Microwave-Assisted Hydrothermal Rapid Synthesis of Calcium Phosphates: Structural Control and Application in Protein Adsorption
Abstract
:1. Introduction
2. Results and Discussion
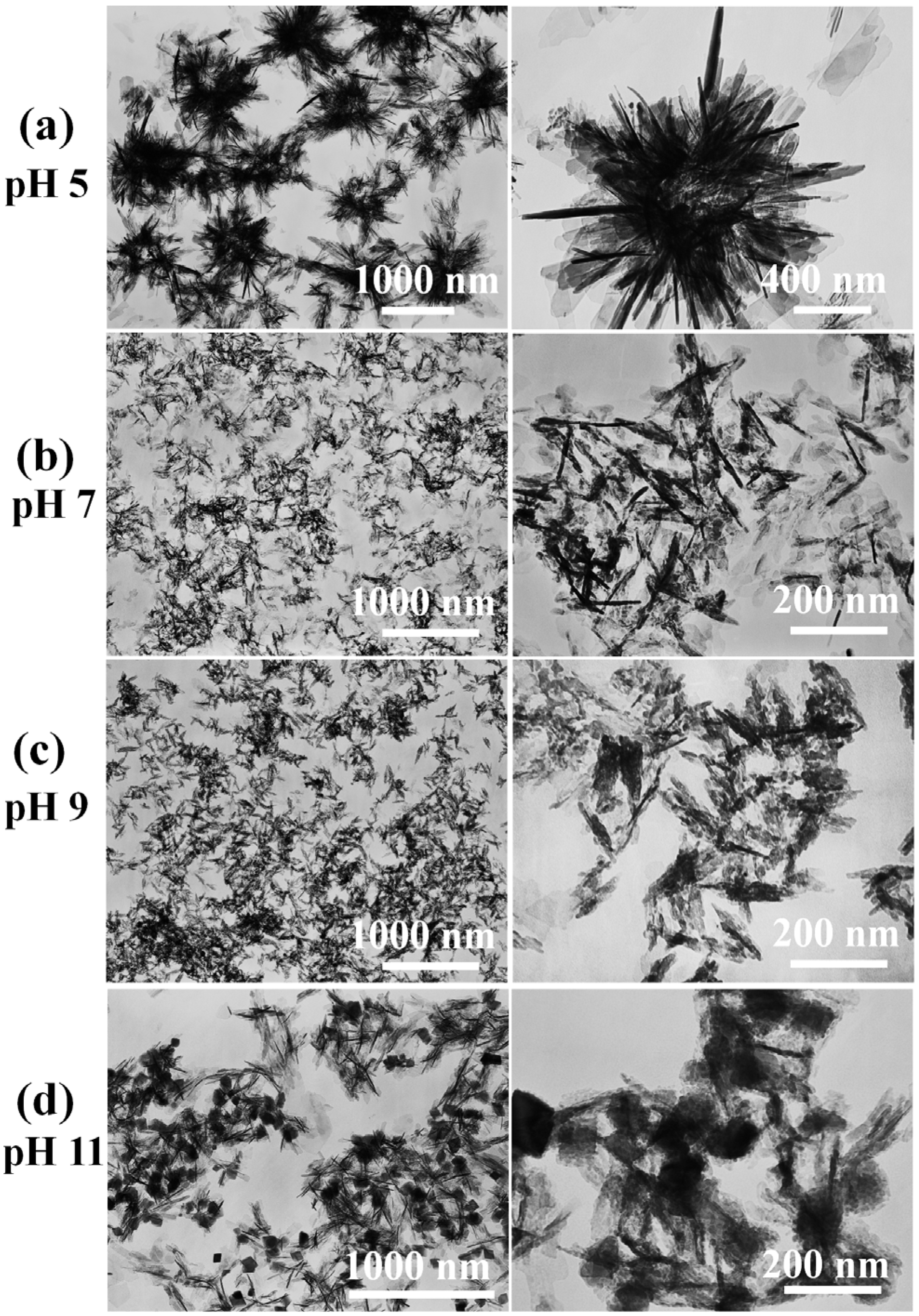
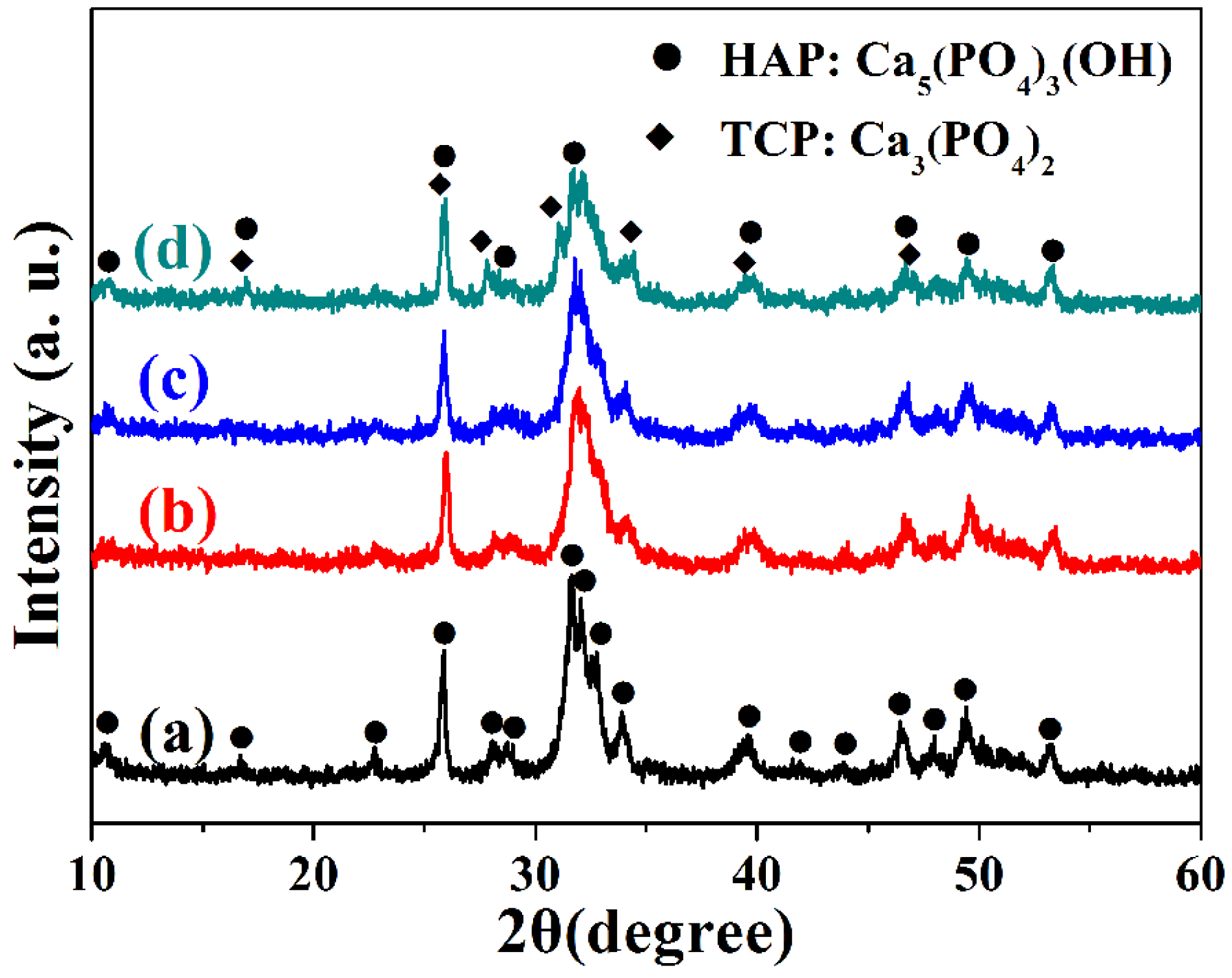

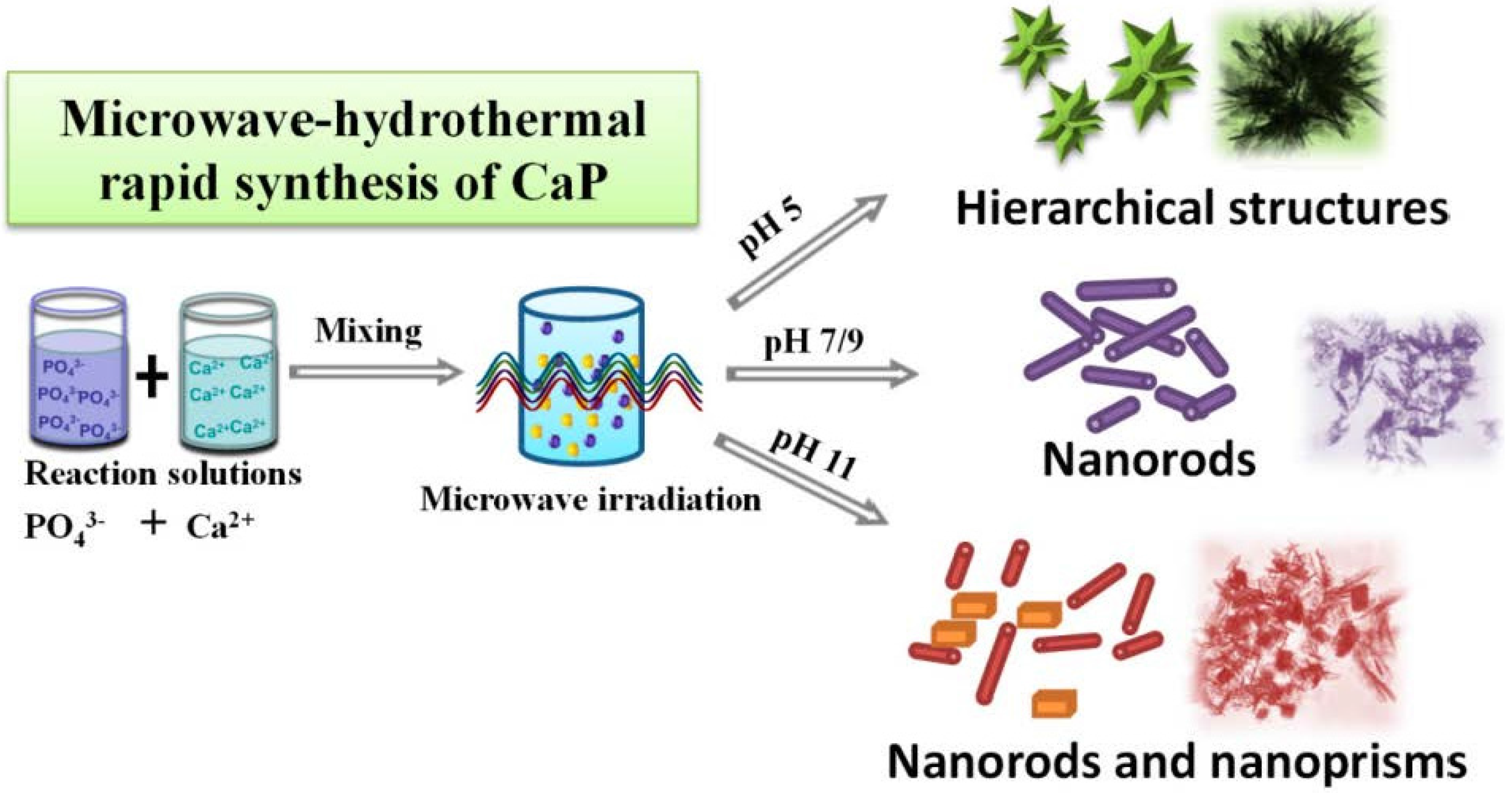
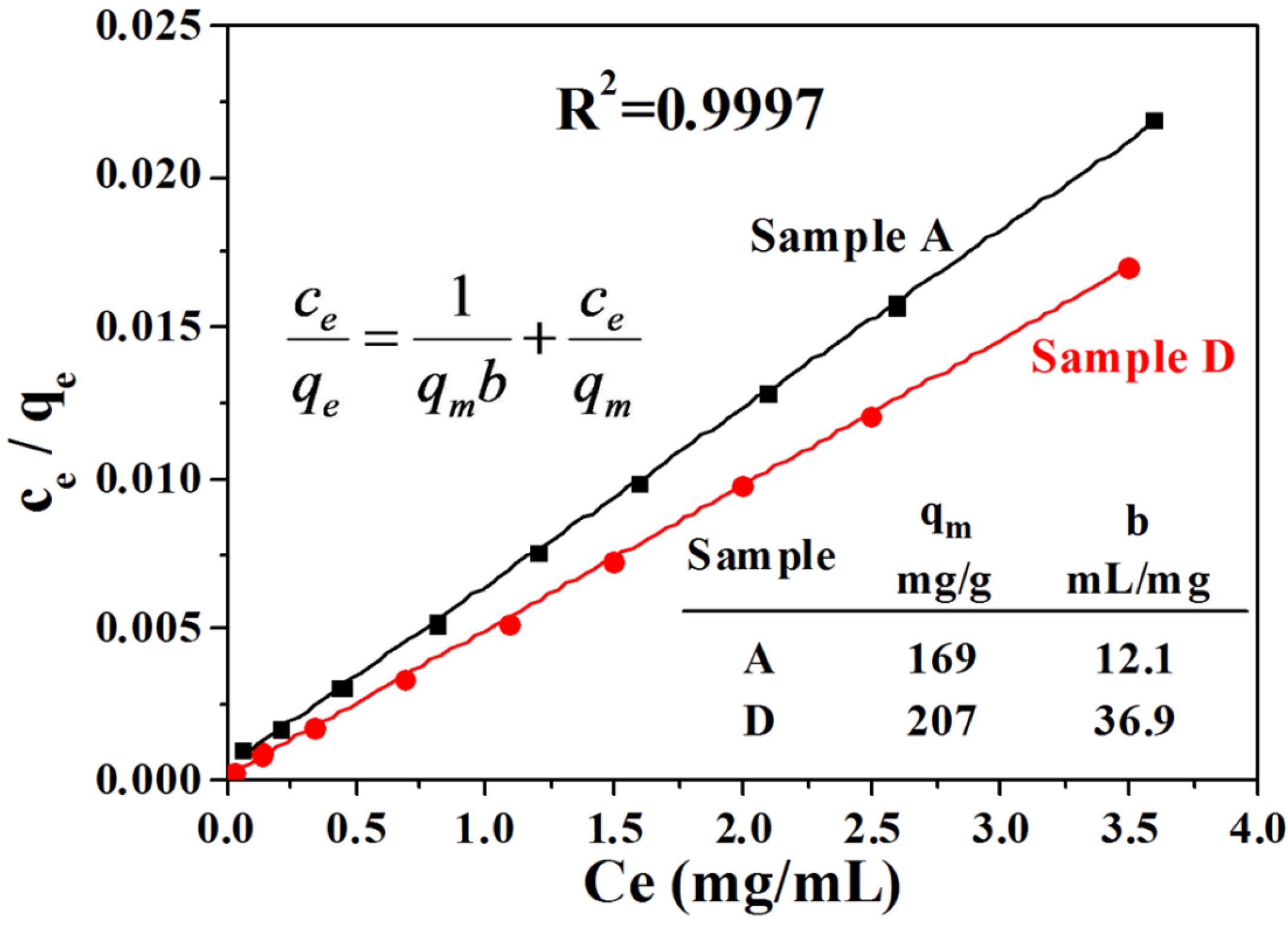
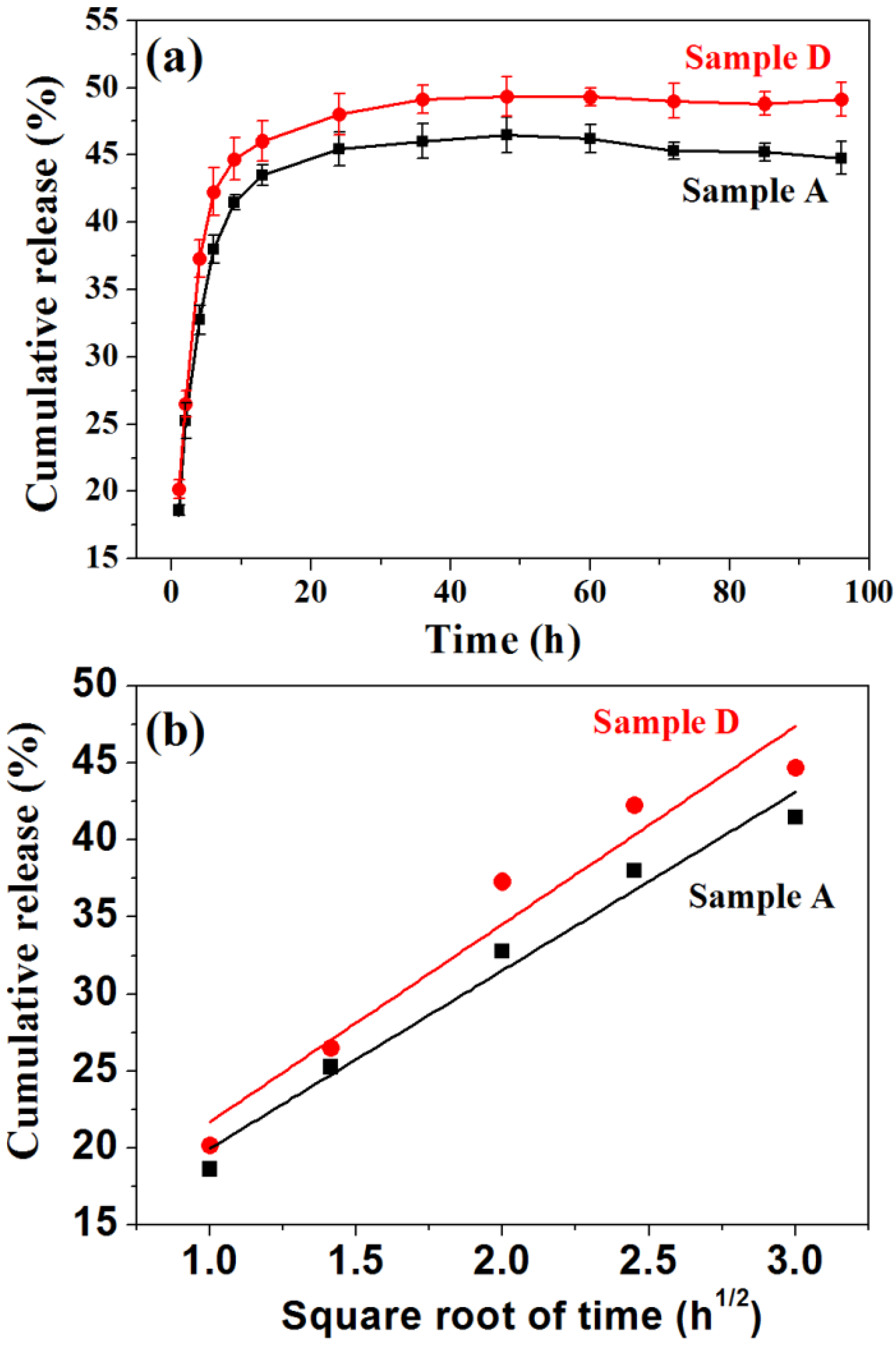
3. Experimental Section
3.1. Preparation of Calcium Phosphate Nanostructures
3.2. Characterization of Samples
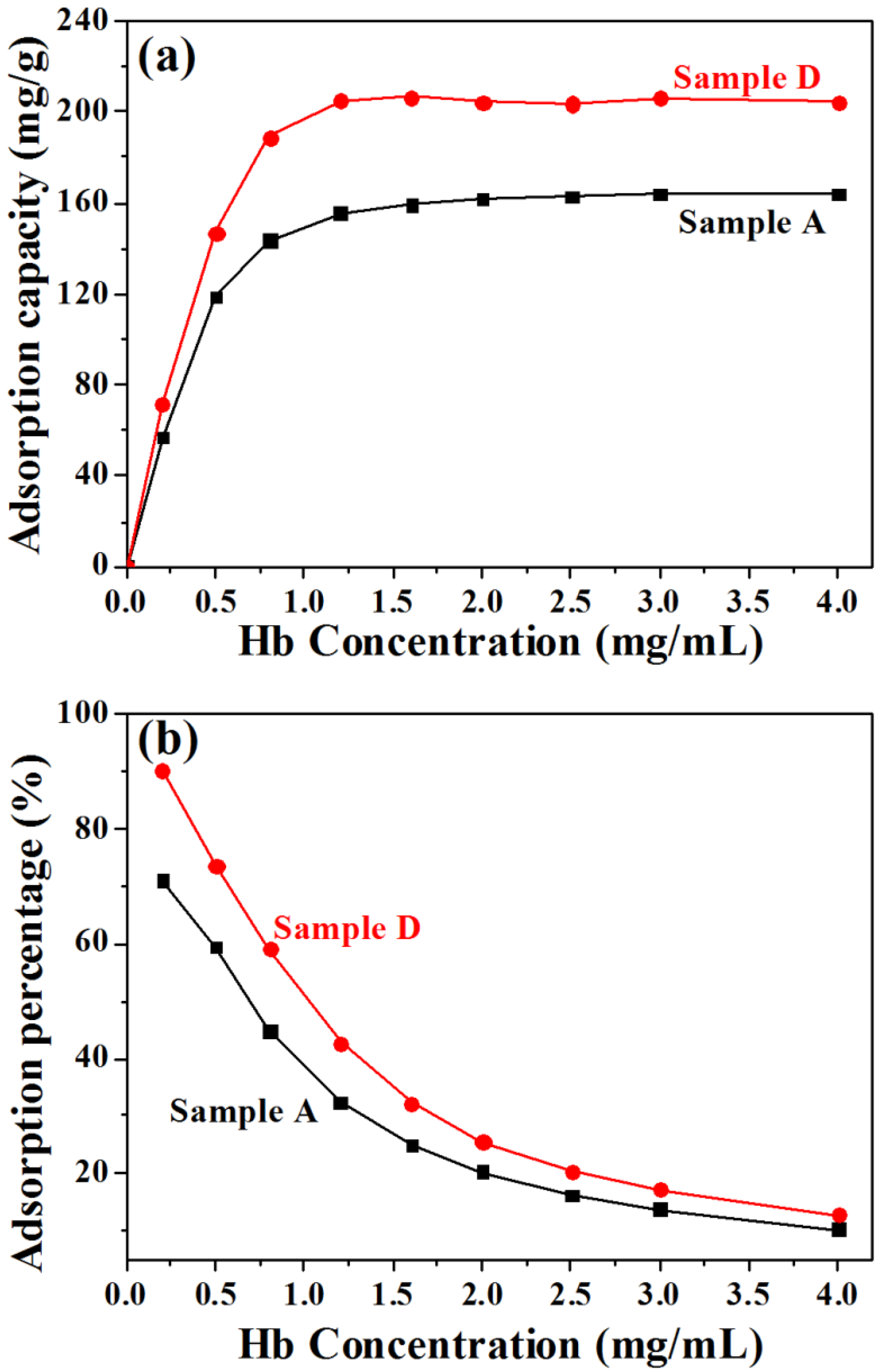
3.3. Protein Adsorption and Release Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, K.L.; Wu, C.T.; Chang, J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014, 10, 4071–4102. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Rubin, M.A.; Jasiuk, L.; Taylor, J.; Rubin, J.; Ganey, T.; Apkarian, R.P. Tem analysis of the nanostructure of normal and osteoporotic human trabecular bone. Bone 2003, 33, 270–282. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Valsami-Jones, E. Bone and tooth mineralization: Why apatite? Elements 2008, 4, 97–104. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, Y.J.; Wu, J.; Huang, P.; Cui, D.X. Nanostructured calcium phosphates: Preparation and their application in biomedicine. Nano. Biomed. Eng. 2012, 4, 41–49. [Google Scholar] [CrossRef]
- Feng, D.S.; Shi, J.; Wang, X.J.; Zhang, L.; Cao, S.K. Hollow hybrid hydroxyapatite microparticles with sustained and ph-responsive drug delivery properties. RSC Adv. 2013, 3, 24975–24982. [Google Scholar] [CrossRef]
- Chen, F.; Li, C.; Zhu, Y.J.; Zhao, X.Y.; Lu, B.Q.; Wu, J. Magnetic nanocomposite of hydroxyapatite ultrathin nanosheets/Fe3O4 nanoparticles: Microwave-assisted rapid synthesis and application in pH-responsive drug release. Biomater. Sci. 2013, 1, 1074–1081. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- He, C.L.; Zhang, F.; Cao, L.J.; Feng, W.; Qiu, K.X.; Zhang, Y.Z.; Wang, H.S.; Mo, X.M.; Wang, J.W. Rapid mineralization of porous gelatin scaffolds by electrodeposition for bone tissue engineering. J. Mater. Chem. 2012, 22, 2111–2119. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y.J. Multifunctional calcium phosphate nanostructured materials and biomedical applications. Curr. Nanosci. 2014, 10, 465–485. [Google Scholar] [CrossRef]
- Chen, F.; Huang, P.; Zhu, Y.J.; Wu, J.; Zhang, C.L.; Cui, D.X. The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031–9039. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef]
- Long, T.; Guo, Y.P.; Liu, Y.Z.; Zhu, Z.A. Hierarchically nanostructured mesoporous carbonated hydroxyapatite microspheres for drug delivery systems with high drug-loading capacity. RSC Adv. 2013, 3, 24169–24176. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y.J.; Wang, K.W.; Zhao, K.L. Surfactant-free solvothermal synthesis of hydroxyapatite nanowire/nanotube ordered arrays with biomimetic structures. CrystEngComm 2011, 13, 1858–1863. [Google Scholar] [CrossRef]
- Kandori, K.; Kuroda, T.; Togashi, S.; Katayama, E. Preparation of calcium hydroxyapatite nanoparticles using microreactor and their characteristics of protein adsorption. J. Phys. Chem. B 2011, 115, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhu, Y.J.; Cheng, G.F.; Ruan, Y.J.; Ding, G.J.; Sun, T.W.; Chen, F.; Wu, J. Microwave-assisted rapid synthesis of magnesium phosphate hierarchical structures using adenosine 5'-triphosphate disodium salt as a phosphorus source. Mater. Lett. 2015, 140, 79–82. [Google Scholar] [CrossRef]
- Ma, M.G.; Zhu, J.F.; Jia, N.; Li, S.M.; Sun, R.C.; Cao, S.W.; Chen, F. Rapid microwave-assisted synthesis and characterization of cellulose-hydroxyapatite nanocomposites in N,N-dimethylacetamide solvent. Carbohyd. Res. 2010, 345, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, S.M.; Ma, M.G.; Sun, R.C. Rapid microwave-assisted fabrication of cellulose/F-substituted hydroxyapatite nanocomposites using green ionic liquids as additive. Mater. Lett. 2012, 68, 44–46. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Luo, H.M.; Zheng, L.W.; Wang, K.J.; Li, H.X.; Wang, Y.; Feng, H.X. Utilization of waste phosphogypsum to prepare hydroxyapatite nanoparticles and its application towards removal of fluoride from aqueous solution. J. Hazard. Mater. 2012, 241, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.; Mazloumi, M.; Mohajerani, M.S.; Zanganeh, S.; Shayegh, M.R.; Kajbafvala, A.; Arami, H.; Sadrnezhaad, S.K. Rapid formation of mono-dispersed hydroxyapatite nanorods with narrow-size distribution via microwave irradiation. J. Am. Ceram. Soc. 2008, 91, 3580–3584. [Google Scholar] [CrossRef]
- Kumar, G.S.; Girija, E.K. Flower-like hydroxyapatite nanostructure obtained from eggshell: A candidate for biomedical applications. Ceram. Int. 2013, 39, 8293–8299. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, L.; Zheng, H.; Wang, Z. Hydroxyapatite nanocrystals for biomedical applications. J. Phys. Chem. C 2010, 114, 18352–18357. [Google Scholar] [CrossRef]
- Jevtić, M.; Mitrić, M.; Škapin, S.; Jančar, B.; Ignjatović, N.; Uskoković, D. Crystal structure of hydroxyapatite nanorods synthesized by sonochemical homogeneous precipitation. Cryst. Growth Des. 2008, 8, 2217–2222. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.J.; Zhao, X.Y.; Zhao, J.; Chen, F.; Cheng, G.F.; Ruan, Y.J. High surface area carbonate apatite nanorod bundles: Surfactant-free sonochemical synthesis and drug loading and release properties. Mater. Res. Bull. 2013, 48, 1536–1540. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yao, A.H.; Huang, W.H.; Wang, D.P.; Zhou, J. In situ fabrication of hollow hydroxyapatite microspheres by phosphate solution immersion. J. Cryst. Growth 2011, 327, 245–250. [Google Scholar] [CrossRef]
- Pan, H.; Liu, X.Y.; Tang, R.; Xu, H.Y. Mystery of the transformation from amorphous calcium phosphate to hydroxyapatite. Chem. Commun. 2010, 46, 7415–7417. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, M.; Tang, P.F.; Li, G. Novel application of HA-TCP biomaterials in distraction osteogenesis shortened the lengthening time and promoted bone consolidation. J. Orthop. Res. 2009, 27, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Mayr, H.; Ziegler, G. Formation of osteoclast-like cells on HA and TCP ceramics. Acta Biomater. 2008, 4, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Uskokovic, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. B 2011, 96, 152–191. [Google Scholar] [CrossRef] [PubMed]
- Morcol, T.; Nagappan, P.; Nerenbaum, L.; Mitchell, A.; Bell, S.J.D. Calcium phosphate-PEG-insulin-casein (CAPIC) particles as oral delivery systems for insulin. Int. J. Pharm. 2004, 277, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Yamaguchi, S.; Kusunose, T.; Toyonaga, T.; Hamada, Y.; Takahashi, J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials 2004, 25, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Joosten, U.; Joist, A.; Frebel, T.; Brandt, B.; Diederichs, S.; von Eiff, C. Evaluation of an in situ setting injectable calcium phosphate as a new carrier material for gentamicin osteomyelitis: Studies in the treatment of chronic in vitro and in vivo. Biomaterials 2004, 25, 4287–4295. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Mechanism of sustained-action medication-theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Rosenholm, J.; Areva, S.; Linden, M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro- and mesoporous silica matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.