Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles
Abstract
:1. Introduction
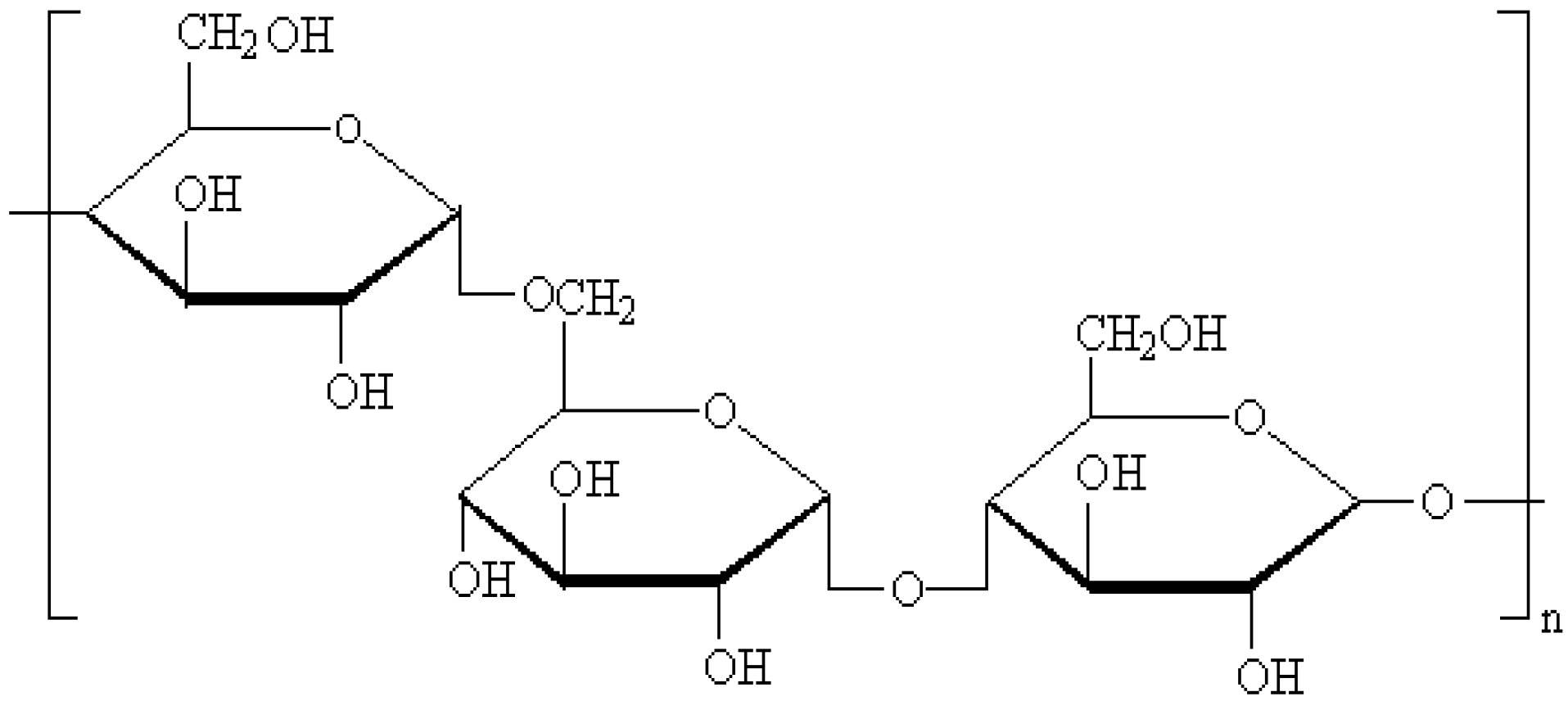

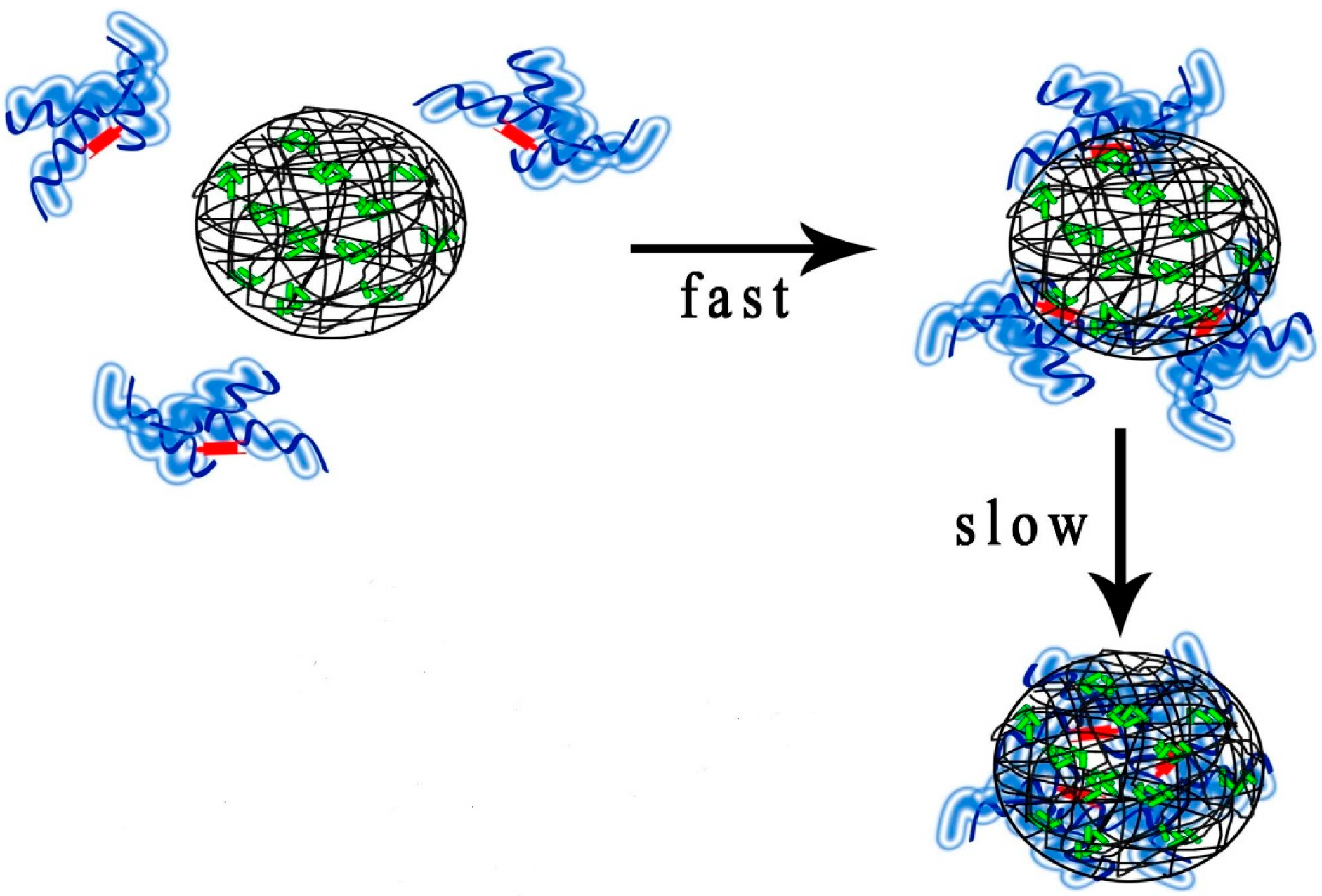
2. Results and Discussion
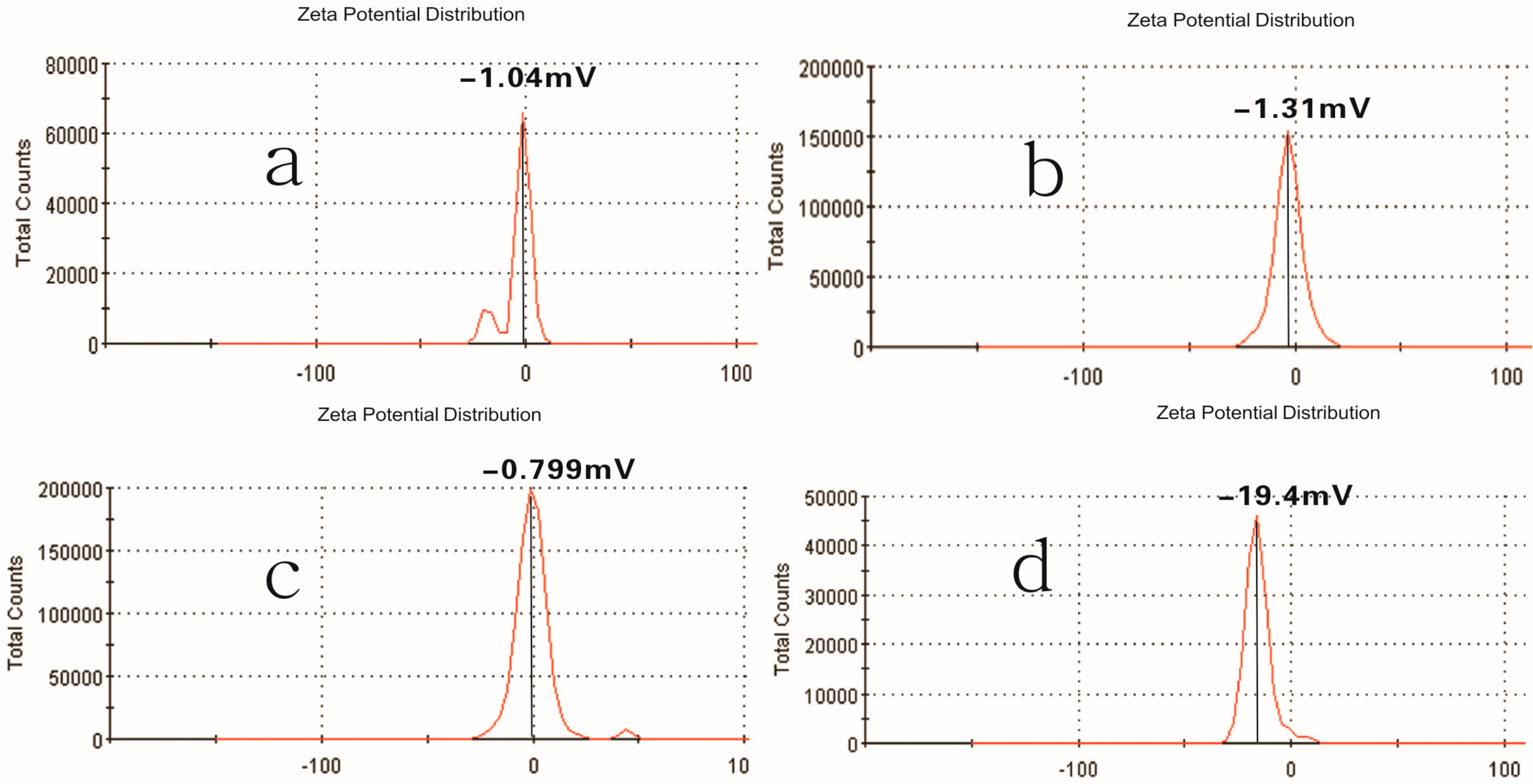
2.1. Properties of Pullulan Nanoparticles
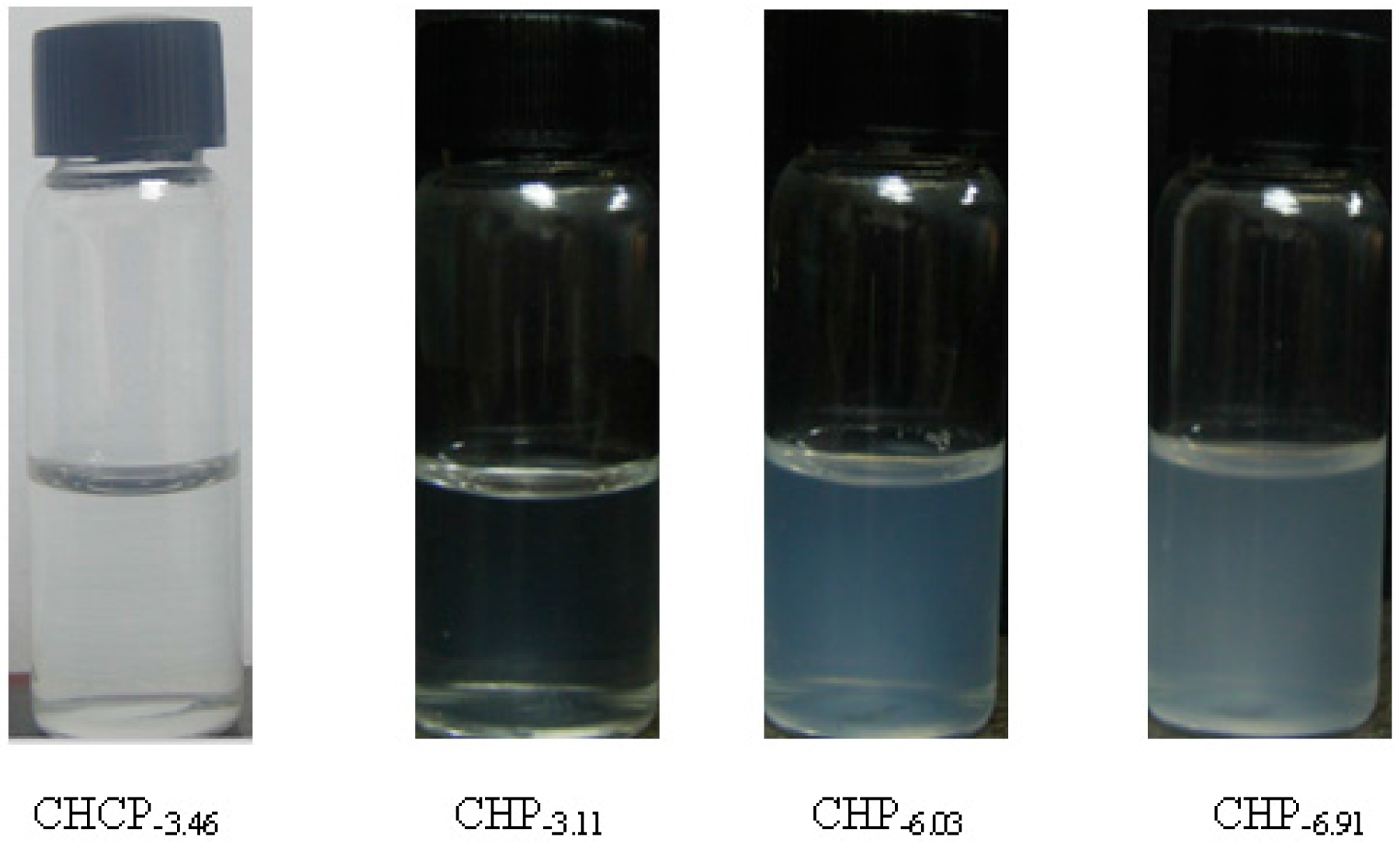
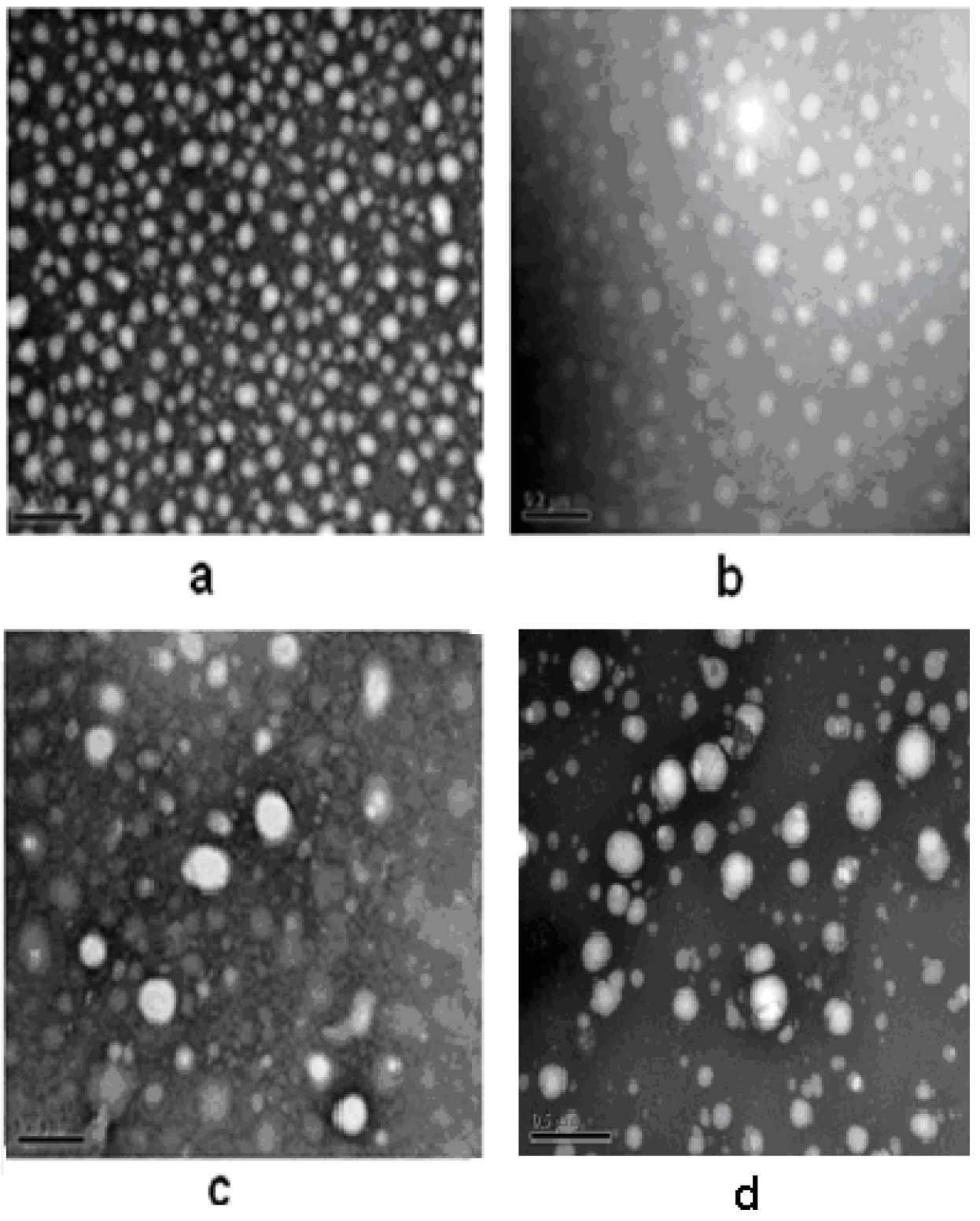

2.2. Drug-Loading of Pullulan Nanoparticles
| Sample | C/D (w/w) a | EE (%) b | LC (%) c | Dh (nm) d |
|---|---|---|---|---|
| CHP−3.11 | 1/10 | 52.4 ± 2.03 | 4.45 ± 0.26 | 162.3 ± 4.6 |
| CHCP | 1/10 | 50.1 ± 1.92 | 4.25 ± 0.23 | 213.5 ± 6.2 |
| CHP−6.03 | 1/10 | 85.1 ± 1.92 | 8.82 ± 0.23 | 142.6 ± 4.2 |
| CHP−6.91 | 1/10 | 90.2 ± 2.43 | 10.1 ± 0.32 | 128.5 ± 3.6 |

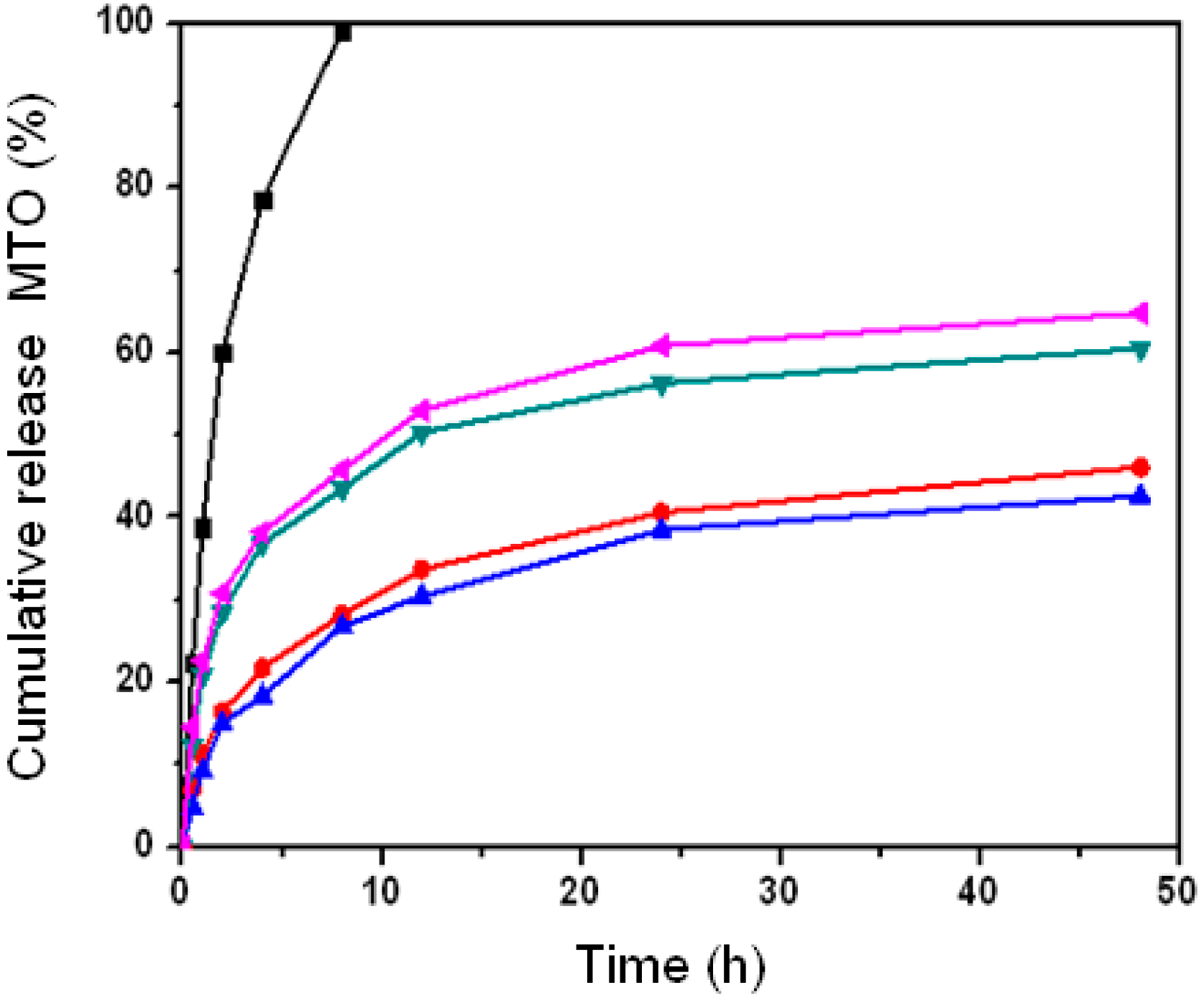
2.3. Effect of Nanoparticle Properties on Drug Release
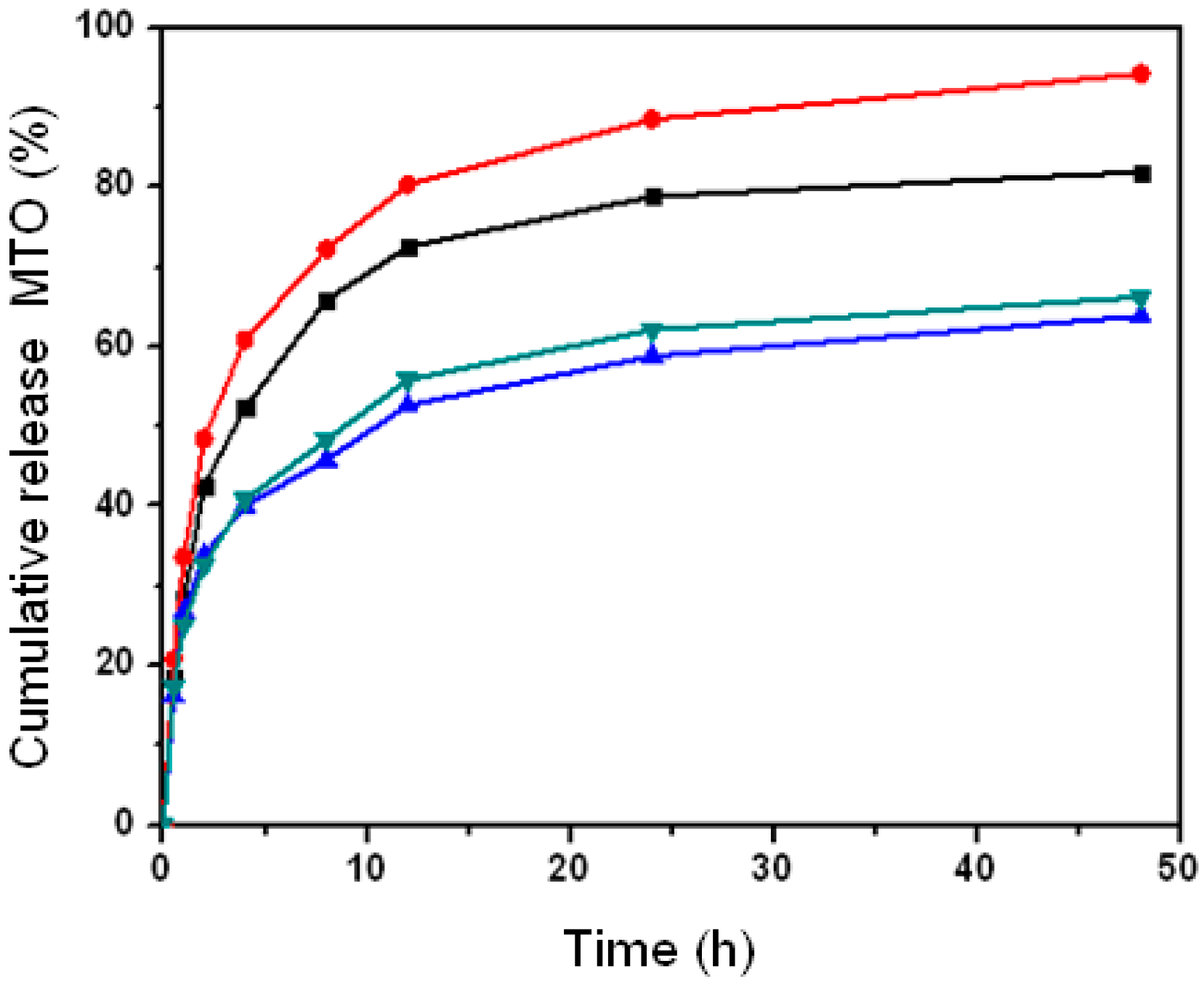
2.4. Effect of Nanoparticle Property on Drug Release with Different pH
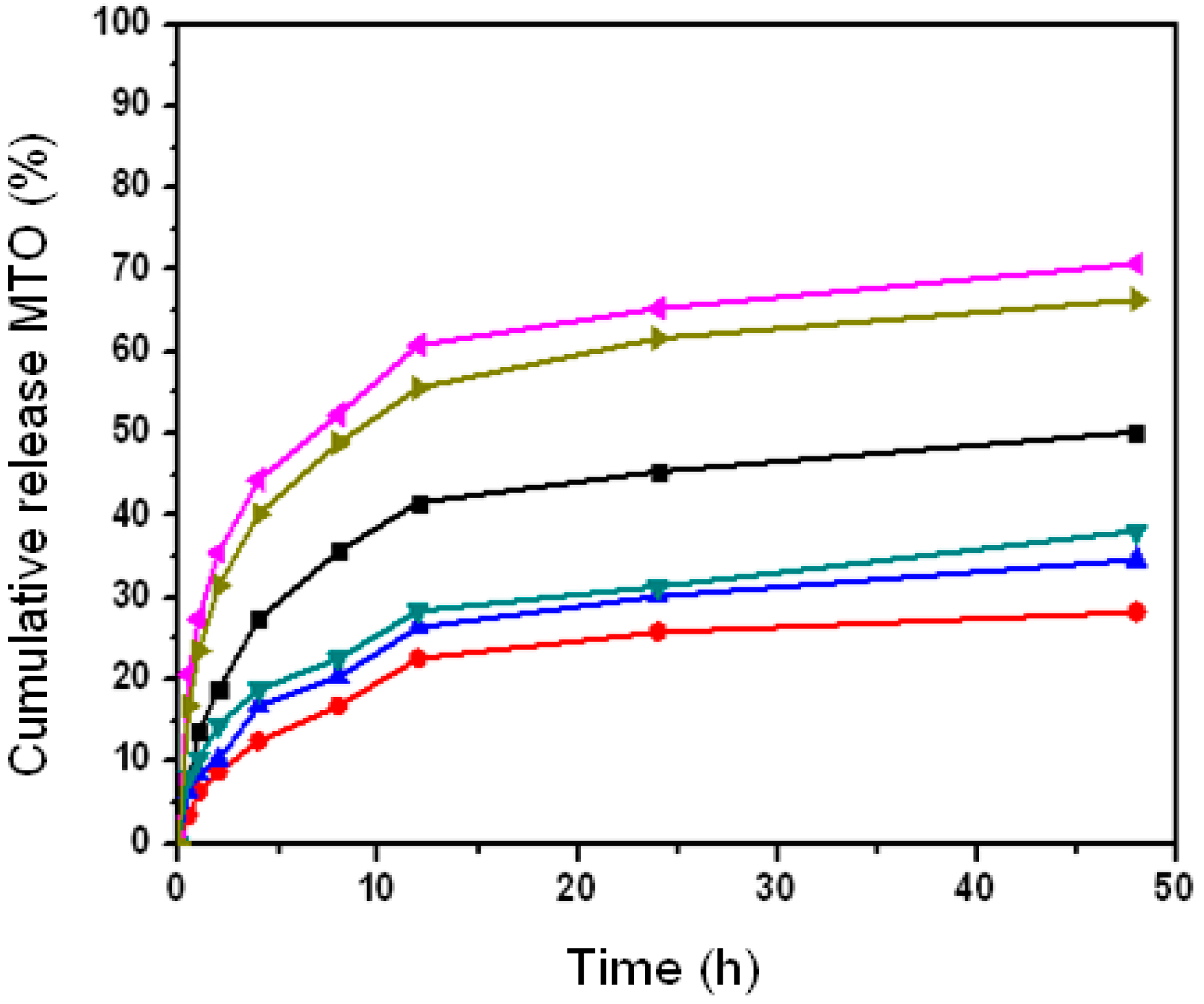
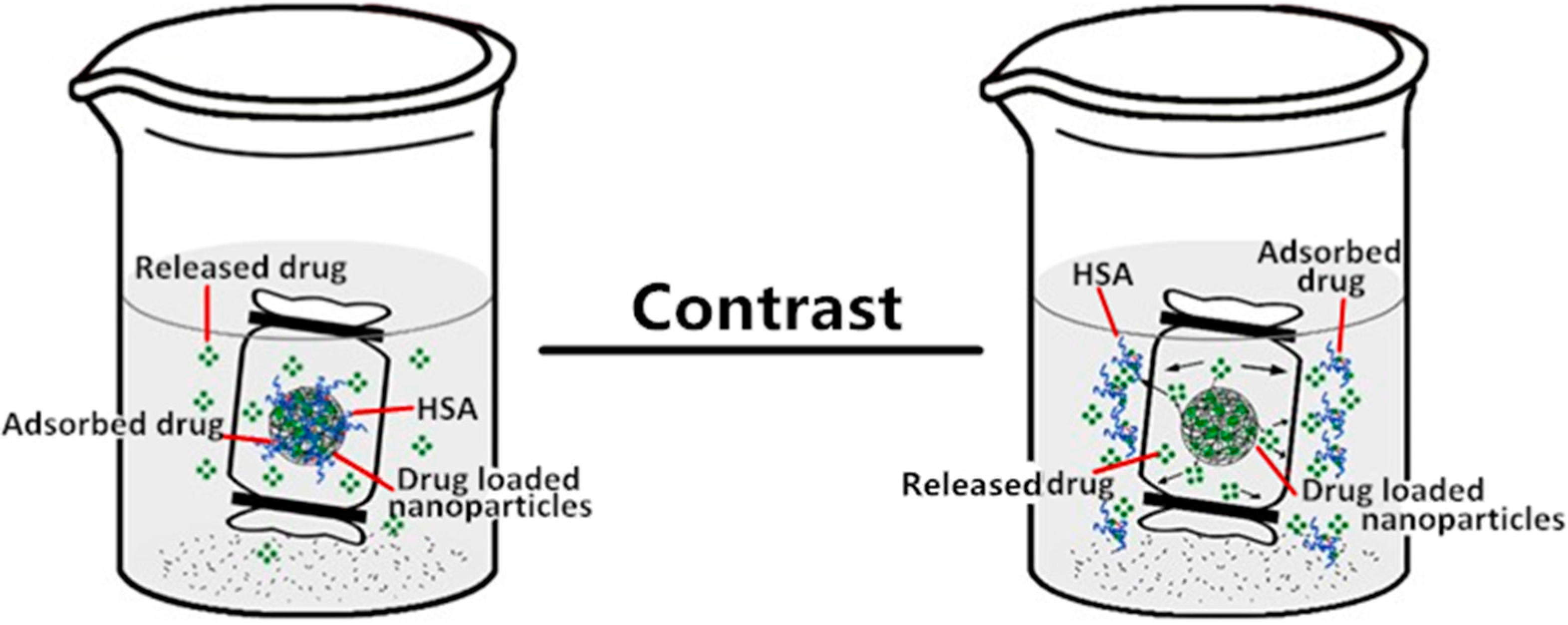
2.5. The Effect of Nanoparticle Properties on Drug Release upon HSA Complexation
3. Experimental Section
3.1. Materials
3.2. CHP and CHCP Nanoparticle Characterization
3.3. Mitoxantrone-Loaded Nanoparticle Characterization
3.4. In Vitro Drug Release of Pullulan Nanoparticles with Different Properties
3.5. In Vitro Drug Release of Pululllan Nanoparticles in Release Media with Different pH
3.6. Drug Release of Pulullan Nanoparticles with HSA Complexation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, T.; Choi, M.; Cui, F.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J. Control. Release 2007, 120, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Koneru, B.; Shi, Y.; Wang, Y.C.; Chavala, S.H.; Miller, M.L.; Holbert, B.; Conson, M.; Ni, A.; Pasqua, A.J.D. Tetracycline-Containing MCM-41 Mesoporous Silica Nanoparticles for the Treatment of Escherichia coli. Molecules 2015, 20, 19690–19698. [Google Scholar] [CrossRef] [PubMed]
