization through incorporationof pH/thermo-responsive microgels. Part II: Polyester functionalization and characterization. Mater. Sci. 2012, 47, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, D.; Saponjic, Z.; Radoicic, M.; Radetic, T.; Jovancic, P.; Nedeljkovic, J.; Radetic, M. Functionalization of polyester fabrics with alginates and TiO2 nanoparticles. Carbohydr. Polym. 2010, 79, 526–532. [Google Scholar] [CrossRef]
- Lam, Y.L.; Kan, C.W.; Yuen, C.W.M. Effect of concentration of titanium dioxide acting as catalyst or co-catalyst on the wrinkle-resistant finishing of cotton fabric. Fiber Polym. 2010, 11, 551–558. [Google Scholar] [CrossRef]
- Radetic, M. Functionalization of textile materials with TiO2 nanoparticles. J. Photochem. Photobiol. C 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Ilic, V.; Saponjic, Z.; Vodnik, V.; Lazovic, S.; Dimitrijevic, S.; Jovancic, P.; Nedeljkovic, J.M.; Radetic, M. Bactericidal efficiency of silver nanoparticles deposited onto radio frequency plasma pretreated polyester fabrics. Ind. Eng. Chem. Res. 2010, 49, 7287–7293. [Google Scholar] [CrossRef]
- Cai, Y.; Stromme, M.; Welch, K. Photocatalytic antibacterial effects are maintained on resin-based TiO2 nanocomposites after cessation of UV irradiation. PLoS ONE 2013, 8, e75929. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Champagne, P.; Campagne, C.; Perwuelz, A.; Dumont, F.; Leriche, A. Study the multi self-cleaning characteristics of ZnO nanorods functionalized polyester fabric. J. Ind. Text. 2016, 45, 1440–1456. [Google Scholar] [CrossRef]
- Vucetic, S.B.; Rudic, O.L.; Markov, S.L.; Bera, O.J.; Vidakovic, A.M.; Skapin, A.S.S.; Ranogajec, J.G. Antifungal efficiency assessment of the TiO2 coating on facade paints. Environ. Sci. Pollut. Res. 2014, 21, 11228–11237. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, F.; Mohammadi, S.R.; Mohammadi, P.; Hosseinkhani, S.; Shidpour, R. Antifungal activity of TiO2 nanoparticles and EDTA on Candida albicans biofilms. Infect. Epidemiol. Med. 2013, 1, 33–38. [Google Scholar]
- Tang, B.; Wang, J.; Xu, S.; Afrin, T.; Xu, W.; Sun, L.; Wang, X. Application of anisotropic silver nanoparticles: Multifunctionalization of wool fabric. J. Colloid Interface Sci. 2011, 356, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.R.; Mahmed, N.; Abdullah, M.M.B.; Sandu, A.V. Self-cleaning technology in fabric: A review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012028. [Google Scholar] [CrossRef]
- Haji, A.; Shoushtari, A.M.; Mazaheri, F.; Tabatabaeyan, S.E. Environmentally Friendly Pretreatment for Enhanced Coating of TiO2 Nanoparticles on PET/Wool Fabric. In Proceedings of the International Conference on Chemical, Civil and Environmental Engineering (CCEE’2014), Singapore, Singapore, 18–19 November 2014.
