Boron Phosphide: A Comprehensive Overview of Structures, Properties, Synthesis, and Functional Applications
Abstract
1. Introduction

2. Structures of Boron Phosphide
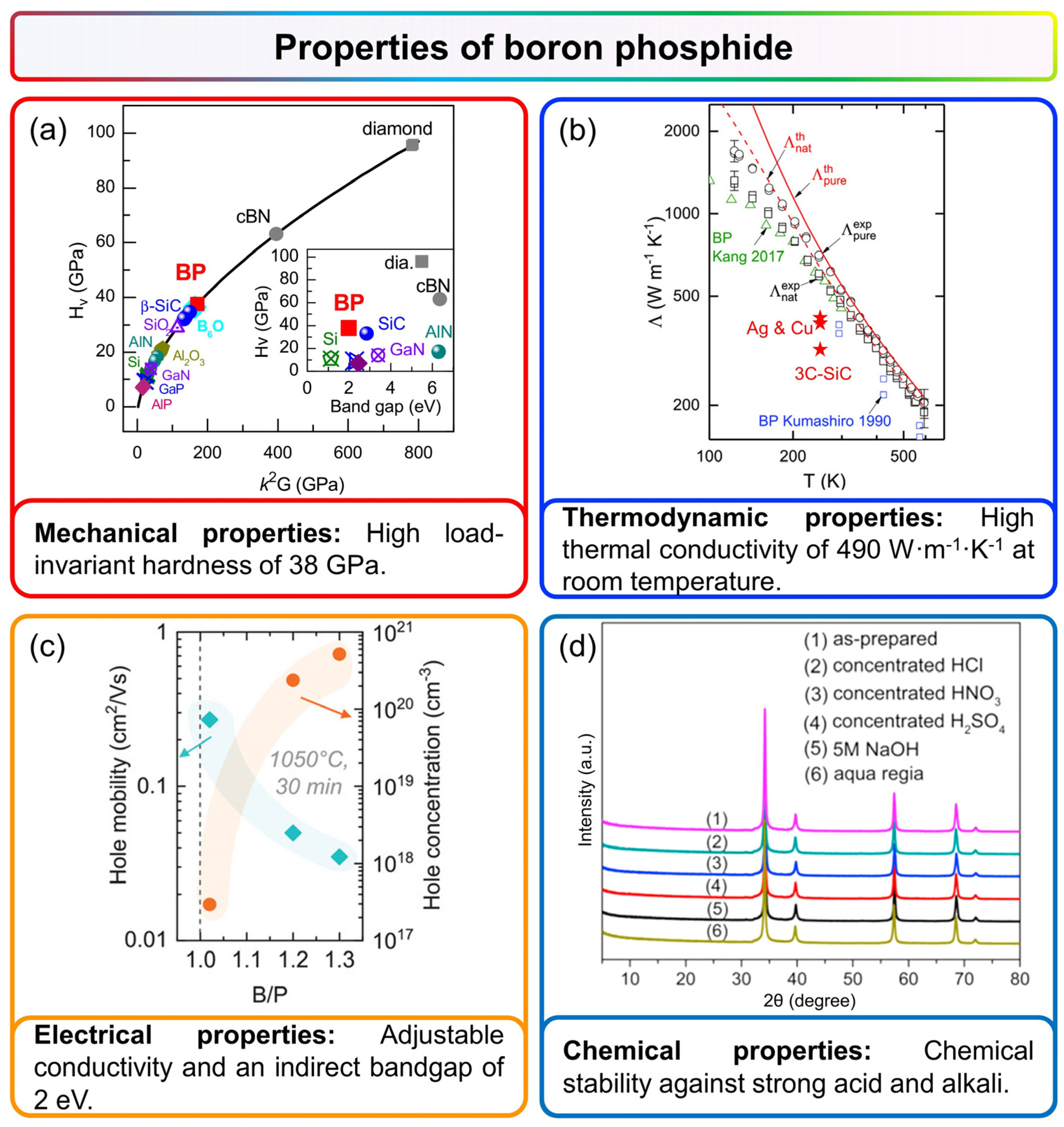
3. Properties of Boron Phosphide
