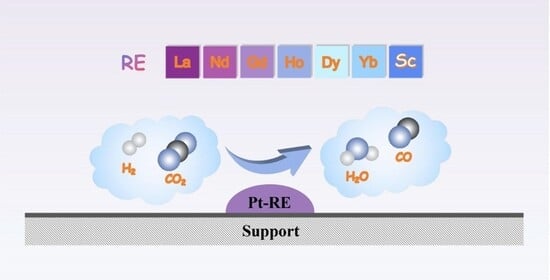
Pt-Rare Earth Subnanometric Bimetallic Clusters Efficiently Catalyze the Reverse Water–Gas Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalytic Performance Testing
2.4. Catalyst Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Sun, J.; Wang, H.; Cui, X. Recent Advances in Hydrogenation of CO2 to CO with Heterogeneous Catalysts Through the RWGS Reaction. J. Chem. Asian 2024, 19, e202300971. [Google Scholar] [CrossRef]
- Garcia, J.; Villen-Guzman, M.; Rodriguez-Maroto, J.; Paz-Garcia, J. Comparing CO2 Storage and Utilization: Enhancing Sustainability through Renewable Energy Integration. Sustainability 2024, 16, 6639. [Google Scholar] [CrossRef]
- Bashir, A.; Ali, M.; Patil, S.; Aljawad, M.; Mahmoud, M.; Al-Shehri, D.; Hoteit, H.; Kamal, M.S. Comprehensive review of CO2 geological storage: Exploring principles, mechanisms, and prospects. Earth-Sci. Rev. 2024, 249, 104672. [Google Scholar] [CrossRef]
- Ali, A.; Haupt, J.; Werra, M.; Gernuks, S.; Wiegel, M.; Rueggeberg, M.; Cerdas, F.; Herrmann, C. Life cycle assessment of carbon dioxide removal and utilisation strategies: Comparative analysis across Europe. Resour. Conserv. Recy. 2024, 211, 107837. [Google Scholar] [CrossRef]
- Tsoy, N.; Steubing, B.; Guinée, J. Ex-ante life cycle assessment of polyols using carbon captured from industrial process gas. Green Chem. 2023, 25, 5526–5538. [Google Scholar] [CrossRef]
- Kamkeng, A.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Bai, Y.; Ding, X.; Sun, Y.; Song, Y.; Wang, Y. Safe and efficient catalytic reaction for direct synthesis of CO from methylcyclohexane and CO2. Chem. Eng. J. 2023, 451, 138967. [Google Scholar] [CrossRef]
- Bahmanpour, A.; Signorile, M.; Kröcher, O. Recent progress in syngas production via catalytic CO2 hydrogenation reaction. Appl. Catal. B Environ. 2021, 295, 120319. [Google Scholar] [CrossRef]
- Ai, X.; Xie, H.; Chen, S.; Zhang, G.; Xu, B.; Zhou, G. Highly dispersed mesoporous Cu/γ-Al2O3 catalyst for RWGS reaction. Int. J. Hydrogen Energy 2022, 47, 14884–14895. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, L.; Yuan, H.; Liu, H.; Wu, P.; Fu, M.; Wu, J.; Chen, P.; Qiu, Y.; Ye, D.; et al. Reciprocal regulation between support defects and strong metal-support interactions for highly efficient reverse water gas shift reaction over Pt/TiO2 nanosheets catalysts. Appl. Catal. B Environ. 2021, 298, 120507. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, M.; Ma, P.; Zheng, Y.; Chen, J.; Li, H.; Zhang, X.; Zheng, K.; Kuang, Q.; Xie, Z. Atomically dispersed Pt/CeO2 catalyst with superior CO selectivity in reverse water gas shift reaction. Appl. Catal. B Environ. 2021, 291, 120101. [Google Scholar] [CrossRef]
- Goguet, A.; Meunier, F.; Breen, J.; Burch, R.; Petch, M.; Ghenciu, A. Study of the origin of the deactivation of a Pt/CeO2 catalyst during reverse water gas shift (RWGS) reaction. J. Catal. 2004, 226, 382–392. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, W.; Lin, S. Study of reverse water gas shift reaction by TPD, TPR and CO2 hydrogenation over potassium-promoted Cu/SiO2 catalyst. Appl. Catal. A Gen. 2003, 238, 55–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, L.; Chen, Z.; Wen, J.; Zhong, W.; Zou, S.; Fu, M.; Chen, L.; Ye, D. Highly efficient Cu/CeO2-hollow nanospheres catalyst for the reverse water-gas shift reaction: Investigation on the role of oxygen vacancies through in situ UV-Raman and DRIFTS. Appl. Surf. Sci. 2020, 516, 146035. [Google Scholar] [CrossRef]
- Yang, S.; Pang, S.; Sulmonetti, T.; Su, W.; Le, J.; Hwang, B.; Jones, C. Synergy between Ceria Oxygen Vacancies and Cu Nanoparticles Facilitates the Catalytic Conversion of CO2 to CO under Mild Conditions. ACS Catal. 2018, 8, 12056–12066. [Google Scholar] [CrossRef]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S. Catalytic activity of rare earth and alkali metal promoted (Ce, La, Mg, K) Ni/Al2O3 nanocatalysts in reverse water gas shift reaction. Res. Chem. Intermed. 2019, 45, 5125–5141. [Google Scholar] [CrossRef]