- Saravanan, D. UV protection textile materials. AUTEX Res. J. 2007, 7, 53–62. [Google Scholar]
- Zgura, I.; Frunza, S.; Frunza, L.; Enculescu, M.; Florica, C.; Cotorobai, V.F.; Ganea, C.P. Polyester fabrics covered with amorphous titanium dioxide layers: Combining wettability measurements and photoinduced hydrophylicity to assess their surface properties. Romanian Rep. Phys. 2016, 68, 259–269. [Google Scholar]
- Ma, H.; Gao, Q.; Gao, C.; Bao, W.; Ge, M. Facile synthesis of electroconductive AZO@TiO2 whiskers and their application in textiles. J. Nanomater. 2016, 2016, 5940618. [Google Scholar] [CrossRef]
- Patra, J.K.; Gouda, S. Application of nanotechnology in textile engineering: An overview. J. Eng. Technol. Res. 2013, 5, 104–111. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176, 396–428. [Google Scholar] [CrossRef]
- Stan, M.S.; Nica, I.C.; Dinischiotu, A.; Varzaru, E.; Iordache, O.G.; Dumitrescu, I.; Popa, M.; Chifiriuc, M.C.; Pircalabioru, G.G.; Lazar, V.; et al. Photocatalytic, antimicrobial and biocompatibility features of cotton knit coated with Fe–N-doped titanium dioxide nanoparticles. Materials 2016, 9, 789. [Google Scholar] [CrossRef]
- Messaoud, M.; Chadeau, E.; Brunon, C.; Ballet, T.; Rappenne, L.; Roussel, F.; Leonard, D.; Oulahal, N.; Langlet, M. Photocatalytic generation of silver nanoparticles and application to the antibacterial functionalization of textile fabrics. J. Photochem. Photobiol. A 2010, 215, 147–156. [Google Scholar] [CrossRef]
- Hsu, B.C. Preparation, Characterisation and Visible Light Responsive Photocatalytic Activity of Iron/Nitrogen Doped Titanium Dioxide Nanocrystalline. Ph.D. Thesis, National Taipei University of Technology, Taipei, Taiwan, 2009. [Google Scholar]
- Zhanga, Y.; Chenga, K.; Lva, F.; Huanga, H.; Feib, B.; Hea, Y.; Yec, Z.; Shen, B. Photocatalytic treatment of 2,4,6-trinitotoluene in red water by multi-doped TiO2 with enhanced visible light photocatalytic activity. Colloids Surf. A 2014, 452, 103–108. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, photocatalytic activity, and mechanism of nano-TiO2 co-doped with nitrogen and iron (III). Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials; Wiley: New York, NY, USA, 1966; p. 491. [Google Scholar]