- Bennet, D.; Marimuthu, M.; Kim, S.; An, J. Dual drug-loaded nanoparticles on self-integrated scaffold for controlled delivery. Int. J. Nanomed. 2012, 7, 3399–3419. [Google Scholar]
- Bhirde, A.A.; Kapoor, A.; Liu, G.; Iglesias-Bartolome, R.; Jin, A.; Zhang, G.F.; Xing, R.J.; Lee, S.; Leapman, R.D.; Gutkind, S.J.; Chen, X.Y. Nuclear mapping of nano-drug delivery systems in dynamic cellular environments. ACS Nano. 2012, 6, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Ptail, Y.; Sadhukha, T.; Ma, L.; Panyam, J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tarquidar overcomes tumor drug resistance. J. Control. Release 2009, 136, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Alakhova, D.Y.; Kim, J.O.; Bronich, T.K.; Kabanov, A.V. A simple way to enhance doxil® therapy: Drug release from liposomes at the tumor site by amphiphilic block copolymer. J. Control. Release 2013, 168, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Andriyanov, A.V.; Koren, E.; Barenholz, Y.; Goldberg, S.N. Therapeutic efficacy of combining PEGylated liposomal doxorubicin and radiofrequency (RF) ablation: Comparison between slow-drug-releasing, non-thermosensitive and fast-drug-releasing, thermosensitive nano-liposomes. PLoS ONE 2014, 9, 92555–92567. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, H.S.; Nukolova, N.V.; Alexander, V.; Kabanov, A.V.; Bronich, T.K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv. Rev. 2013, 65, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.T.; Huang, L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. 