3.1. Mechanical Properties
3.2. Thermodynamic Properties
3.3. Electrical Properties
3.4. Chemical Stability
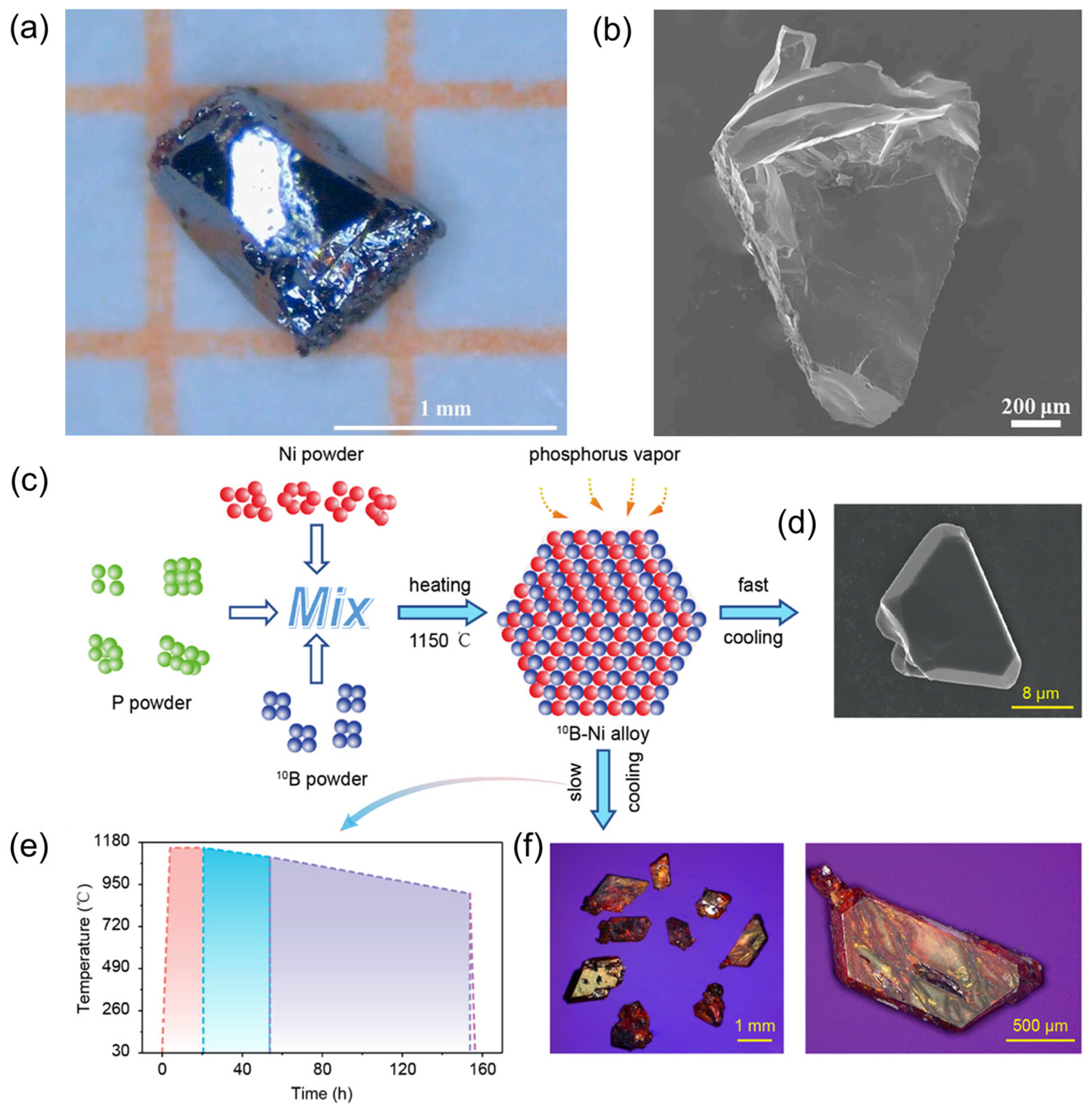
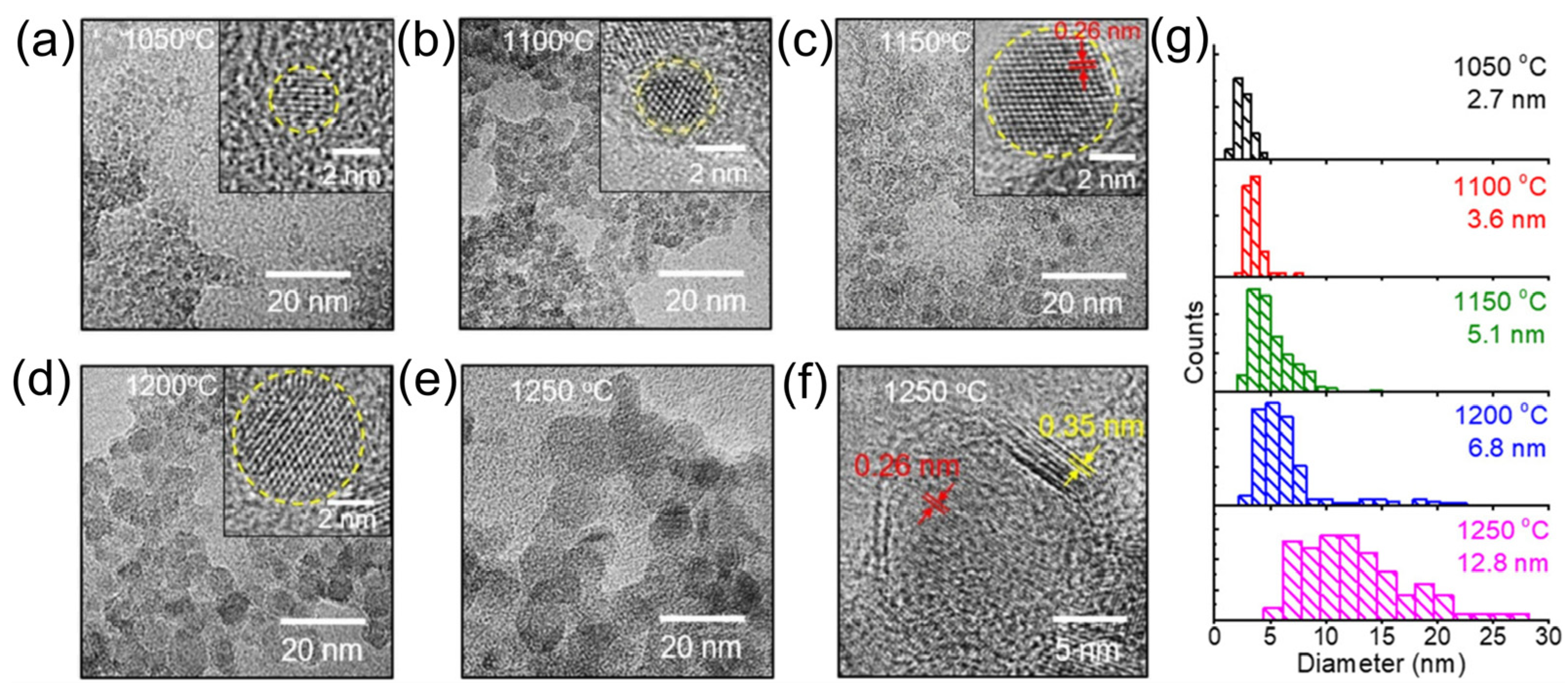
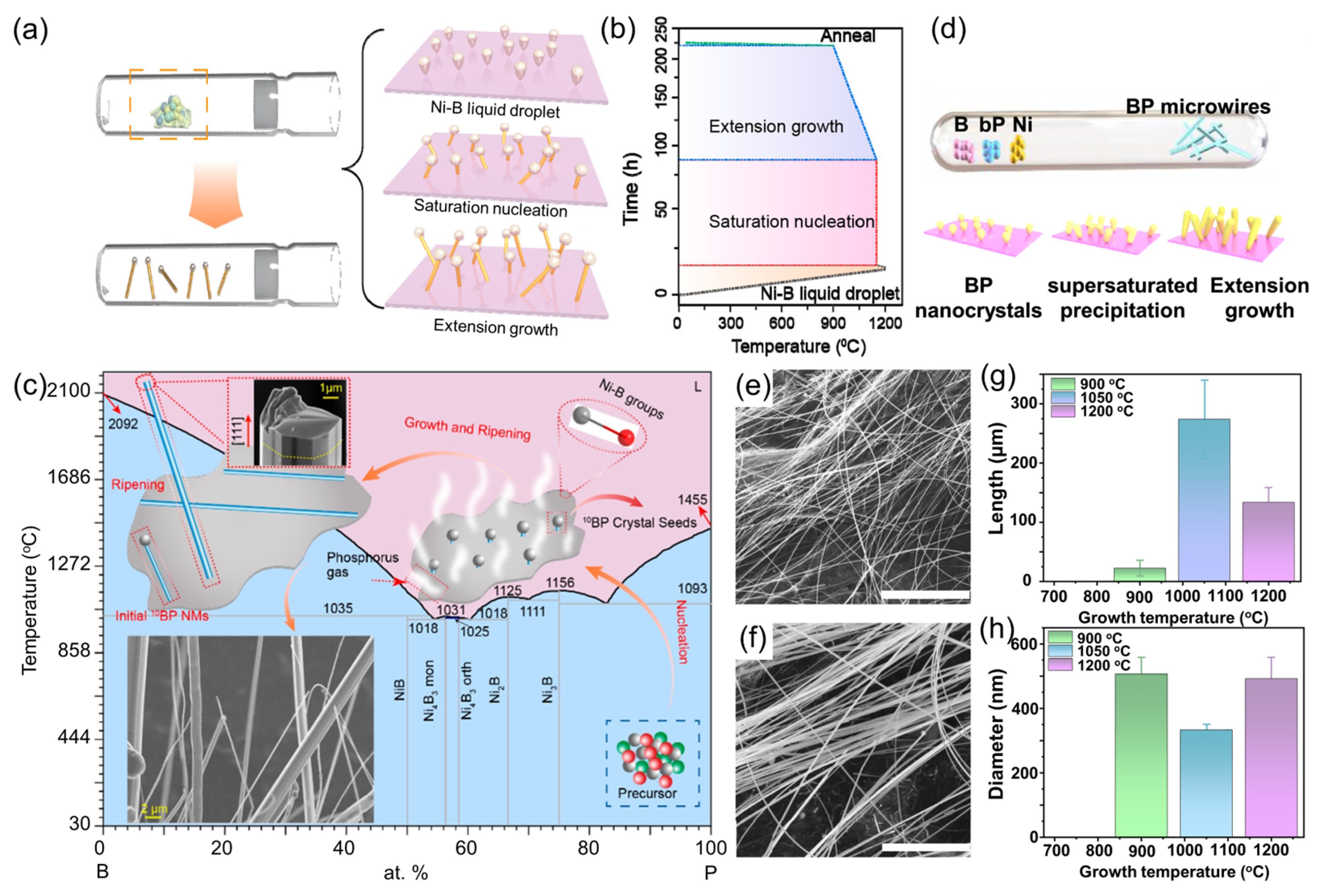