- Lu, B.; Kawamoto, K. Preparation of mesoporous CeO2 and monodispersed NiO particles in CeO2, and enhanced selectivity of NiO/CeO2 for reverse water gas shift reaction. Mater. Res. Bull. 2014, 53, 70–78. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, T.; Xie, H.; Zheng, X. Effects of structure on the carbon dioxide methanation performance of Co-based catalysts. Int. J. Hydrogen Energy 2013, 38, 10012–10018. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Chen, J.; Liu, P. CO2 hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of synergy between Pt and oxide support. J. Catal. 2016, 343, 115–126. [Google Scholar] [CrossRef]
- Kwak, J.; Kovarik, L.; Szanyi, J. Heterogeneous catalysis on atomically dispersed supported metals: CO2 reduction on multifunctional Pd catalysts. ACS Catal. 2013, 3, 2094–2100. [Google Scholar] [CrossRef]
- Duyar, M.; Ramachandran, A.; Wang, C.; Farrauto, R. Kinetics of CO2 methanation over Ru/γ-Al2O3 and implications for renewable energy storage applications. J. CO2 Util. 2015, 12, 27–33. [Google Scholar] [CrossRef]
- Tang, R.; Zhu, Z.; Li, C.; Xiao, M.; Wu, Z.; Zhang, D.; Zhang, C.; Xiao, Y.; Chu, M.; Genest, A.; et al. Ru-Catalyzed Reverse Water Gas Shift Reaction with Near-Unity Selectivity and Superior Stability. ACS Mater. Lett. 2021, 3, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Mathew, T.; Saju, S.; Raveendran, S. Survey of heterogeneous catalysts for the CO2 reduction to CO via reverse water gas shift. J. Energy Chem. 2021, 12, 281−316. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, X.; Qiu, S.; Wang, L.; Hao, L.; Yu, Y. Stable CuIn alloy for electrochemical CO2 reduction to CO with high-selectivity. Mater. Today Phys. 2023, 33, 101050. [Google Scholar] [CrossRef]
- Seo, J.; Park, G.; Arshad, M.; Zhang, C.; Kim, S.; Kim, S. Active and selective reverse water-gas shift reaction over Pt/Na-Zeolite catalysts. J. CO2 Util. 2022, 66, 102291. [Google Scholar] [CrossRef]
- Zhao, J.; Bao, R.; Sun, R.; Zhang, X.; Zhang, T.; Wang, C. Silica-supported Pt-In intermetallic alloy for low-temperature reverse water-gas shift reaction. Mol. Catal. 2025, 574, 114890. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, X.; Yu, Y.; Chen, L.; Cheng, T.; Huang, J.; Liu, Y.; Harada, M.; Ishihara, A.; Wang, Y. Indium oxide supported Pt-In alloy nanocluster catalysts with enhanced catalytic performance toward oxygen reduction reaction. J. Power Sources 2020, 446, 227332. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Wang, X.; Nuzzo, R.; Frenkel, A.; Liu, P.; Gersappe, D. Revealing Intermetallic Active Sites of PtNi Nanocatalysts for Reverse Water Gas Shift Reaction. J. Phys. Chem. C 2023, 127, 22067–22075. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Frenkel, A.; Liu, P. Rationalization of promoted reverse water gas shift reaction by Pt3Ni alloy: Essential contribution from ensemble effect. J. Chem. Phys. 2021, 154, 014702. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Li, H.; Wei, Y.; Li, Y.; Li, H.; Yu, H.; Li, K.; Li, H.; Yin, H. Pt-Fe Bimetallic Nanoparticles Confined in Nitrogen-Doped Carbon Nanoshells as a Catalyst for the Reverse Water-Gas Shift Reaction. ACS Appl. Nano Mater. 2025, 8, 10315–10325. [Google Scholar] [CrossRef]
- Wang, H.; Bootharaju, M.; Kim, J.; Wang, Y.; Wang, K.; Zhao, M.; Zhang, R.; Xu, J.; Hyeon, T.; Wang, X.; et al. Synergistic Interactions of Neighboring Platinum and Iron Atoms Enhance Reverse Water-Gas Shift Reaction Performance. Am. Chem. Soc. 2023, 145, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Wang, H.; Zhang, T. Synthesis of Pt-Rare Earth Metal Alloys and Their Applications. Chem. Eur. J. 2024, 30, e202402750. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Sudalai, A.; Jo, C.; Park, S. Ethane Oxidative Dehydrogenation over Pt-Rare Earth Intermetallic Compounds with CO2 as Soft Oxidant. Ind. Eng. Chem. Res. 2023, 62, 10382–10390. [Google Scholar] [CrossRef]