2014, 32, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-based micelles for drug delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; Ibrahim, N.A.; Shameli, K.; Zainuddin, N.; Yunus, W.M.W. Copper Nanoparticles Mediated by Chitosan: Synthesis and Characterization via Chemical Methods. Molecules 2012, 17, 14928–14936. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.F.; Zheng, F.C.; Zhong, S.P.; Tao, X.J.; Zhang, Y.M.; Gao, F.F.; Yao, F.; Chen, J.X.; Chen, Y.C.; Shi, G.G. Self-assembled nanoparticles of glycyrrhetic acid-modified pullulan as a novel of curcumin. Molecules 2014, 19, 13305–13318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Jiang, Q.; Li, R.S.; Liu, L.L.; Zhang, Q.Q.; Wang, Y.M.; Zhao, J. Self-assembled nanoparticles of cholesterol-modified O-carboxymethyl chitosan as a novel carrier for paclitaxel. Nanotechnology 2008, 19, 145101–145109. [Google Scholar] [CrossRef] [PubMed]
- Boudou, T.; Kharkar, P.; Jing, J.; Guillot, R.; Paintrand, I.; Auzely-Velty, R.; Picart, C. Polyelectrolyte multilayer nanoshells with hydrophobic nanodomains for delivery of Paclitaxel. J. Control. Release 2012, 159, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Zhang, Q.Q.; Wang, Y.S.; Chen, H.; Zhang, H.Z.; Gao, F.P.; Liu, L.R. Self-aggregated nanoparticles from methoxy poly(ethylene glycol)-modified chitosan: Synthesis; characterization; aggregation and methotrexate release in vitro. Colloids Surf. B 2008, 61, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Li, X.M.; Liu, L.R.; Zhang, Q.Q. Cellular uptake mechanism and intracellular fate of hydrophobically modified pullulan nanoparticles. Int. J. Nanomed. 2013, 8, 1825–1834. [Google Scholar]
