Solvent-Engineered PEACl Passivation: A Pathway to 24.27% Efficiency and Industrially Scalable Perovskite Solar Cells
Abstract
1. Introduction
- (1)
- We establish a universal “solvent coordination-volatilization kinetics” model, experimentally validated through in situ XRD and XPS, that links solvent properties to passivation layer quality.
- (2)
- DMSO/IPA-engineered devices achieve a PCE of 24.27% (VOC = 1.15 V) with 90% efficiency retention after 1344 h in inert atmospheres, outperforming single-solvent control systems.
- (3)
- By replacing toxic chlorobenzene with a low-DMSO green solvent system, this strategy aligns with industrial requirements for scalable, eco-friendly manufacturing.
2. Experimental Section
2.1. Materials
2.2. Device Fabrication
2.3. Characterization and Test
3. Results and Discussion
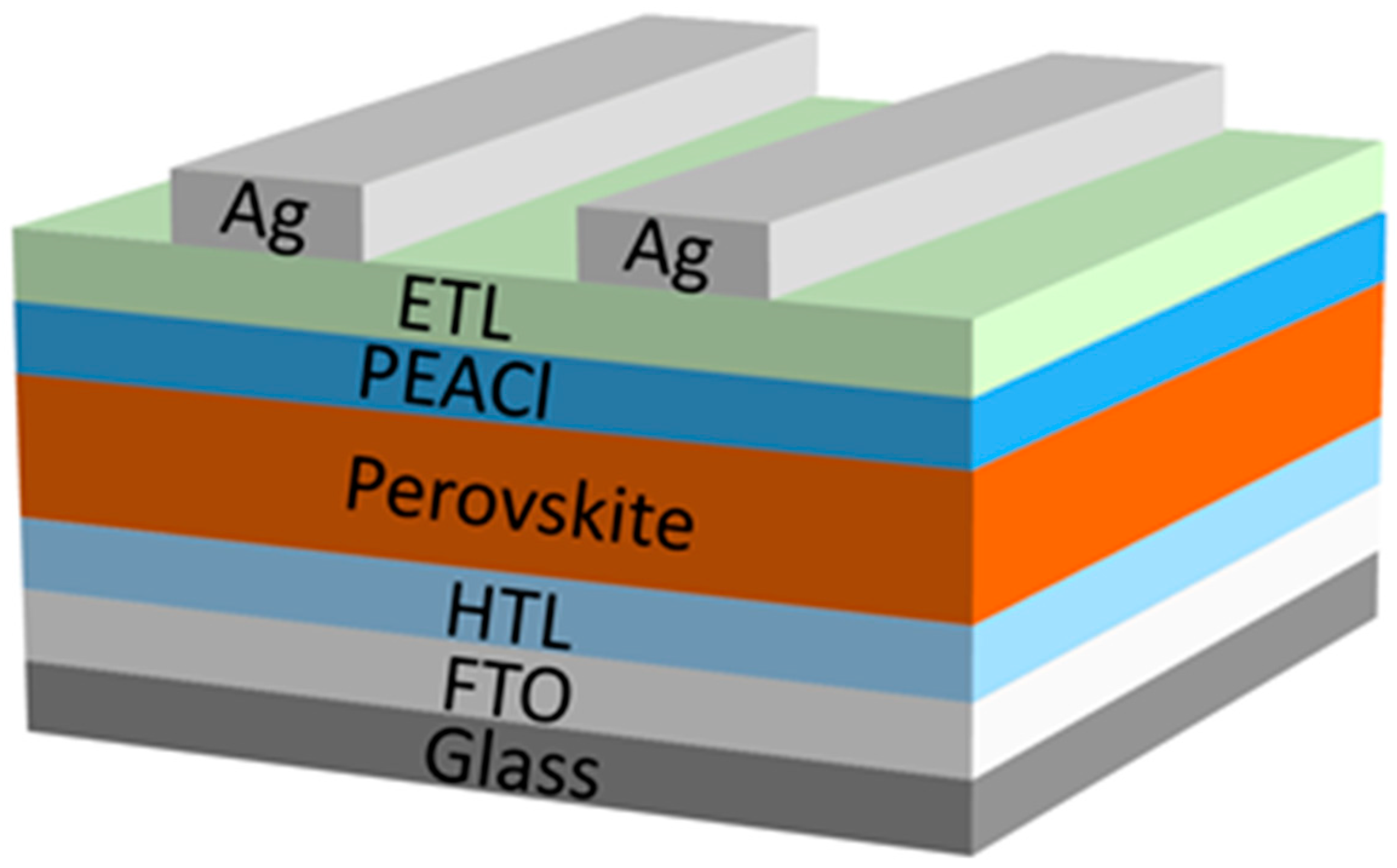
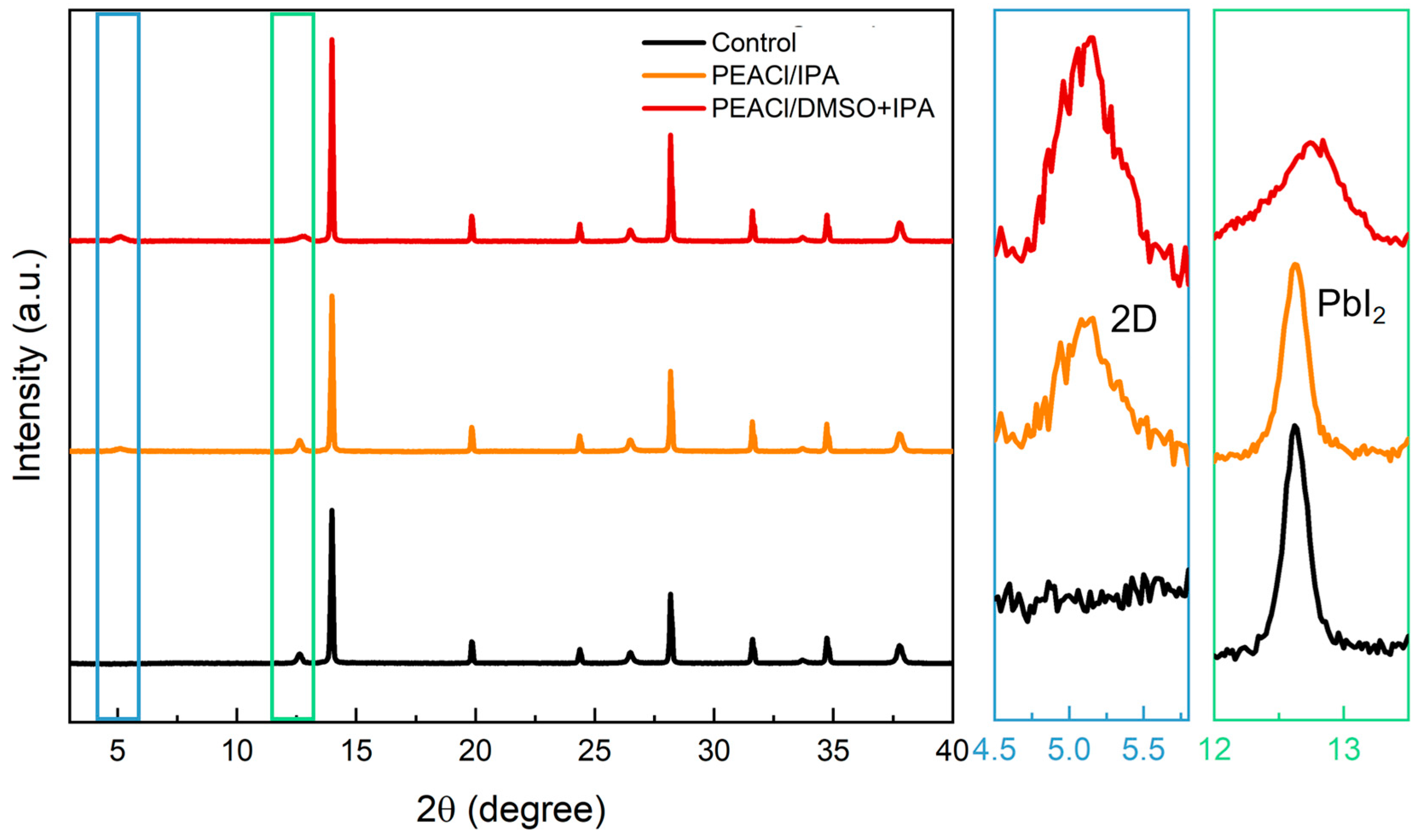