4. Synthesis of Boron Phosphide
4.1. Bulk Boron Phosphide
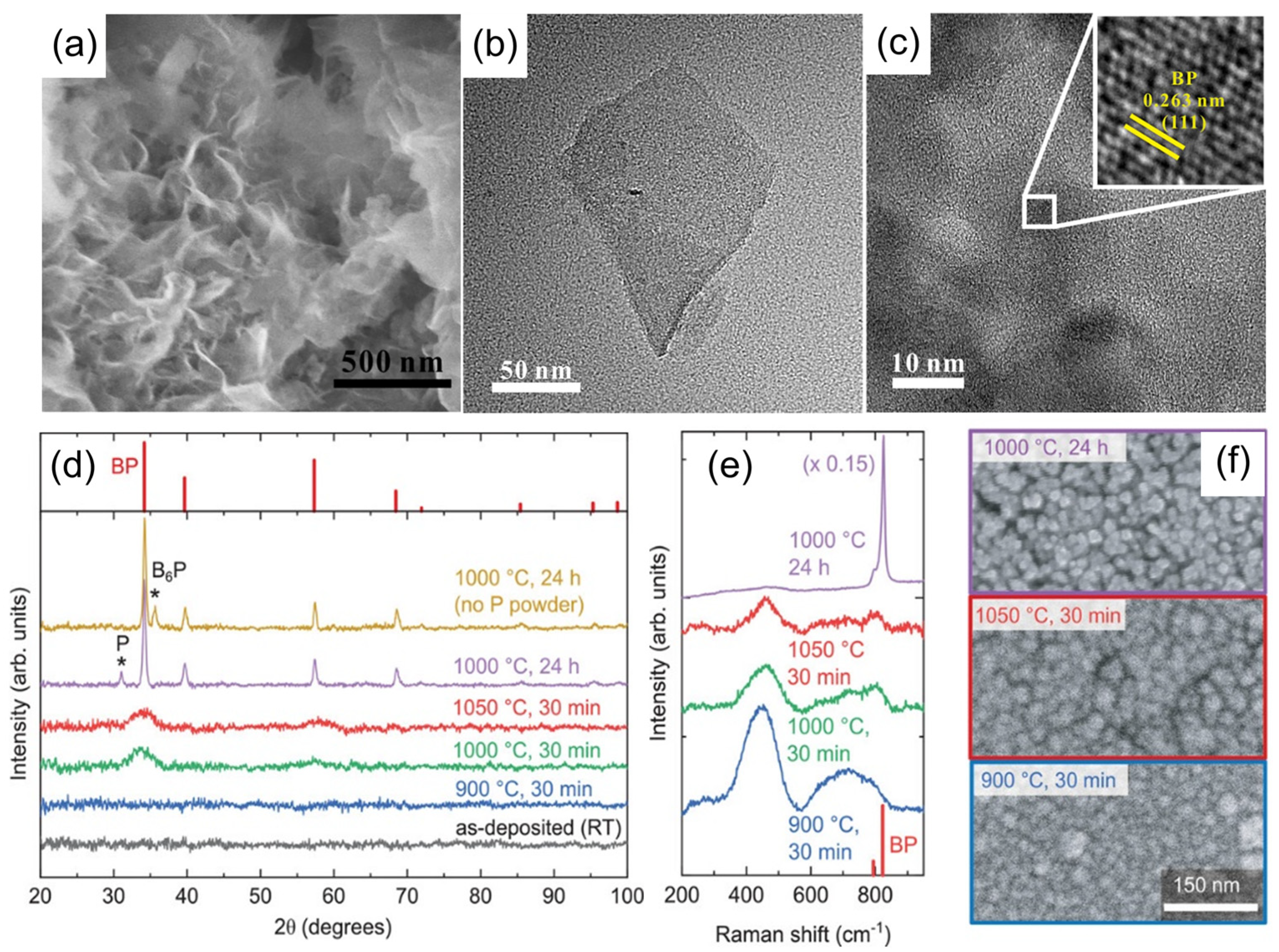
4.2. Boron Phosphide Nanoparticles
4.3. Boron Phosphide Nanowires
4.4. Boron Phosphide Films
5. Applications of Boron Phosphide