- Dulong, V.; Cerf, D.L.; Picton, L.; Muller, G. Carboxymethylpullulan hydrogels with an ionic and/or amphiphilic behavior: Swelling properties and entrapment of cationic and/or hydrophobic molecules. Colloids Surf. A 2006, 274, 163–169. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Ascenzi, P. Preparation and characterization of pH- and temperature-sensitive pullulan microspheres for controlled release of drugs. Biomaterials 2008, 29, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yang, J.; Ji, F.; Liu, Y.X.; Yao, F.L. Drug co-loading and pH-sensitive release core-shell nanoparticles via layer-by-layer assembly. J. Biomat. Sci. 2014, 25, 1573–1589. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.S.; van Kirk, E.A.; Murdoch, W.J.; Zhan, Y.H.; Isaak, D.D.; Radosz, M.; Shen, Y.Q. Anticancer efficacies of cisplatin-releasing pH-responsive nanoparticles. Biomacromolecules 2006, 7, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, E.S.; Bae, Y.H. A driamycin loaded pullulan acetate/sulfonamide conjugate nanoparticles responding to tumor pH: pH-dependent cell interaction, internalization and cytotoxicity in vitro. J. Control. Release 2003, 87, 3–13. [Google Scholar] [CrossRef]
- Na, K.; Lee, E.S.; Bae, Y.H. Self-organized nanogels responding to tumor extracellular pH: pH-dependent drug release and in vitro cytotoxicity against MCF-7 Cells. Bioconjugate Chem. 2007, 18, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Lindman, S.; Lynch, I.; Thulin, E.; Nilsson, H.; Dawson, H.A.; Linse, S. Systematic investigation of the thermodynamics of HSA adsorption N-iso-propylacrylamide/N-tert-butylacrylamide copolymer nanoparticles. Effects of particle size and hydrophobicity. Nano Lett. 2007, 7, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Shubhra, Q.T.H.; Tóth, J.; Gyenis, J.; Feczkó, T. Surface modification of HSA containing magnetic PLGA nanoparticles by poloxamer to decrease plasma protein adsorption. Colloids Surf. B 2014, 122, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Sundaram, S.; Teeguarden, J.; Riley, B.; Fifield, L.; Jacobs, J.; Addleman, S.; Kaysen, G.; Moudgil, B.; Weber, T. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Shivakumar, H.R.; Sheshappa, R.K.; Ganesh, S.; Prasad, P.; Guru, G.S.; Bhavya, B.B. Miscibility and thermal behavior of pullulan/polyacrylamide blends. J. Macromol. Sci. A 2011, 11, 920–926. [Google Scholar] [CrossRef]
- Bulman, S.E.; Coleman, C.M.; Murphy, J.M.; Medcalf, N.; Ryan, A.E.; Barry, F. Pullulan: A new cytoadhesive for cell-mediated cartilage repair. Stem Cell Res. Ther. 2015, 6, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.B.; Li, L.; Chen, H.; Zhou, Z.M.; Chen, H.L.; Li, X.M.; Liu, L.R.; Wang, Y.S.; Zhang, Q.Q. Stability and in vivo evaluation of pullulan acetate as a drug nanocarrier. Drug Deliv. 2010, 17, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, E.; Morimoto, N.; Kujawa, P.; Ozawa, Y.; Winnik, F.M.; Akiyoshi, K. Self-assembled nanogels of cholesteryl-modified polysaccharides: Effect of the polysaccharide structure on their association characteristics in the dilute and semidilute regimes. Biomacromolecules 2007, 8, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.G.; Li, H.Y.; Yang, W.Z. The preliminary evaluation on cholesterol-modified pullulan as a drug nanocarrier. Drug Deliv. 2014, 21, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.; Tyagi, M.; Patel, K.; Gupta, S.; Vavia, P. Self-assembled nanocomplexes of anionic pullulan and polyallylamine for DNA and pH-sensitive intracellular drug delivery. J. Nanopart Res. 2014, 16, 2781–2792. [Google Scholar] [CrossRef]