- Lee, H.L.; Kim, J.; Park, C.H. Fabrication of self-cleaning textiles by TiO2-carbon nanotube treatment. Text. Res. J. 2014, 84, 267–278. [Google Scholar] [CrossRef]
- International Organization for Standardization. Joint ISO/CIE Standard: Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space; ISO 11664–4:2008(E)/CIE S 014–4/E: 2007; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Larumbe, S.; Monge, M.; Gomez-Polo, C. Comparative study of (N, Fe) doped TiO2 photocatalysts. Appl. Surf. Sci. 2015, 327, 490–497. [Google Scholar] [CrossRef]
- Zhuomao, Z.; Baoan, B.; Haifeng, S. Effect of N and Fe codoping on the electronic structure and optical properties of TiO2 from first-principles study. J. Semicond. 2015, 36, 102003. [Google Scholar]
- Zhang, Y.; Shen, H.; Liu, Y. Cooperation between N and Fe in co-doped TiO2 photocatalyst. Res. Chem. Intermed. 2016, 42, 687. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photocatalytic decolourisation and degradation of reactive orange 4 by TiO2-UV process. Dyes Pigment. 2006, 68, 133–142. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Horikoshi, S.; Hidaka, H.; Zhao, J.; Serpone, N. Photocatalyzed N-demethylation and degradation of methylene blue in titania dispersions exposed to concentrated sunlight. Sol. Energy Mater. Sol. Cells 2002, 73, 287–303. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Sohabi, M.R.; Ghavami, M. Photocatalytic degradation of direct red 23 dye using UV/TiO2: Effect of operational parameters. J. Hazard. Mater. 2008, 153, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Meilert, K.T.; Laub, D.; Kiwi, J. Photocatalytic self-cleaning of modified cotton textiles by TiO2 clusters attached by chemical spacers. J. Mol. Catal. A 2005, 237, 101–108. [Google Scholar] [CrossRef]
- Bozzi, A.; Yuranova, T.; Guasaquillo, I.; Kiwi, J. Self-cleaning of modified cotton textiles by TiO2 at low temperatures under daylight irradiation. J. Photochem. Photobiol. A 2005, 174, 156–164. [Google Scholar] [CrossRef]