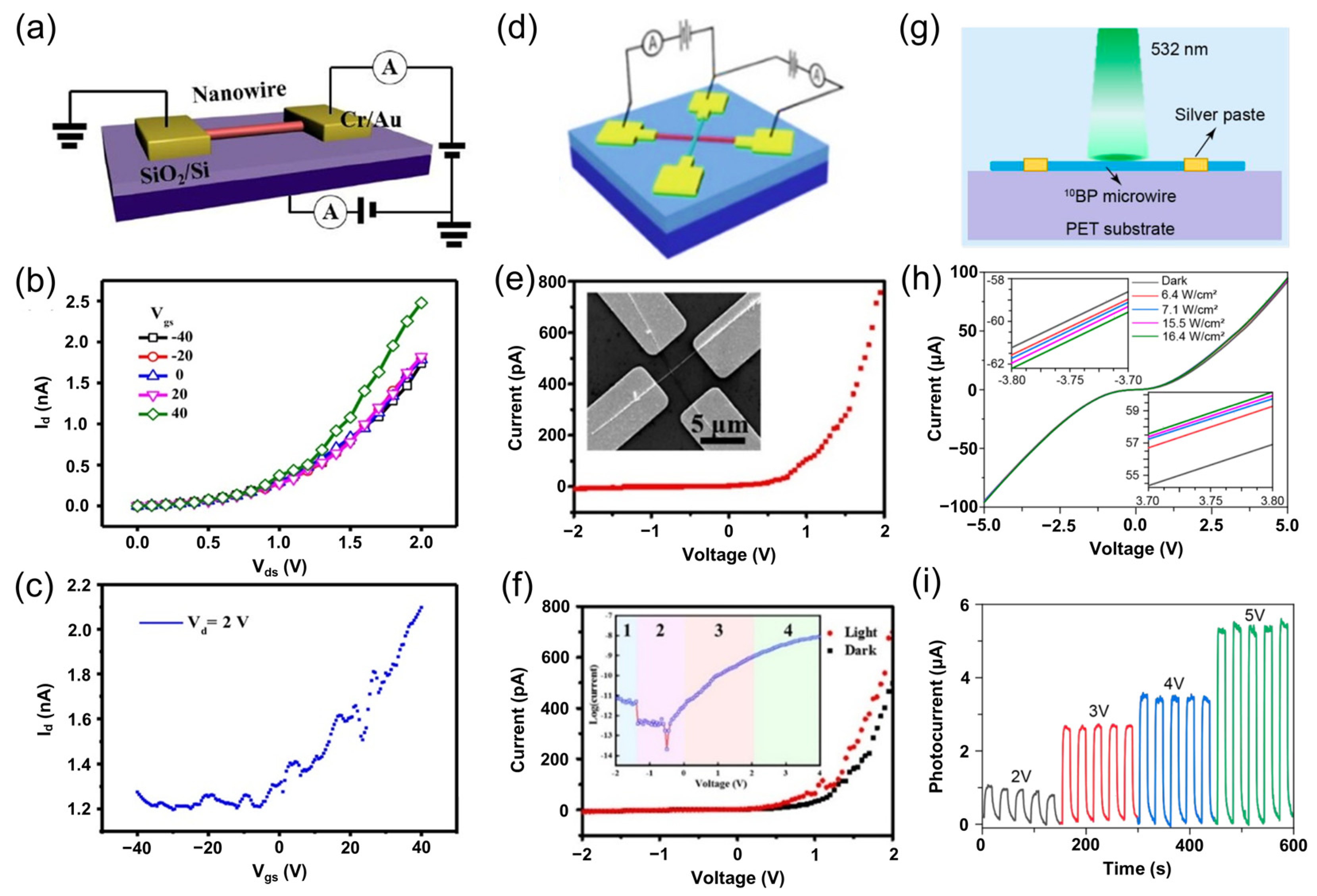
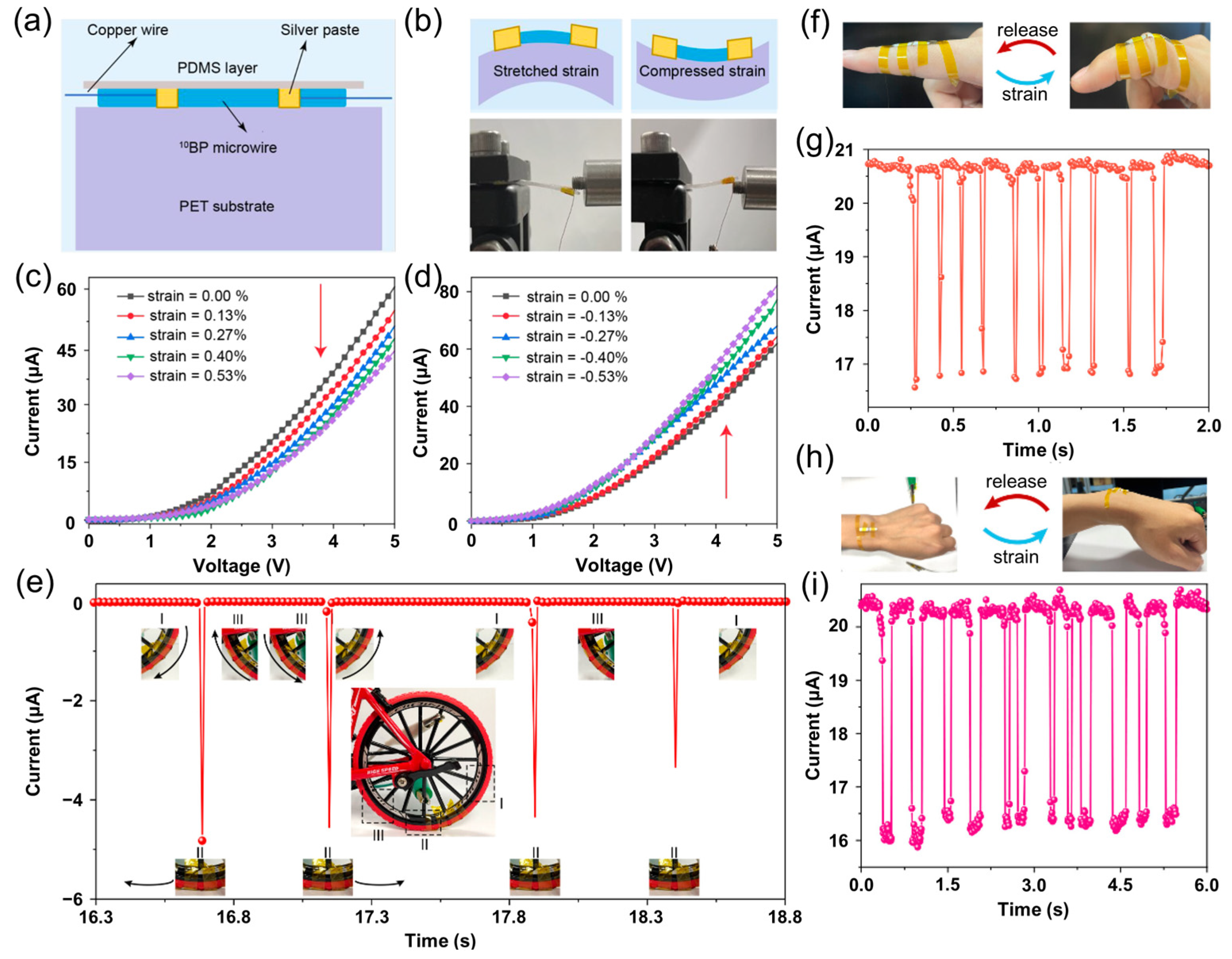
5.1. Boron Phosphide for Photodetectors and Sensors
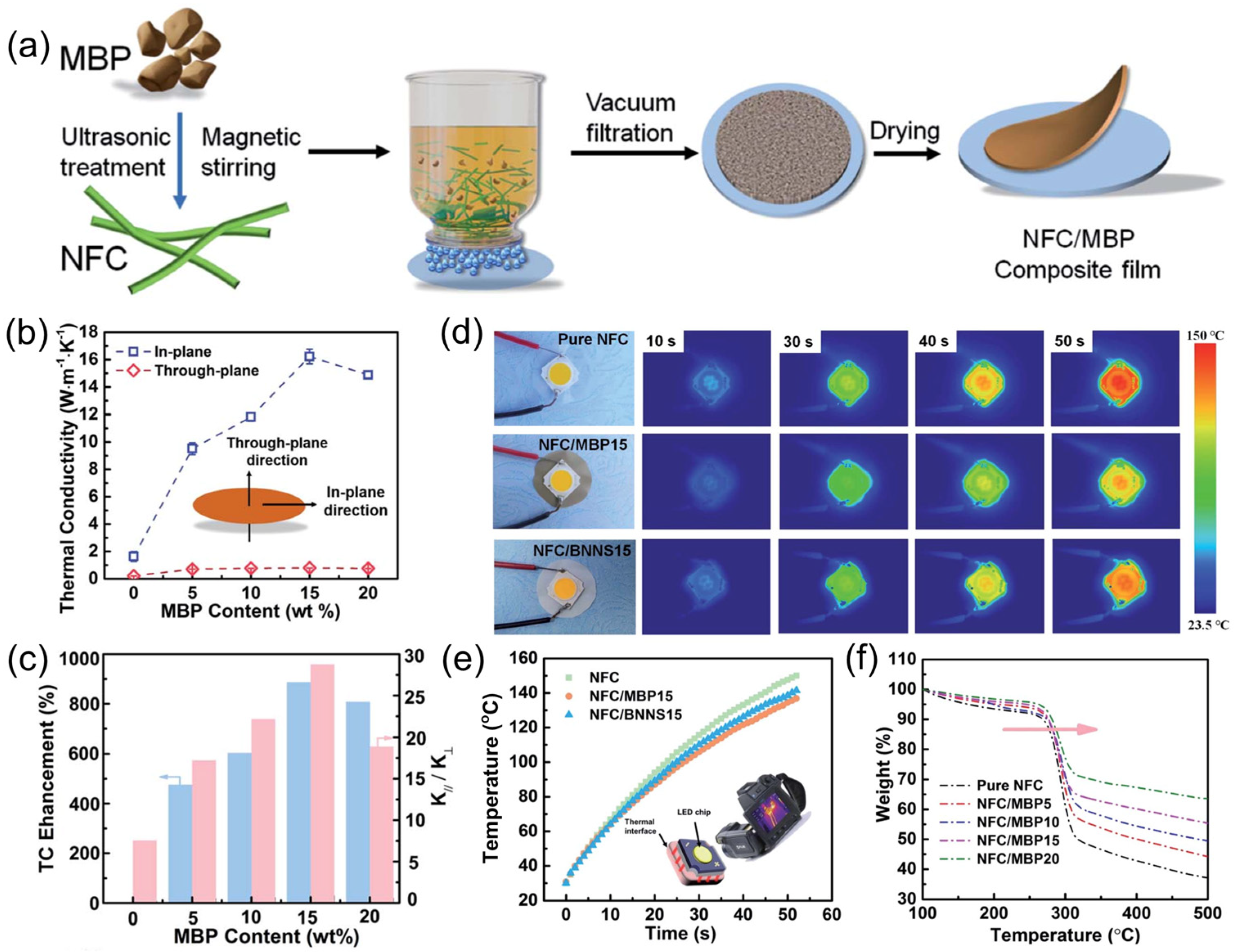
5.2. Boron Phosphide for Thermal Management