- Ryoo, R.; Kim, J.; Jo, C.; Han, S.; Kim, J.; Park, H.; Han, J.; Shin, H.; Shin, J. Rare-earth–platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 2020, 585, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Kanady, J.; Leidinger, P.; Haas, A.; Titlbach, S.; Schunk, S.; Schierle-Arndt, K.; Crumlin, E.; Wu, C.; Alivisatos, A. Synthesis of Pt3Y and other early–late intermetallic nanoparticles by way of a molten reducing agent. J. Am. Chem. Soc. 2017, 139, 5672−5675. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y.; Liang, Z.; Li, Q.; Du, Y.; Liu, J.; Cheng, Y.; Luo, F. Activating PtRu with rare earth alloying for efficient electrocatalytic methanol oxidation reaction. Catal. Sci. Technol. 2025, 15, 2473–2481. [Google Scholar] [CrossRef]
- Malacrida, P.; Escudero-Escribano, M.; Verdaguer-Casadevall, A.; Stephensa, I.; Chorkendorff, I. Enhanced activity and stability of Pt-La and Pt-Ce alloys for oxygen electroreduction: The elucidation of the active surface phase. Mater. Chem. A 2014, 2, 4234–4243. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; Sun, C.; Sun, X.; Li, Z.; Du, Y.; Liu, J.; Luo, F. Enhanced Alkaline Hydrogen Evolution Reaction via Electronic Structure Regulation: Activating PtRh with Rare Earth Tm Alloying. Small 2024, 20, 2400662. [Google Scholar] [CrossRef]
- Li, Q.; Sun, C.; Fu, H.; Zhang, S.; Sun, X.; Liu, J.; Du, Y.; Luo, F. Enhanced Alkaline Hydrogen Evolution Reaction through Lanthanide-Modified Rhodium Intermetallic Catalysts. Small 2024, 20, 2307052. [Google Scholar] [CrossRef]
- Vidales, A.; Omanovic, S.; Li, H.; Hrapovic, S.; Tartakovsky, B. Evaluation of biocathode materials for microbial electrosynthesis of methane and acetate. Bioelectrochemistry 2022, 148, 108246. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Escudero-Escribano, M.; Malacrida, P.; Hansen, M.; Vej-Hansen, U.; Velázquez-Palenzuela, A.; Tripkovic, V.; Schiotz, J.; Rossmeisl, J.; Stephens, I.; Chorkendorff, I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73–76. [Google Scholar] [CrossRef]
- Vidales, A.; Semai, M. Platinum nanoparticles supported on nickel-molybdenum-oxide for efficient hydrogen production via acidic water electrolysis. J. Mol. Struct. 2023, 1290, 135956. [Google Scholar] [CrossRef]
- Szamosvölgyi, A.; Pitó, A.; Efremova, A.; Baán, K.; Kutus, B.; Suresh, M.; Sápi, A.; Szenti, I.; Kiss, J.; Kolonits, T.; et al. Optimized Pt–Co Alloy Nanoparticles for Reverse Water–Gas Shift Activation of CO2. ACS Appl. Nano Mater. 2024, 7, 9968–9977. [Google Scholar] [CrossRef]
- Song, Y.; Kim, D.; Hong, S.; Kim, T.; Kim, K.; Park, J. Bimetallic Synergy from a Reaction-Driven Metal Oxide-Metal Interface of Pt-Co Bimetallic Nanoparticles. ACS Catal. 2023, 13, 13777–13785. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Liang, Z.; Sun, C.; Shen, S.; Li, Q.; Luo, F. Pt-Rare Earth Subnanometric Bimetallic Clusters Efficiently Catalyze the Reverse Water–Gas Reaction. Nanomaterials 2026, 16, 77. https://doi.org/10.3390/nano16010077
Liang Z, Sun C, Shen S, Li Q, Luo F. Pt-Rare Earth Subnanometric Bimetallic Clusters Efficiently Catalyze the Reverse Water–Gas Reaction. Nanomaterials. 2026; 16(1):77. https://doi.org/10.3390/nano16010077
Chicago/Turabian StyleLiang, Zhaolei, Chang Sun, Songhe Shen, Qingqing Li, and Feng Luo. 2026. "Pt-Rare Earth Subnanometric Bimetallic Clusters Efficiently Catalyze the Reverse Water–Gas Reaction" Nanomaterials 16, no. 1: 77. https://doi.org/10.3390/nano16010077
APA StyleLiang, Z., Sun, C., Shen, S., Li, Q., & Luo, F. (2026). Pt-Rare Earth Subnanometric Bimetallic Clusters Efficiently Catalyze the Reverse Water–Gas Reaction. Nanomaterials, 16(1), 77. https://doi.org/10.3390/nano16010077