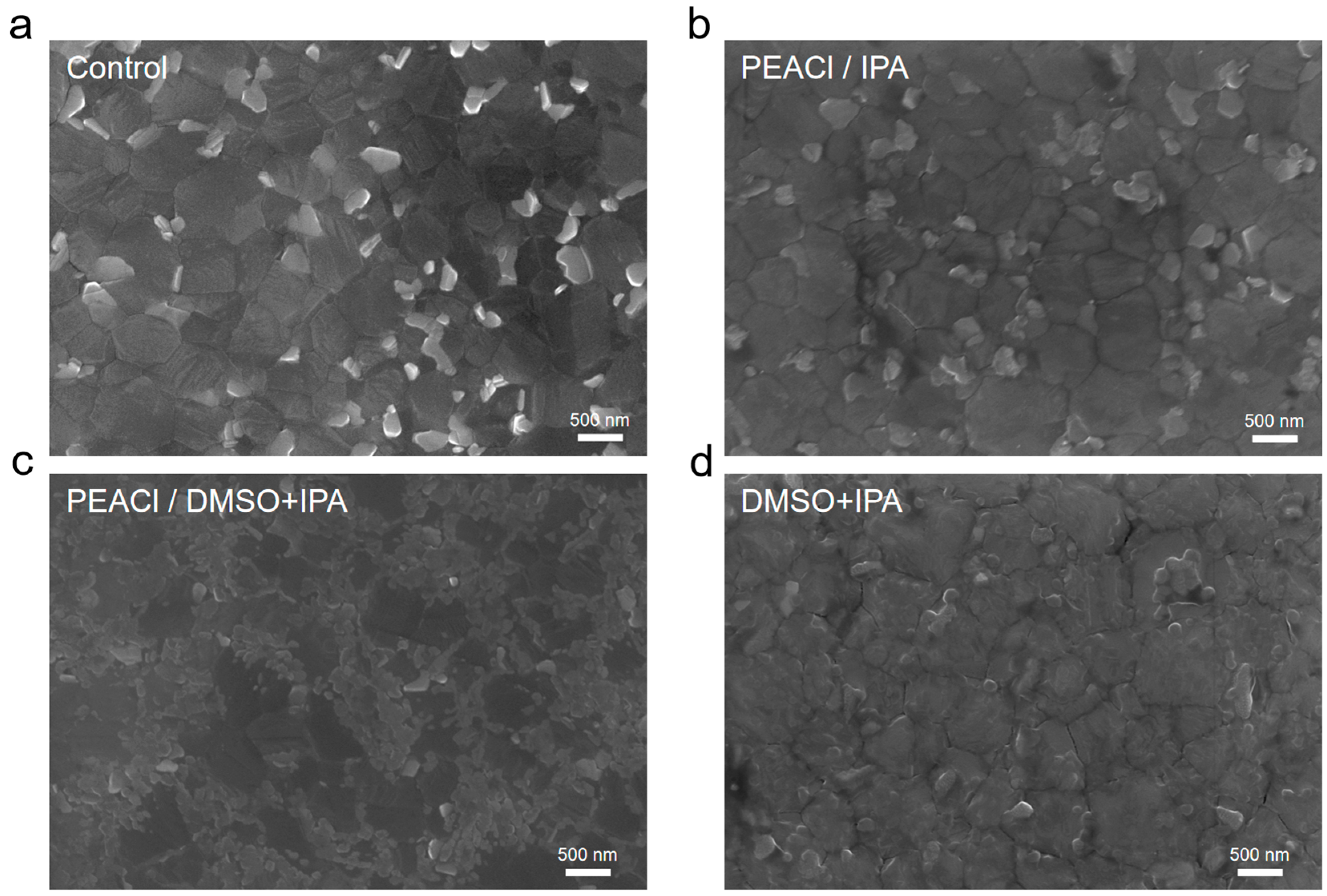
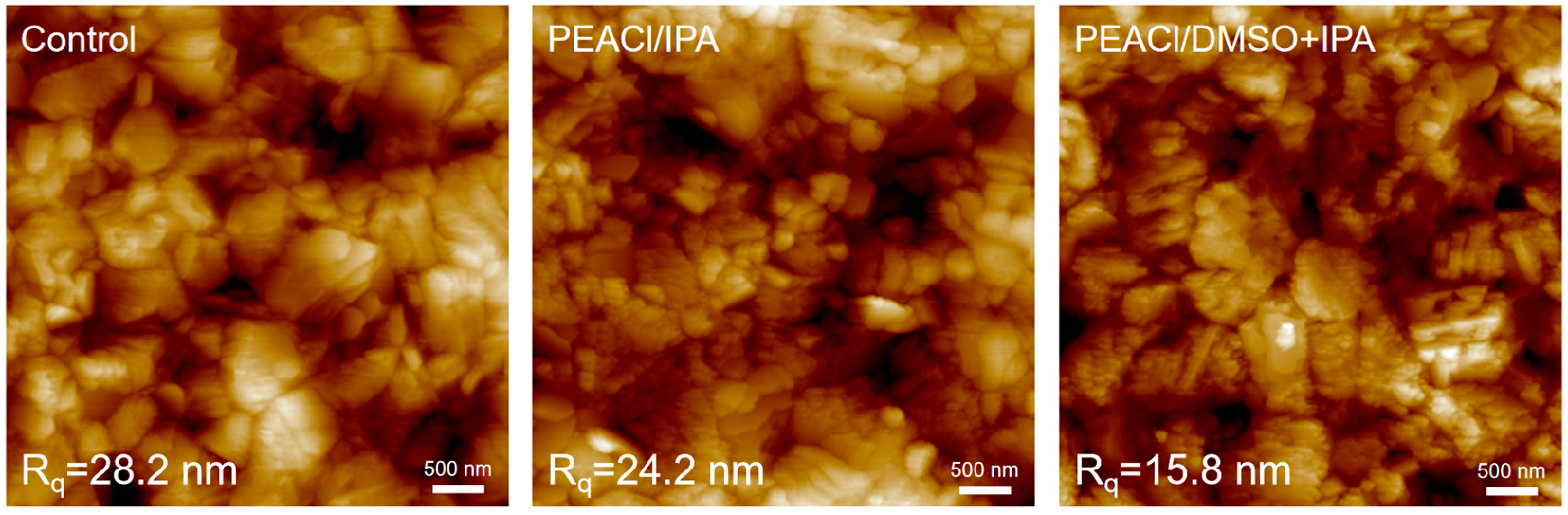
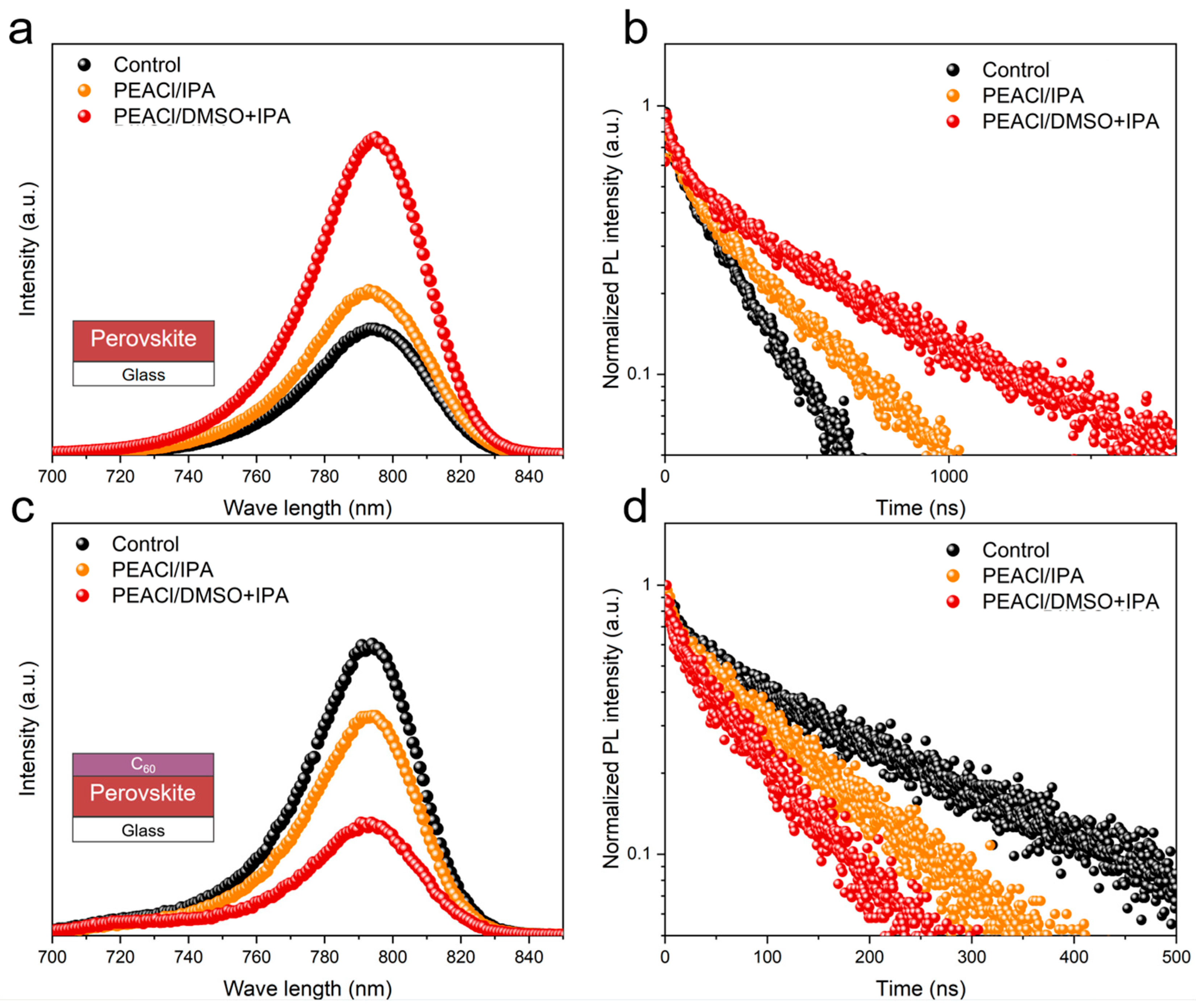
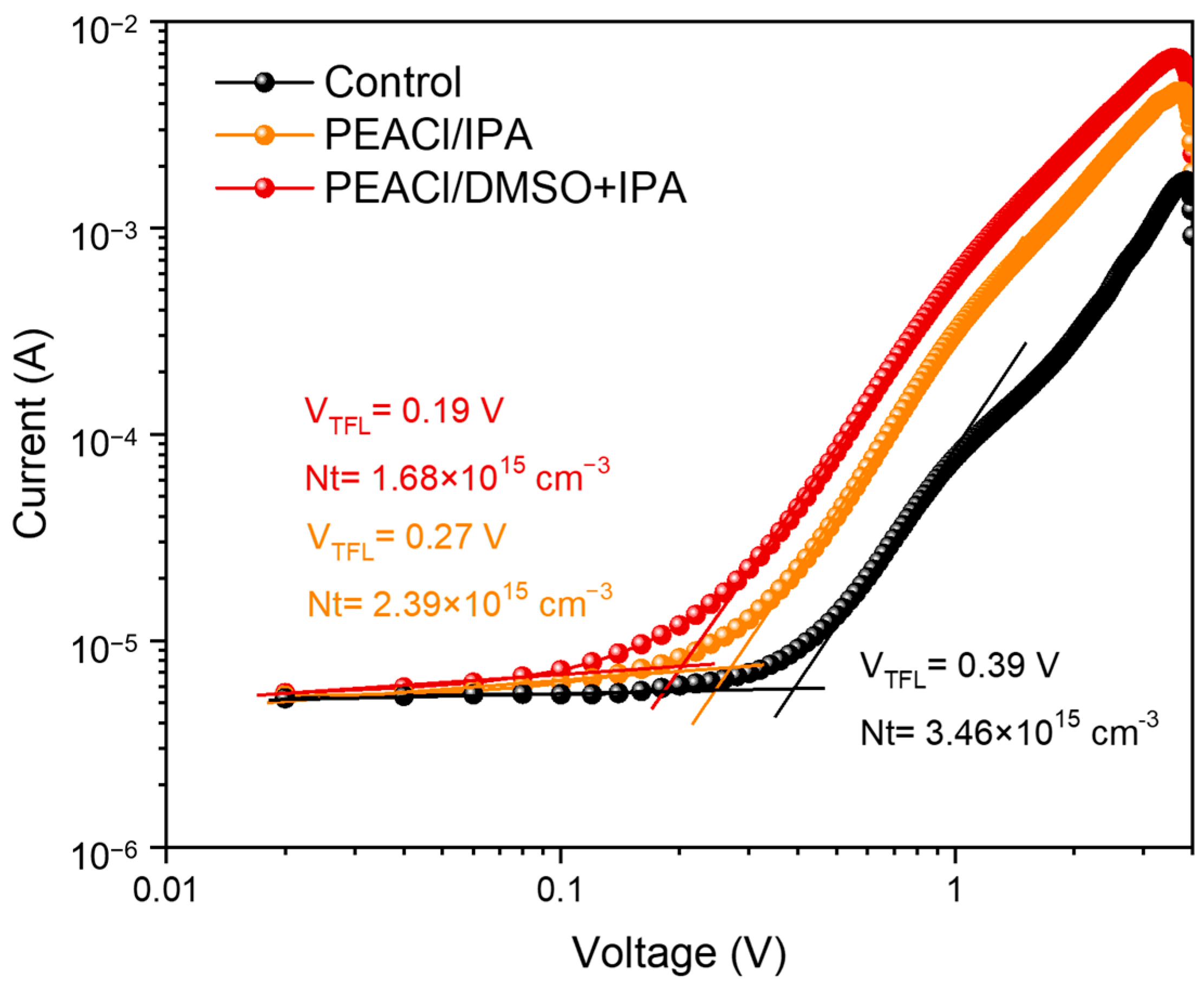
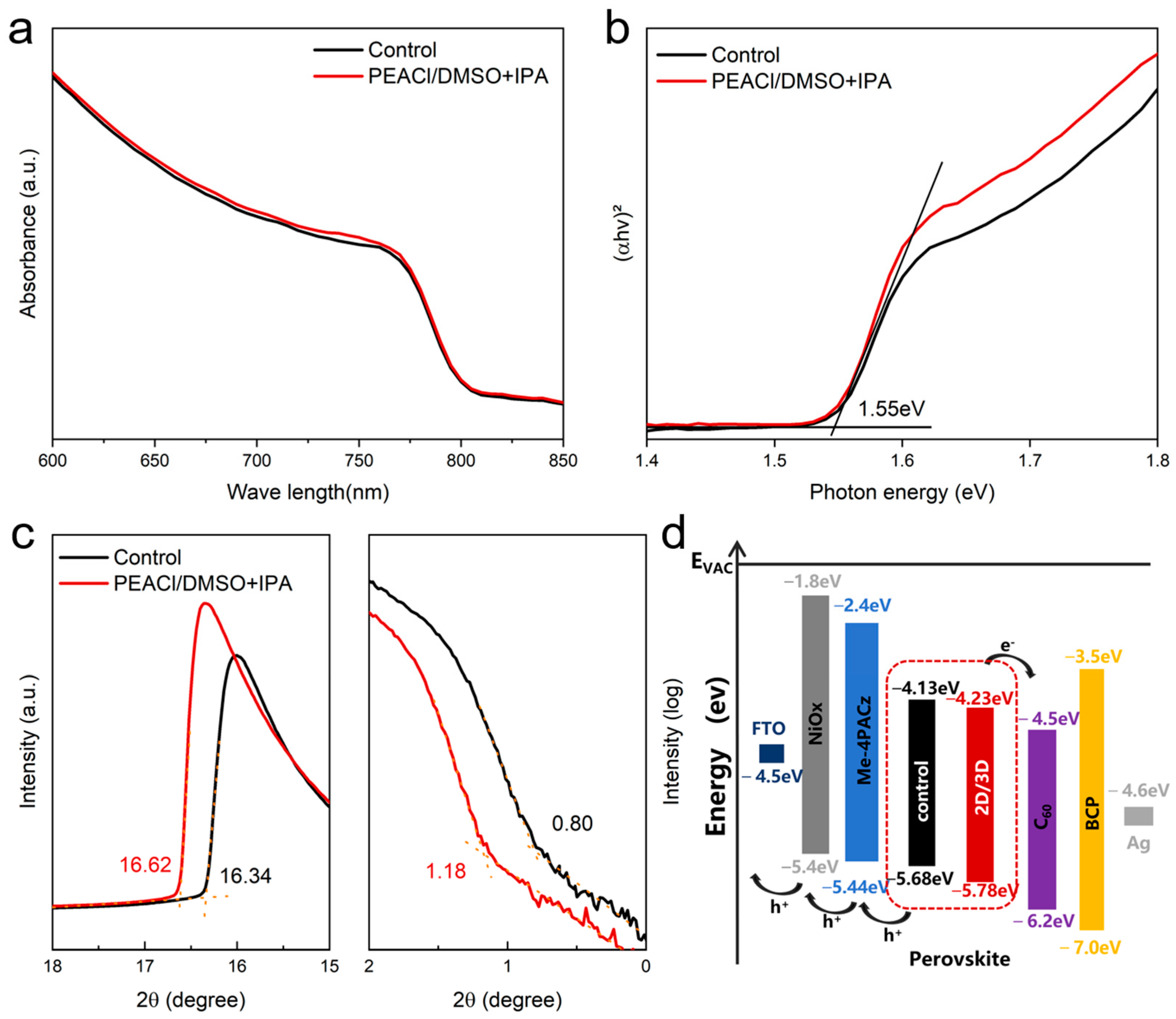
3.1. The Influence of PEACl on Perovskite Films in Different Solvents
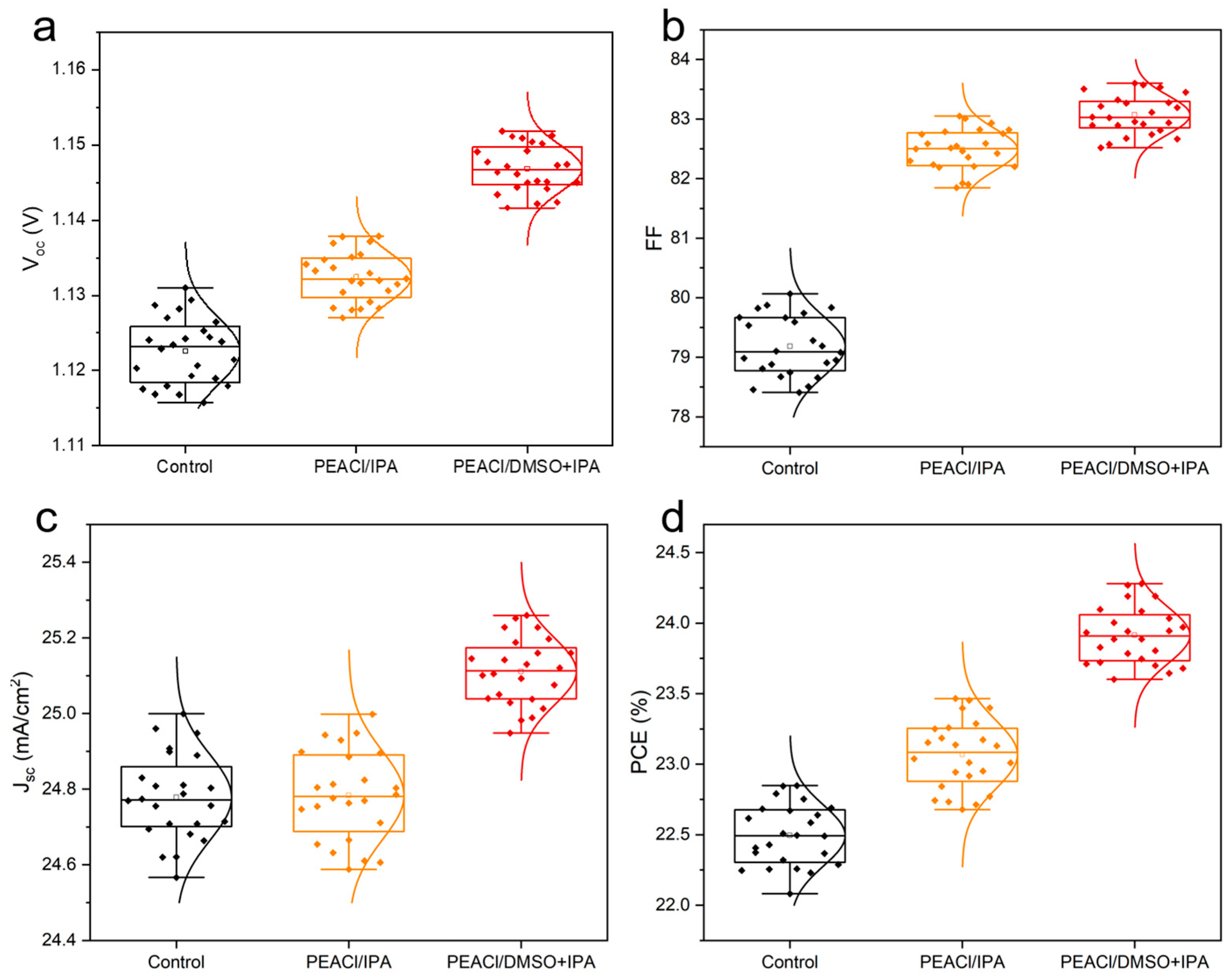