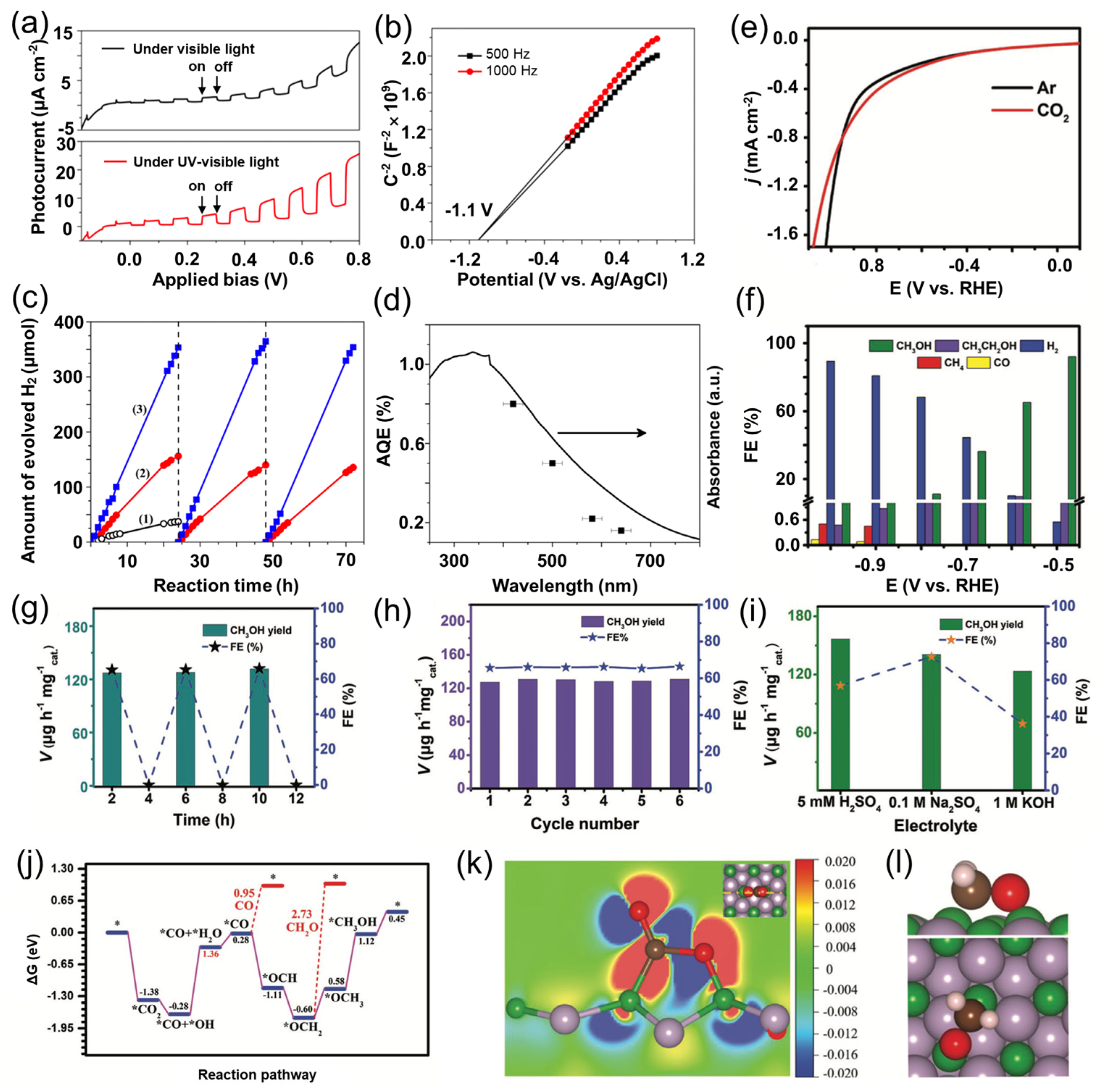
5.3. Boron Phosphide for Energy Conversion
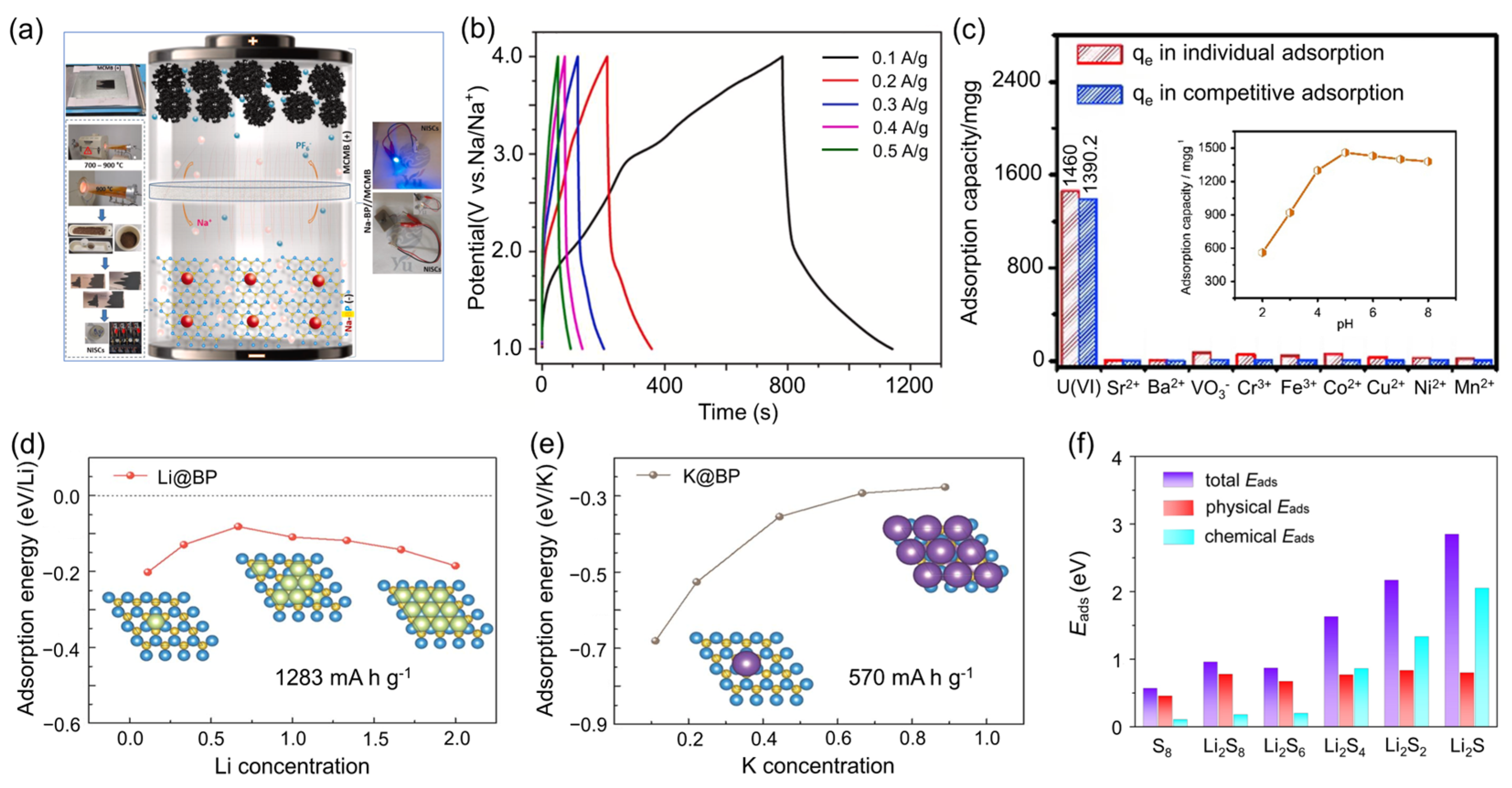
5.4. Boron Phosphide for Energy Storage
6. Summary and Outlook
6.1. Summary
- Progress in synthesis and methodological innovations
- 2.
- Applications of boron phosphide in various fields
6.2. Future Challenges and Outlook
- Synthesis and scalability challenges
- 2.
- Theoretical and computational frontiers
- 3.
- Material performance and application gaps
- 4.