- Adams, D.; Brus, L.; Chidsey, C.; Creager, S.; Creutz, C.; Kagan, C.; Kamat, P.V.; Lieberman, M.; Lindsay, S.; Marcus, R.; et al. Charge transfer on the nanoscale: Current status. J. Phys. Chem. B 2003, 107, 6668–6697. [Google Scholar] [CrossRef]
- Sychev, A.Y.; Isak, V.G. Iron compounds and the mechanisms of the homogeneous catalysis of the activation of O2 and H2O2 and of the oxidation of organic substrates. Russ. Chem. Rev. 1995, 64, 1105–1129. [Google Scholar] [CrossRef]
- Gaynes, R.; Edwards, J.R. National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005, 41, 848–854. [Google Scholar] [PubMed]
- Prabhakar, P.K.; Raj, S.; Anuradha, P.R.; Sawant, S.N.; Doble, M. Biocompatibility studies on polyaniline and polyaniline-silver nanoparticle coated polyurethane composite. Colloids Surf. B 2011, 86, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Vesco, S.; Tagliaferri, V. Self-cleaning and self-sanitizing coatings on plastic fabrics: Design, manufacture and performance. Colloids Surf. B 2014, 120, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, V.; Thilagavathi, G. Developing antiviral surgical gown using nonwoven fabrics for health care sector. Afr. Health Sci. 2013, 13, 327–332. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.V.; Tuckfield, R.C.; Baker-Austin, C. Antimicrobial textiles. Handb. Exp. Pharmacol. 2012, 211, 135–152. [Google Scholar]
- Chattopadhyay, D.P.; Patel, B.H. Effect of nanosized colloidal copper on cotton fabric. J. Eng. Fiber Fabr. 2010, 5, 1–6. [Google Scholar]
- Aslan, N.; Şentürk, K.; Şen, T.; Çoruhlu, T.; Vartürk, İ.; Şeker, S.; Shahidi, S.; Dobrovolskiy, A.M.; Tsiolko, V.V.; Matsevich, S.V.; et al. Investigation of antimicrobial activity and morphological properties of metal coated textile surfaces. Probl. Atom. Sci. Technol. 2014, 20, 208–211. [Google Scholar]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Beyerle, A.; Schulz, H.; Kissel, T.; Stoeger, T. Screening strategy to avoid toxicological hazards of inhaled nanoparticles for drug delivery: The use of α-quartz and nano zinc oxide particles as benchmark. JPCS 2009, 151, 012034. [Google Scholar] [CrossRef]