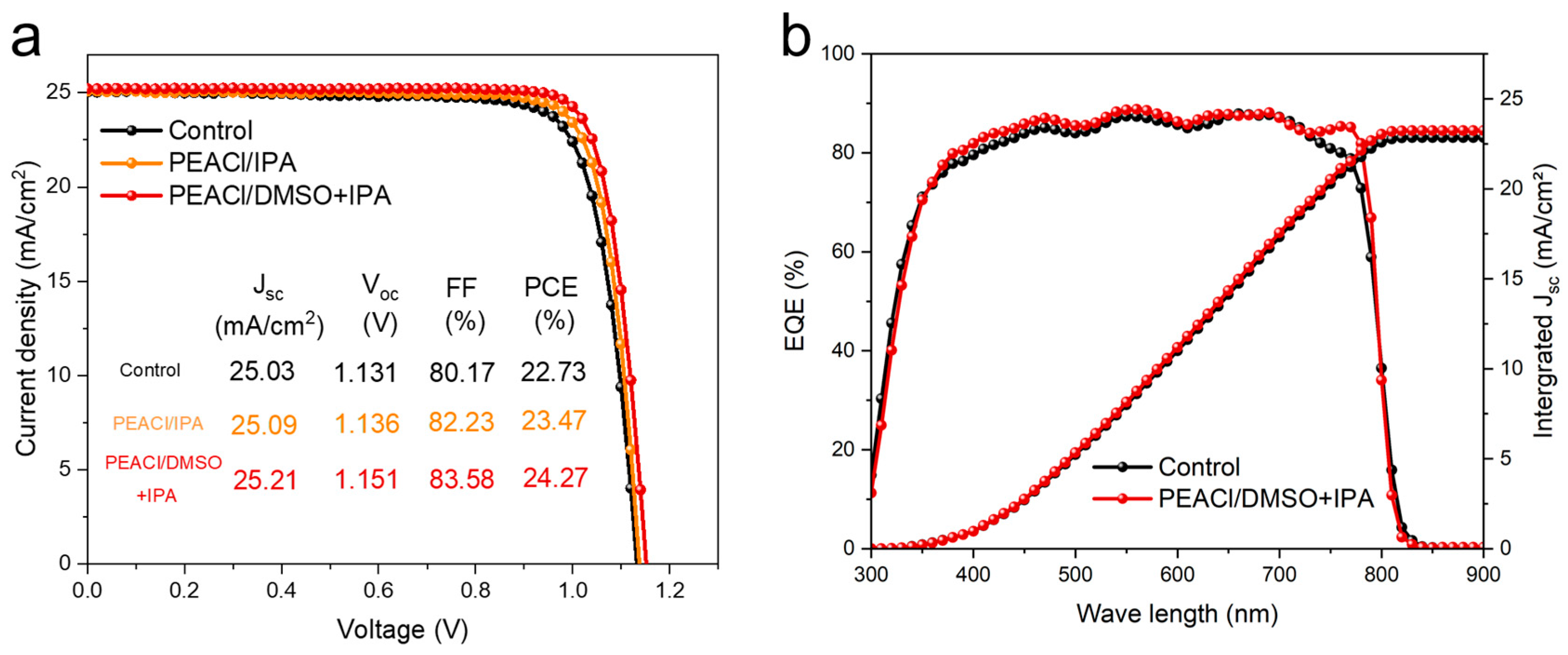
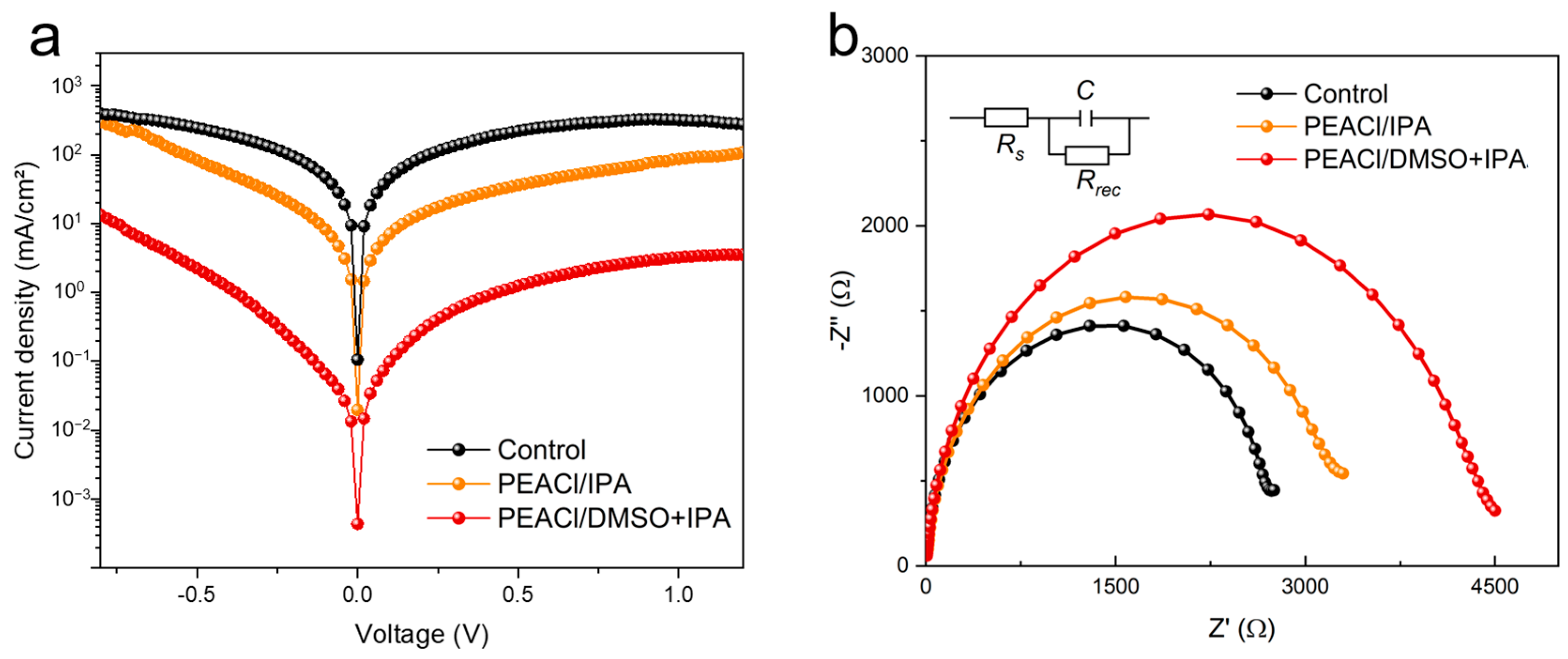
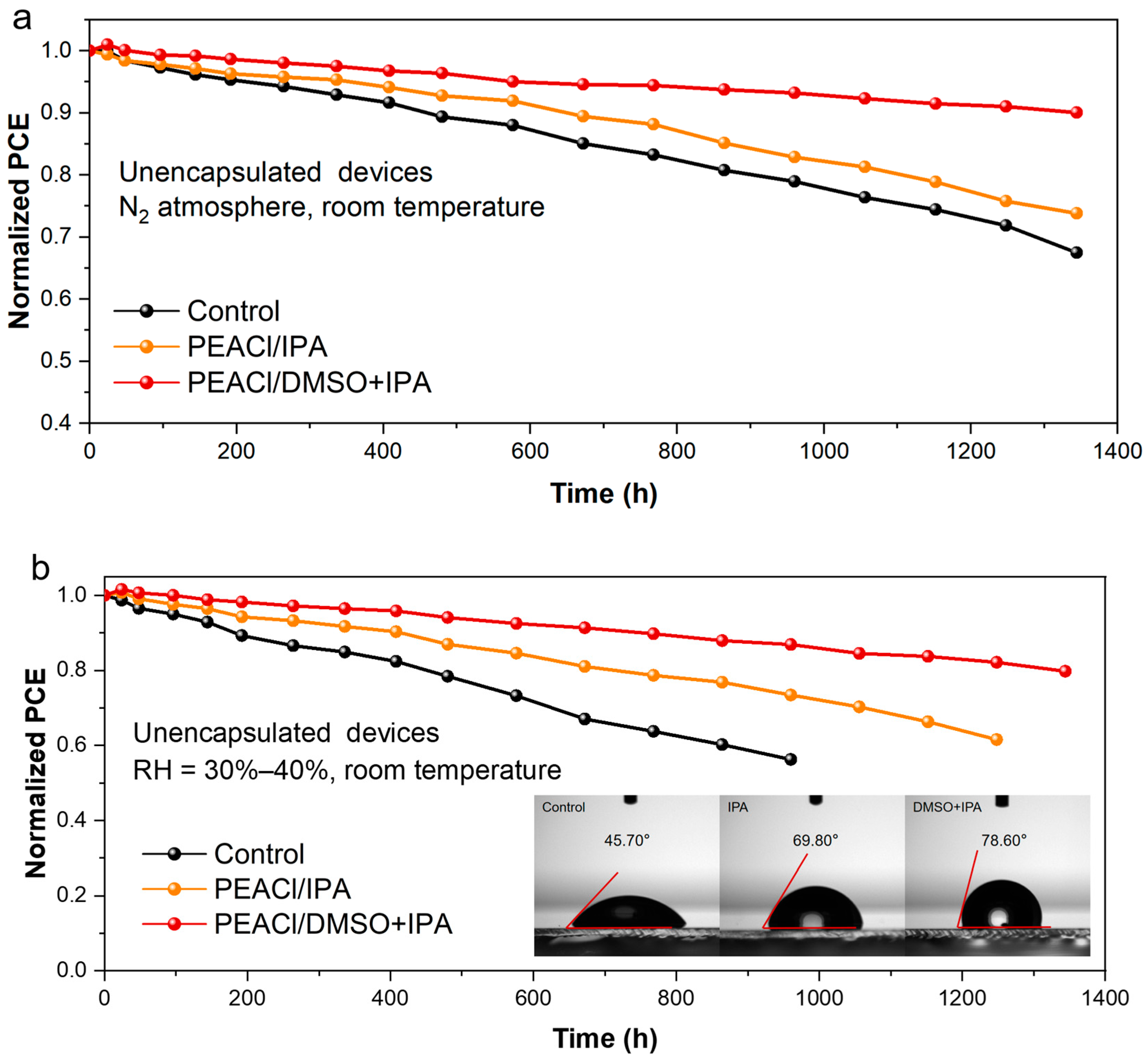
3.2. Performance of PSC Devices
4. Conclusions
- (1)
- Synergistic solvent dynamics: The DMSO: IPA (1:100) mixed solvent establishes a dynamic equilibrium, where DMSO retards the crystallization process, promotes the growth of large grains, and improves the uniformity of thin films through strong coordination, while IPA’s rapid volatilization confines 2D perovskite growth to an ultrathin, pinhole-free morphology. This dual mechanism reduces interfacial defect density by 51% compared to the control.