- Yamagishi, H.; Akiyoshi, K.; Otsuji, E.; Mazda, O.; Shimizu, T.; Kishida, T.; Hasegawa, U.; Ueda, Y.; Imanishi, J. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem. Biophs. Res. Commun. 2008, 67, 330–335. [Google Scholar]
- Ayame, H.; Morimoto, N.; Akiyoshi, K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjugate Chem. 2008, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.J.; Zhang, Q.F.; Yang, W.Z.; Zhang, Q.Q. The interaction between human serum albumin and cholesterol-modified pullulan nanoparticle. Curr. Nanosci. 2012, 8, 830–837. [Google Scholar] [CrossRef]
- Tao, X.J.; Zhang, Q.F.; Ling, K.; Chen, Y.C.; Yang, W.Z.; Gao, F.F.; Shi, G.G. Effect of pullulan nanoparticle surface charges on HSA complexation and drug Release behavior of HSA-bound nanoparticles. PLoS ONE 2012, 7, 49304–49317. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Wang, M.M.; Ma, L.L.; Li, H.Y.; Huang, L. Synthesis and characterization of biotin modified cholesteryl pullulan as a novel anticancer drug carrier. Carbohydr. Polym. 2014, 99, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Park, J.H.; Chung, H.; Kwon, I.C.; Jeong, S.Y.; Kim, I.S. Physicochemical characteristics of self-assembled nanoparticles based on glycol chitosan bearing 5β-cholanic acid. Langmuir 2003, 19, 10188–10193. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Rosfa, S.; Stauber, R.H. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Foy, M.; Berggad, T.; Donnelly, S.; Cagney, G.; Linse, S.; Dawson, K. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 5754–5756. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lee, K.H.; Bae, Y.H. pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate. J. Control. Release 2004, 97, 513–525. [Google Scholar] [CrossRef]