- Bosca, L.; Zeini, M.; Traves, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pasqui, D.; Barbucci, R. Synthesis, characterization and self-cleaning properties of titania nanoparticles grafted on polyester fabrics. J. Photochem. Photobiol. A 2014, 274, 1–6. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.L.; Dondi, M. TiO2 nanosols applied directly on textiles using different purification treatments. Materials 2015, 8, 7988–7996. [Google Scholar] [CrossRef]
- Cui, G.; Xin, Y.; Dong, M.; Li, J.; Wang, P.; Zhai, S.; Dong, Y.; Jia, J.; Yan, B. Safety profile of TiO2-based photocatalytic nanofabrics for indoor formaldehyde degradation. Int. J. Mol. Sci. 2015, 16, 27721–27729. [Google Scholar] [CrossRef] [PubMed]
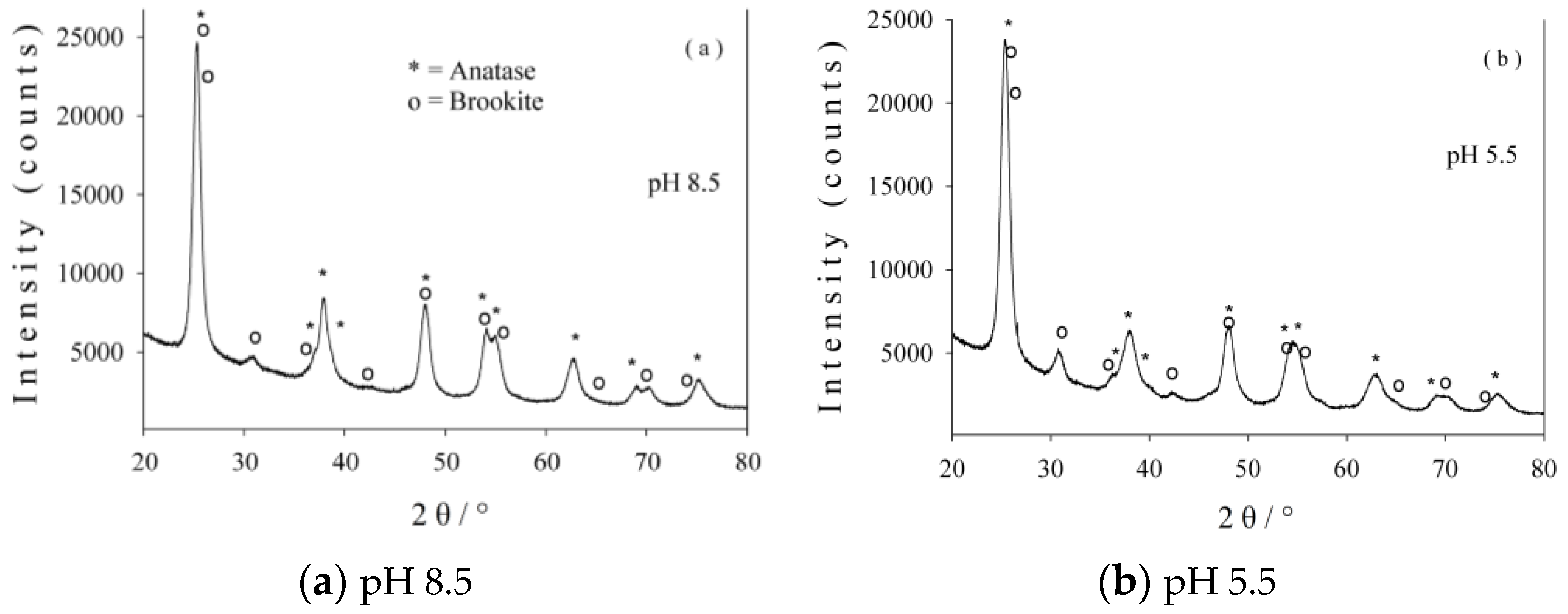
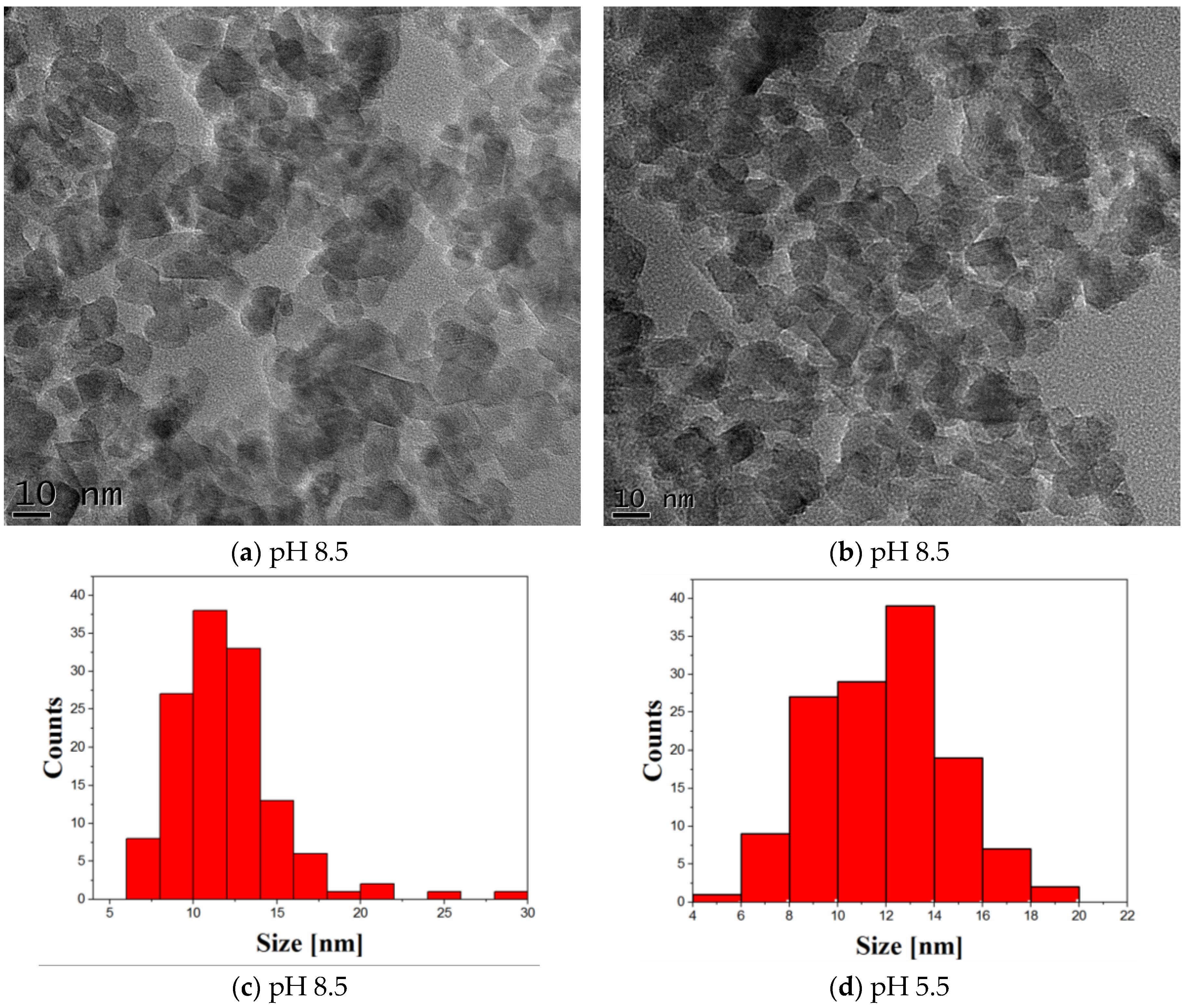


| Sample | Crystallite Size (nm) | Phase Assignment/Abundance (wt %) |
|---|---|---|
| (Photocatalyst 1) | 12.3 | Anatase 85.3 |
| 8.5 | Brookite 14.7 | |
| (Photocatalyst 2) | 10.4 | Anatase 79.4 |
| 11.6 | Brookite 20.6 | |
| Errors | ±1.5 | ±1.4 |
| Parameters | Results for Each Sample | Standard | ||||
|---|---|---|---|---|---|---|
| Initial | HT1 | HT1 ITO | HT2 | HT2 ITO | ||
| Weight (g/m2) | 142 | 149.65 | 150 | 150.55 | 150 | SR EN ISO 12127/2003 |
| Thickness (mm) (surface: 20 cm2, ∆pres: 100 Pa/200 Pa, t: 19.2 °C, RH: 63.2%) | 0.295 | 0.36 | 0.36 | 0.39 | 0.36 | SR EN ISO 5084/2001 |
| Fiber diameter (μm) | 11.69 | 11.13 | 10.72 | 11.33 | 11.18 | Projection microscope |
| Air permeability (L/m2/s) (20 cm2, 100 Pa, t: 19.2 °C, RH: 63.2%) | 173.3 | 153 | 106.33 | 86.33 | 99.86 | SR EN ISO9237:1999 |
| Water vapor resistance (m2Pa/W) | 6.519 | 6.439 | 6.679 | 5.999 | 6.678 | SR EN 31092/A1/2013 ISO 11092 /1997 |
| Surface resistivity (×1014 Ω) (t: 24.1 °C, RH: 45.4%) | 1.75 | 2.2 | 2.6 | 1.3 | 2.7 | SR EN 1149-1:2006 |
| Element (wt %) | Control | HT1 | HT1 ITO | HT2 | HT2 ITO |
|---|---|---|---|---|---|