- (2)
- Efficiency–stability co-optimization: The optimized device achieves a PCE of 24.27% (VOC = 1.15 V, FF = 83.5%) and retains 90% of its initial efficiency after 1344 h in inert atmospheres, addressing the perennial efficiency–stability trade-off.
- (3)
- Universal design principles: The proposed “solvent coordination-volatilization kinetics” model transcends PEACl-based systems, offering a blueprint for tailoring passivation layers in diverse perovskite compositions. This model hinges on two transferable parameters: (a) coordination strength—solvents with Lewis base functionality (e.g., DMSO and dimethylformamide) can tune passivator–substrate bonding to align molecular orientation, and (b) volatilization gradient—low-boiling-point solvents (e.g., IPA and ethyl acetate) regulate phase separation kinetics, preventing excessive 2D phase growth. For instance, substituting PEA+ with bulkier ammonium cations (e.g., butylammonium) would require adjusting the DMSO/IPA ratio to balance steric effects and crystallization dynamics. Similarly, replacing Pb2+ with Sn2+ in lead-free perovskites could leverage alternative coordination motifs (e.g., Sn-S bonds with thiourea-based solvents).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hinken, D.; Rauer, M.; Hohl-Ebinger, J.; Hao, X. Solar cell efficiency tables (Version 64). Prog. Photovolt. Res. Appl. 2024, 32, 425–441. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhu, K. Rapid advances enabling high-performance inverted perovskite solar cells. Nat. Rev. Mater. 2024, 9, 399–419. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Xu, J.; Maxwell, A.; Zhou, W.; Yang, Y.; Zhou, Q.; Bati, A.S.R.; Wan, H.; Wang, Z.; et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 2024, 384, 189–193. [Google Scholar] [CrossRef]
- Wu, J.; Chen, P.; Xu, H.; Yu, M.; Li, L.; Yan, H.; Huangfu, Y.; Xiao, Y.; Yang, X.; Zhao, L.; et al. Ultralight flexible perovskite solar cells. Sci. China Mater. 2022, 65, 2319–2324. [Google Scholar] [CrossRef]
- Príncipe, J.; Duarte, V.C.M.; Andrade, L. Inverted Perovskite Solar Cells: The Emergence of a Highly Stable and Efficient Architecture. Energy Technol. 2022, 10, 2100952. [Google Scholar] [CrossRef]
- Azmi, R.; Ugur, E.; Seitkhan, A.; Aljamaan, F.; Subbiah, A.S.; Liu, J.; Harrison, G.T.; Nugraha, M.I.; Eswaran, M.K.; Babics, M.; et al. Damp heat–stable perovskite solar cells with tailored-dimensionality 2D/3D heterojunctions. Science 2022, 376, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Wu, X.; Sheppard, S.A.; Zhang, S.; Gao, D.; Long, N.J.; Zhu, Z. Organometallic-functionalized interfaces for highly efficient inverted perovskite solar cells. Science 2022, 376, 416–420. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, Y.; Li, S.; Jia, X.; Luo, D.; Zhang, W.; Snaith, H.J.; Gong, Q.; Zhu, R. The Promise and Challenges of Inverted Perovskite Solar Cells. Chem. Rev. 2024, 124, 10623–10700. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Xiao, W.; Chen, R.; Sun, Z.; Zhang, Y.; Lei, X.; Hu, S.; Kober-Czerny, M.; Wang, J.; et al. Buried interface molecular hybrid for inverted perovskite solar cells. Nature 2024, 632, 536–542. [Google Scholar] [CrossRef]
- Baumann, S.; Eperon, G.E.; Virtuani, A.; Jeangros, Q.; Kern, D.B.; Barrit, D.; Schall, J.; Nie, W.; Oreski, G.; Khenkin, M.; et al. Stability and reliability of perovskite containing solar cells and modules: Degradation mechanisms and mitigation strategies. Energy Environ. Sci. 2024, 17, 7566–7599. [Google Scholar] [CrossRef]
- Turedi, B.; Yeddu, V.; Zheng, X.; Kim, D.Y.; Bakr, O.M.; Saidaminov, M.I. Perovskite Single-Crystal Solar Cells: Going Forward. ACS Energy Lett. 2021, 6, 631–642. [Google Scholar] [CrossRef]
- Wang, J.; Bi, L.; Fu, Q.; Jen, A.K.Y. Methods for Passivating Defects of Perovskite for Inverted Perovskite Solar Cells and Modules. Adv. Energy Mater. 2024, 14, 2401414. [Google Scholar] [CrossRef]
- Chowdhury, T.A.; Bin Zafar, M.A.; Sajjad-Ul Islam, M.; Shahinuzzaman, M.; Islam, M.A.; Khandaker, M.U. Stability of perovskite solar cells: Issues and prospects. RSC Adv. 2023, 13, 1787–1810. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Shi, J.; Chen, Y.; Miao, Y.; Zhao, Y. Cs-content-dependent organic cation exchange in FA1-xCsxPbI3 perovskite. J. Energy Chem. 2022, 72, 539–544. [Google Scholar] [CrossRef]
- Wang, M.; Shi, Z.; Fei, C.; Deng, Z.J.D.; Yang, G.; Dunfield, S.P.; Fenning, D.P.; Huang, J. Ammonium cations with high pKa in perovskite solar cells for improved high-temperature photostability. Nat. Energy 2023, 8, 1229–1239. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Wang, S.; Lyu, M.; Yun, J.-H.; Wang, L. Perovskite Solar Cells: In Situ Growth of 2D Perovskite Capping Layer for Stable and Efficient Perovskite Solar Cells (Adv. Funct. Mater. 17/2018). Adv. Funct. Mater. 2018, 28, 1870113. [Google Scholar] [CrossRef]
- Cho, K.T.; Grancini, G.; Lee, Y.; Oveisi, E.; Ryu, J.; Almora, O.; Tschumi, M.; Schouwink, P.A.; Seo, G.; Heo, S.; et al. Selective growth of layered perovskites for stable and efficient photovoltaics. Energy Environ. Sci. 2018, 11, 952–959. [Google Scholar] [CrossRef]