- Interdisciplinary opportunities inspired by 2D Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Boron phosphide |
| CVD | Chemical vapor deposition |
| h-BN | Hexagonal boron nitride |
| 2D | Two-dimensional |
| c-BP | Cubic boron phosphide |
| h-BP | Hexagonal boron phosphide |
| TG-DTA | Thermal gravimetry/differential thermal analysis |
| TDTR | Time-domain thermoreflectance |
| c-BN | Cubic boron nitride |
| DFT | Density functional theory |
| BAs | Boron arsenide |
| PL | Photoluminescence |
| HCl | Hydrochloric acid |
| H2SO4 | Sulfuric acid |
| HNO3 | Nitric acid |
| NCs | Nanocrystals |
| BPSG | Borophosphosilicate glass |
| 1D | One-dimensional |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| EDX | Energy-dispersive X-ray |
| XRD | X-ray diffraction |
| VLS | Vapor–liquid–solid |
| VS | Vapor–solid |
| FETs | Field-effect transistors |
| PDMS | Polydimenthylsiloxane |
| PI | Polyimide |
| MBP | Milled boron phosphide |
| NFC | Nanofibrillated cellulose |
| BNNS | boron nitride nanosheets |
| 3D | Three-dimensional |
| HER | Hydrogen evolution reactions |
| M-S | Mott–Schottky |
| AQE | Apparent quantum efficiency |
| CO2RR | CO2 reduction reaction |
| UV-Vis | Ultraviolet–visible |
| NISCs | Sodium-ion hybrid supercapacitors |
| MCMB | Mesoporous carbon microspheres |
| CDI | Capacitive deionization |
| VI | Uranium |
References
- Sergeeva, A.P.; Popov, I.A.; Piazza, Z.A.; Li, W.L.; Romanescu, C.; Wang, L.S.; Boldyrev, A.I. Understanding boron through size-selected clusters: Structure, chemical bonding, and fluxionality. Acc. Chem. Res. 2014, 47, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Piazza, Z.A.; Hu, H.S.; Li, W.L.; Zhao, Y.F.; Li, J.; Wang, L.S. Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat. Commun. 2014, 5, 3113. [Google Scholar] [CrossRef]
- Chen, T.A.; Chuu, C.P.; Tseng, C.C.; Wen, C.K.; Wong, H.S.P.; Pan, S.; Li, R.; Chao, T.A.; Chueh, W.C.; Zhang, Y.; et al. Wafer-scale single-crystal hexagonal boron nitride monolayers on Cu (111). Nature 2020, 579, 219–223. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, properties and applications of two-dimensional hexagonal boron nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef] [PubMed]
- Tai, G.; Hu, T.; Zhou, Y.; Wang, X.; Kong, J.; Zeng, T.; You, Y.; Wang, Q. Synthesis of atomically thin boron films on copper foils. Angew. Chem. Int. Ed. 2015, 54, 15473–15477. [Google Scholar] [CrossRef] [PubMed]
- Mannix, A.J.; Zhou, X.F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.; Liu, Y.; Wu, Z.; Liang, X.; Liu, X. Borophene-based materials for energy, sensors and information storage applications. Nano Res. Energy 2023, 2, e9120051. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.; Hao, J.; Sheng, L.; Liu, B.; Wu, Z. Ultrastable crystalline semiconducting hydrogenated borophene. Angew. Chem. Int. Ed. 2020, 59, 10819–10825. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.; Wu, Z.; Hao, J. Borophene: Current status, challenges and opportunities. ChemPluschem 2020, 85, 2186–2196. [Google Scholar] [CrossRef]
- Tian, H.; Wang, J.; Lai, G.; Dou, Y.; Gao, J.; Duan, Z.; Feng, X.; Wu, Q.; He, X.; Yao, L.; et al. Renaissance of elemental phosphorus materials: Properties, synthesis, and applications in sustainable energy and environment. Chem. Soc. Rev. 2023, 52, 5388–5484. [Google Scholar]
- Han, X.; Han, J.; Liu, C.; Sun, J. Promise and challenge of phosphorus in science, technology, and application. Adv. Funct. Mater. 2018, 28, 1803471. [Google Scholar] [CrossRef]
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Castro Neto, A.H. Phosphorene: From theory to applications. Nat. Rev. Mater. 2016, 1, 16061. [Google Scholar] [CrossRef]
- Power, P.P. Boron-phosphorus compounds and multiple bonding. Angew. Chem. Int. Ed. 1990, 29, 449–460. [Google Scholar] [CrossRef]
- Pestana, D.C.; Power, P.P. Nature of the boron-phosphorus bond in monomeric phosphinoboranes and related compounds. J. Am. Chem. Soc. 1991, 113, 8426–8437. [Google Scholar] [CrossRef]
- Popper, P.; Ingles, T.A. Boron phosphide, a III–V compound of zinc-blende structure. Nature 1957, 179, 1075. [Google Scholar] [CrossRef]
- Stone, B.; Hill, D. Semiconducting properties of cubic boron phosphide. Phys. Rev. Lett. 1960, 4, 282–284. [Google Scholar] [CrossRef]
- Chu, T.L.; Gill, M.; Smeltzer, R.K. Growth of boron monophosphide crystals with the accelerated container rotation technique. J. Cryst. Growth 1976, 33, 53–57. [Google Scholar] [CrossRef]
- Kumashiro, Y.; Mitsuhashi, T.; Okaya, S.; Muta, F.; Koshiro, T.; Takahashi, Y.; Mirabayashi, M. Thermal conductivity of a boron phosphide single-crystal wafer up to high temperature. J. Appl. Phys. 1989, 65, 2147–2148. [Google Scholar] [CrossRef]
- Gu, Y.L.; Zheng, H.G.; Guo, F.; Qian, Y.T.; Yang, Z.P. A benzene-thermal synthesis of cubic boron phosphide (BP) ultrafine powders. Chem. Lett. 2002, 31, 724–725. [Google Scholar] [CrossRef]
- Kang, J.S.; Wu, H.; Hu, Y.J. Thermal properties and phonon spectral characterization of synthetic boron phosphide for high thermal conductivity applications. Nano Lett. 2017, 17, 7507–7514. [Google Scholar] [CrossRef]
- Ryan, F.M.; Miller, R.C. Photoluminescence and pair spectrum in boron phosphide. Phys. Rev. 1966, 148, 858–862. [Google Scholar] [CrossRef]
- Alves, H.W.L.; Kunc, K. Lattice dynamics of boron phosphide. Phys.-Condens. Matter 1992, 4, 6603–6612. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Kurakevych, O.O.; Le Godec, Y.; Kurnosov, A.V.; Oganov, A.R. Boron phosphide under pressure: In situ study by Raman scattering and X-ray diffraction. J. Appl. Phys. 2014, 116, 033501. [Google Scholar] [CrossRef]
- Liu, T.H.; Song, B.; Meroueh, L.; Ding, Z.W.; Song, Q.C.; Zhou, J.W.; Li, M.D.; Chen, G. Simultaneously high electron and hole mobilities in cubic boron-V compounds: BP, BAs, and BSb. Phys. Rev. B 2018, 98, 081203. [Google Scholar] [CrossRef]
- Chu, T.L.; Jackson, J.M.; Smeltzer, R.K. The growth of boron monophosphide crystals by chemical transport. J. Cryst. Growth 1972, 15, 254–258. [Google Scholar] [CrossRef]