| C K | 61.51 | 65.43 | 58.57 | 62.12 | 53.74 |
| O K | 38.49 | 32.58 | 35.78 | 34.34 | 36.99 |
| Ti K | 0 | 1.99 | 5.66 | 3.54 | 9.27 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Sample | Stained with Methylene Blue | Stained with Coffee | ||||
|---|---|---|---|---|---|---|
| dE* Vis | dE* UV | dE* Solar | dE* Vis | dE* UV | dE* Solar | |
| Control | 3.34 | 3.21 | 4.77 | 4.92 | 1.3 | 1.62 |
| HT1 | 7.64 | 3.21 | 8.47 | 4.92 | 2.01 | 2.47 |
| HT2 | 9.06 | 6.2 | 6.35 | 2.84 | 2.96 | 6.42 |
| HT1 ITO | 7.51 | 5.91 | 1.69 | 1.77 | ||
| HT2 ITO | 1.86 | 2.4 | 2.45 | 1.48 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, I.C.; Stan, M.S.; Dinischiotu, A.; Popa, M.; Chifiriuc, M.C.; Lazar, V.; Pircalabioru, G.G.; Bezirtzoglou, E.; Iordache, O.G.; Varzaru, E.; et al. Innovative Self-Cleaning and Biocompatible Polyester Textiles Nano-Decorated with Fe–N-Doped Titanium Dioxide. Nanomaterials 2016, 6, 214. https://doi.org/10.3390/nano6110214
Nica IC, Stan MS, Dinischiotu A, Popa M, Chifiriuc MC, Lazar V, Pircalabioru GG, Bezirtzoglou E, Iordache OG, Varzaru E, et al. Innovative Self-Cleaning and Biocompatible Polyester Textiles Nano-Decorated with Fe–N-Doped Titanium Dioxide. Nanomaterials. 2016; 6(11):214. https://doi.org/10.3390/nano6110214
Chicago/Turabian StyleNica, Ionela Cristina, Miruna Silvia Stan, Anca Dinischiotu, Marcela Popa, Mariana Carmen Chifiriuc, Veronica Lazar, Gratiela G. Pircalabioru, Eugenia Bezirtzoglou, Ovidiu G. Iordache, Elena Varzaru, and et al. 2016. "Innovative Self-Cleaning and Biocompatible Polyester Textiles Nano-Decorated with Fe–N-Doped Titanium Dioxide" Nanomaterials 6, no. 11: 214. https://doi.org/10.3390/nano6110214
APA StyleNica, I. C., Stan, M. S., Dinischiotu, A., Popa, M., Chifiriuc, M. C., Lazar, V., Pircalabioru, G. G., Bezirtzoglou, E., Iordache, O. G., Varzaru, E., Dumitrescu, I., Feder, M., Vasiliu, F., Mercioniu, I., & Diamandescu, L. (2016). Innovative Self-Cleaning and Biocompatible Polyester Textiles Nano-Decorated with Fe–N-Doped Titanium Dioxide. Nanomaterials, 6(11), 214. https://doi.org/10.3390/nano6110214