- Gharibzadeh, S.; Fassl, P.; Hossain, I.M.; Rohrbeck, P.; Frericks, M.; Schmidt, M.; Duong, T.; Khan, M.R.; Abzieher, T.; Nejand, B.A.; et al. Two birds with one stone: Dual grain-boundary and interface passivation enables >22% efficient inverted methylammonium-free perovskite solar cells. Energy Environ. Sci. 2021, 14, 5875–5893. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Z.; Ning, Z.; Li, T.; Guo, X.; Ma, J.; Su, J.; Zhang, C.; Zhang, J.; Liu, S.; et al. Highly Efficient and Stable Planar Perovskite Solar Cells with Modulated Diffusion Passivation Toward High Power Conversion Efficiency and Ultrahigh Fill Factor. Sol. RRL 2019, 3, 1900293. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Yang, Y.; Wang, M.; Qu, S.; Lan, Z.; Jiang, T.; Wang, Z.; Du, S.; Lu, Y.; et al. A Universal Ternary Solvent System of Surface Passivator Enables Perovskite Solar Cells with Efficiency Exceeding 26%. Adv. Mater. 2024, 36, 2410390. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Kulkarni, A.; Sarkar, R.; Akel, S.; Häser, M.; Klingebiel, B.; Wuttig, M.; Wiegand, S.; Chakraborty, S.; Saliba, M.; Kirchartz, T. A Universal Strategy of Perovskite Ink-Substrate Interaction to Overcome the Poor Wettability of a Self-Assembled Monolayer for Reproducible Perovskite Solar Cells. Adv. Funct. Mater. 2023, 33, 2305812. [Google Scholar] [CrossRef]
- Liu, F.; Xu, J.; Ma, Y.; Ahn, Y.; Du, X.; Yang, E.; Xia, H.; Lee, B.R.; Hangoma, P.M.; Park, S.H. Functional design and understanding of effective additives for achieving high-quality perovskite films and passivating surface defects. J. Energy Chem. 2025, 102, 597–608. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Wang, Y.; Yang, Z.; Dong, P.; Feng, Y.; Jiang, Y.; Feng, S.-P.; Zhou, G.; Liu, J.-M.; et al. Co-Solvent Engineering Contributing to Achieve High-Performance Perovskite Solar Cells and Modules Based on Anti-Solvent Free Technology. Small 2023, 19, 2301323. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Ma, C.; Kang, M.-C.; Lee, S.-H.; Kwon, S.J.; Cha, H.-W.; Yang, C.-W.; Park, N.-G. Photovoltaically top-performing perovskite crystal facets. Joule 2022, 6, 2626–2643. [Google Scholar] [CrossRef]
- Sui, Y.; Zhou, W.; Khan, D.; Wang, S.; Zhang, T.; Yu, G.; Huang, Y.; Yang, X.; Chang, K.; He, Y.; et al. Understanding the Role of Crown Ether Functionalization in Inverted Perovskite Solar Cells. ACS Energy Lett. 2024, 9, 1518–1526. [Google Scholar] [CrossRef]
- Liang, J.; Hu, X.; Wang, C.; Liang, C.; Chen, C.; Xiao, M.; Li, J.; Tao, C.; Xing, G.; Yu, R. Origins and influences of metallic lead in perovskite solar cells. Joule 2022, 6, 816–833. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing non-radiative recombination losses in perovskite solar cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Herz, L.M. Charge-Carrier Dynamics in Organic-Inorganic Metal Halide Perovskites. Annu. Rev. Phys. Chem. 2016, 67, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wu, M.; Liu, Z.; Yang, S.; Li, Y.; Wang, J.; Yang, L.; Han, Y.; Liu, S. Orientation Engineering via 2D Seeding for Stable 24.83% Efficiency Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2204260. [Google Scholar] [CrossRef]
- Le Corre, V.M.; Duijnstee, E.A.; El Tambouli, O.; Ball, J.M.; Snaith, H.J.; Lim, J.; Koster, L.J.A. Revealing Charge Carrier Mobility and Defect Densities in Metal Halide Perovskites via Space-Charge-Limited Current Measurements. ACS Energy Lett. 2021, 6, 1087–1094. [Google Scholar] [CrossRef]
- Xu, G.; Muhammad, I.; Zhang, Y.; Zheng, X.; Xin, M.; Gao, H.; Li, J.; Liu, C.; Chen, W.; Tang, J.; et al. Amidinopyridine Ion Docking in Crown Ether Cavity to Modulate the Top Interface in Inverted Perovskite Solar Cells. Adv. Energy Mater. 2025, 513, 2405088. [Google Scholar] [CrossRef]
- Xu, G.; Muhammad, I.; Khan, D.; Zheng, X.; Zhang, Y.; Xin, M.; Gao, H.; Li, J.; Wang, J.; Lu, D.; et al. Exploring the role of dipole moment at the upper interface of inverted perovskite solar cells through a bi-molecular host–guest strategy. Chem. Eng. J. 2025, 511, 161865. [Google Scholar] [CrossRef]
- Zarazua, I.; Han, G.; Boix, P.P.; Mhaisalkar, S.; Fabregat-Santiago, F.; Mora-Seró, I.; Bisquert, J.; Garcia-Belmonte, G. Surface Recombination and Collection Efficiency in Perovskite Solar Cells from Impedance Analysis. J. Phys. Chem. Lett. 2016, 7, 5105–5113. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, M.; Ghani, I.; Zhang, Y.; Gao, H.; Khan, D.; Yang, X.; Tang, Z. Solvent-Engineered PEACl Passivation: A Pathway to 24.27% Efficiency and Industrially Scalable Perovskite Solar Cells. Nanomaterials 2025, 15, 699. https://doi.org/10.3390/nano15090699
Xin M, Ghani I, Zhang Y, Gao H, Khan D, Yang X, Tang Z. Solvent-Engineered PEACl Passivation: A Pathway to 24.27% Efficiency and Industrially Scalable Perovskite Solar Cells. Nanomaterials. 2025; 15(9):699. https://doi.org/10.3390/nano15090699
Chicago/Turabian StyleXin, Min, Ihtesham Ghani, Yu Zhang, Huaxi Gao, Danish Khan, Xin Yang, and Zeguo Tang. 2025. "Solvent-Engineered PEACl Passivation: A Pathway to 24.27% Efficiency and Industrially Scalable Perovskite Solar Cells" Nanomaterials 15, no. 9: 699. https://doi.org/10.3390/nano15090699
APA StyleXin, M., Ghani, I., Zhang, Y., Gao, H., Khan, D., Yang, X., & Tang, Z. (2025). Solvent-Engineered PEACl Passivation: A Pathway to 24.27% Efficiency and Industrially Scalable Perovskite Solar Cells. Nanomaterials, 15(9), 699. https://doi.org/10.3390/nano15090699