-Y.; Peng, F.; Zi, Y.-P.; Chen, F.; Qian, Q.-R. Microwave-Assisted Hydrothermal Rapid Synthesis of Calcium Phosphates: Structural Control and Application in Protein Adsorption. Nanomaterials 2015, 5, 1284-1296. https://doi.org/10.3390/nano5031284
Cai Z-Y, Peng F, Zi Y-P, Chen F, Qian Q-R. Microwave-Assisted Hydrothermal Rapid Synthesis of Calcium Phosphates: Structural Control and Application in Protein Adsorption. Nanomaterials. 2015; 5(3):1284-1296. https://doi.org/10.3390/nano5031284
Chicago/Turabian StyleCai, Zhu-Yun, Fan Peng, Yun-Peng Zi, Feng Chen, and Qi-Rong Qian. 2015. "Microwave-Assisted Hydrothermal Rapid Synthesis of Calcium Phosphates: Structural Control and Application in Protein Adsorption" Nanomaterials 5, no. 3: 1284-1296. https://doi.org/10.3390/nano5031284
APA StyleCai, Z.-Y., Peng, F., Zi, Y.-P., Chen, F., & Qian, Q.-R. (2015). Microwave-Assisted Hydrothermal Rapid Synthesis of Calcium Phosphates: Structural Control and Application in Protein Adsorption. Nanomaterials, 5(3), 1284-1296. https://doi.org/10.3390/nano5031284