- Lin, M.M.; Kang, Y.J.; Sohn, Y.J.; Kim, D.K. Dual targeting strategy of magnetic nanoparticle-loaded and RGD peptide-activated stimuli-sensitive polymeric micelles for delivery of paclitaxel. J. Nanopart Res. 2015, 17, 248–257. [Google Scholar] [CrossRef]
- Bikiaris, D.; Karavelidis, V.; Karavas, E. Novel Biodegradable Polyesters. Synthesis and Application as Drug Carriers for the Preparation of Raloxifene HCl Loaded Nanoparticles. Molecules 2009, 14, 2410–2430. [Google Scholar] [CrossRef] [PubMed]
- Guven, A.; Rusakova, A.; Lewis, M.T.; Wilson, L.J. Cisplatin@US-tube carbon nanocapsules for enhanced chemotherapeutic delivery. Biomaterials 2012, 33, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Probing the interactions of proteins and nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2029–2030. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.L.; He, Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expert Opin. Drug Deliv. 2014, 11, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Tehrani, Z.M.; Mahmoudi, N. The effect of protein corona on doxorubicin release from the magnetic mesoporous silica nanoparticles with polyethylene glycol coating. J. Nanopart Res. 2015, 17, 197–209. [Google Scholar] [CrossRef]
- Kreuter, J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J. Microencapsul. 2013, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gessner, A.; Waicz, R.; Lieske, A.; Paulke, B.R.; Mäder, K.; Müller, R.H. Nanoparticles with decreasing surface hydrophobicities: Influence on plasma protein adsorption. Int. J. Pharm. 2000, 196, 245–249. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2011, 134, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Islam, B.; Yennamalli, R.; Sultan, A.; Khan, A.U. Interaction of mitoxantrone with human serum albumin: Spectroscopic and molecular modeling studies. Eur. J. Pharm. Sci. 2008, 35, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, V.A.E.; Maldar, N.N.; Lonikar, S.V.; Rajan, C.R.; Ponrathnam, S. Thermotropic behavior of cholesterol-linked polysaccharides. J. Appl. Polym. Sci. 1998, 7, 195–201. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, L.R.; Weng, J.; Zhang, Q.Q. Preparation and characterization of self-aggregated nanoparticles of cholesterol-modified O-carboxymethyl chitosan conjugates. Carbohydr. Polym. 2007, 69, 597–606. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Jin, S.; Wu, D.; Ling, K.; Yuan, L.; Lin, P.; Xie, Y.; Yang, X. Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles. Nanomaterials 2016, 6, 2. https://doi.org/10.3390/nano6010002
Tao X, Jin S, Wu D, Ling K, Yuan L, Lin P, Xie Y, Yang X. Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles. Nanomaterials. 2016; 6(1):2. https://doi.org/10.3390/nano6010002
Chicago/Turabian StyleTao, Xiaojun, Shu Jin, Dehong Wu, Kai Ling, Liming Yuan, Pingfa Lin, Yongchao Xie, and Xiaoping Yang. 2016. "Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles" Nanomaterials 6, no. 1: 2. https://doi.org/10.3390/nano6010002
APA StyleTao, X., Jin, S., Wu, D., Ling, K., Yuan, L., Lin, P., Xie, Y., & Yang, X. (2016). Effects of Particle Hydrophobicity, Surface Charge, Media pH Value and Complexation with Human Serum Albumin on Drug Release Behavior of Mitoxantrone-Loaded Pullulan Nanoparticles. Nanomaterials, 6(1), 2. https://doi.org/10.3390/nano6010002