- Slack, G.A.; McNelly, T.F.; Taft, E.A. Melt growth and properties of B6P crystals. J. Phys. Chem. Solids 1983, 44, 1009–1013. [Google Scholar] [CrossRef]
- Archer, R.J.; Koyama, R.Y.; Loebner, E.E.; Lucas, R.C. Optical absorption, electroluminescence, and the band gap of BP. Phys. Rev. Lett. 1964, 12, 538–540. [Google Scholar] [CrossRef]
- Fomichev, V.A.; Zhukova, I.I.; Polushina, I.K. Investigation of the energy band structure of boron phosphide by ultra-soft X-ray spectroscopy. J. Phys. Chem. Solids 1968, 29, 1025–1032. [Google Scholar] [CrossRef]
- Shi, L.; Li, P.; Zhou, W.; Wang, T.; Chang, K.; Zhang, H.; Kako, T.; Liu, G.; Ye, J. n-type boron phosphide as a highly stable, metal-free, visible-light-active photocatalyst for hydrogen evolution. Nano Energy 2016, 28, 158–163. [Google Scholar] [CrossRef]
- Gui, R.; Xue, Z.; Zhou, X.; Gu, C.; Ren, X.; Cheng, H.; Ma, D.; Qin, J.; Liang, Y.; Yan, X.; et al. Strain stiffening, high load-invariant hardness, and electronic anomalies of boron phosphide under pressure. Phys. Rev. B 2020, 101, 035302. [Google Scholar] [CrossRef]
- Lindsay, L.; Broido, D.A.; Reinecke, T.L. First-principles determination of ultrahigh thermal conductivity of boron arsenide: A competitor for diamond? Phys. Rev. Lett. 2013, 111, 025901. [Google Scholar] [CrossRef] [PubMed]
- López-Castillo, A. Prediction of boron-phosphorous nanographene-like material. Int. J. Quantum Chem. 2012, 112, 3152–3157. [Google Scholar] [CrossRef]
- Baei, M.T.; Peyghan, A.A.; Moghimi, M. Electronic structure study of Si-doped (4,4) armchair single-walled boron phosphide nanotube as a semiconductor. Monatsh. Chem. 2012, 143, 1627–1635. [Google Scholar] [CrossRef]
- Lakel, S.; Okbi, F.; Meradji, H. Optical and electronic properties of BxAl1−xP alloys: A first principles study. Optik 2016, 127, 3755–3761. [Google Scholar] [CrossRef]
- Boutaleb, M.; Doumi, B.; Mokaddem, A.; Sayede, A.; Tadjer, A. First-principle study of electronic and half-metallic ferromagnetic properties of vanadium (V)-doped cubic BP and InP. J. Supercond. Nov. Magn. 2017, 30, 2855–2864. [Google Scholar] [CrossRef]
- Wang, T.; Gong, J. Single-crystal semiconductors with narrow band gaps for solar water splitting. Angew. Chem. Int. Ed. 2015, 54, 10718–10732. [Google Scholar] [CrossRef]
- Sharma, A.; Shinde, D.D.; Mahajan, C.; Dambhare, N.V.; Biswas, A.; Mitra, A.; Girade, V.S.; Rath, A.K. Synergistic improvement of narrow bandgap PbS quantum dot solar cells through surface ligand engineering, near-infrared spectral matching, and enhanced electrode transparency. ACS Appl. Mater. Interfaces 2025, 17, 6614–6625. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, H.; Zou, Y.; Wang, R.; Lyu, Y.; Jiang, S.P.; Wang, S. Efficiency and stability of narrow-gap semiconductor-based photoelectrodes. Energy Environ. Sci. 2019, 12, 2345–2374. [Google Scholar] [CrossRef]
- Çakir, D.; Kecik, D.; Sahin, H.; Durgun, E.; Peeters, F.M. Realization of a p-n junction in a single layer boron-phosphide. Phys. Chem. Chem. Phys. 2015, 17, 13013–13020. [Google Scholar] [CrossRef]
- Crovetto, A.; Adamczyk, J.M.; Schnepf, R.R.; Perkins, C.L.; Hempel, H.; Bauers, S.R.; Toberer, E.S.; Tamboli, A.C.; Unold, T.; Zakutayev, A. Boron phosphide films by reactive sputtering: Searching for a p-type transparent conductor. Adv. Mater. Interfaces 2022, 9, 2200031. [Google Scholar] [CrossRef]
- Nwagwu, U. Flux Growth and Characteristics of Cubic Boron Phosphide. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2013. [Google Scholar]
- Liang, W.; Zhang, L.; Xiang, X.; Wang, J.; Zhang, L.; Wu, B.; Wang, Y.; Zeng, Y.; Guan, S.; Tang, Q.; et al. Growth of millimeter-size single-crystal boron phosphide by eutectic melt at 5.0 GPa and 3000 °C. Solid State Commun. 2021, 327, 114206. [Google Scholar] [CrossRef]
- Padavala, B.; Frye, C.D.; Wang, X.; Raghothamachar, B.; Edgar, J.H. CVD growth and properties of boron phosphide on 3C-SiC. J. Cryst. Growth 2016, 449, 15–21. [Google Scholar] [CrossRef]
- Ding, N.; Xu, J.; Zhang, Q.; Su, J.; Gao, Y.; Zhou, X.; Zhai, T. Controllable carrier type in boron phosphide nanowires toward homostructural optoelectronic devices. ACS Appl. Mater. Interfaces 2018, 10, 10296–10303. [Google Scholar] [CrossRef]
- Lu, X.; Lin, R.; Zhu, S.; Song, X.; Xu, C.; Huang, F.; Zheng, W. Millimeter-sized monoisotopic cubic 10boron phosphide. Cryst. Growth Des. 2023, 23, 2812–2817. [Google Scholar] [CrossRef]
- Hu, J.; Wang, K.; Hou, X.; Yang, T.; Xia, H. Boron phosphide with high thermal conductivity: Synthesis by molten salt method and thermal management performance. J. Inorg. Mater. 2022, 37, 933–940. [Google Scholar] [CrossRef]
- Hu, J.J.; Xia, H.Y.; Hou, X.G.; Yang, T.; Si, K.; Wang, Y.; Wang, L.L.; Shi, Z.Q. Enhanced thermal management performance of nanofibrillated cellulose composite with highly thermally conductive boron phosphide. J. Mater. Chem. A 2021, 9, 27049–27060. [Google Scholar] [CrossRef]
- Zhu, X.J.; Wu, T.W.; Ji, L.; Li, C.B.; Wang, T.; Wen, S.H.; Gao, S.Y.; Shi, X.F.; Luo, Y.L.; Peng, Q.L.; et al. Ambient electrohydrogenation of N2 for NH3 synthesis on non-metal boron phosphide nanoparticles: The critical role of P in boosting the catalytic activity. J. Mater. Chem. A 2019, 7, 16117–16121. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, C.; Gong, H.; Liu, Q.; Huang, C. Water-dispersible boron phosphide nanosheets for enhancing the thermal conductivity of nanofluids. J. Mol. Liq. 2020, 309, 113023. [Google Scholar] [CrossRef]
- Lu, X.; Lin, R.; Li, Y.; Ding, Y.; Liang, Y.; Jia, L.; Lin, Z.; Zhu, S.; Huang, F.; Zheng, W. Ultra-hard (41 GPa) isotopic pure 10BP semiconductor microwires for flexible photodetection and pressure sensing. Acs Nano 2022, 16, 4004–4013. [Google Scholar] [CrossRef]
- Thirumal, V.; Rajkumar, P.; Xiao, W.; Yoo, K.; Kim, J. Preparation of two-dimensional sodium-boron phosphide nanosheets used for Na-ion hybrid supercapacitor devices. Flatchem 2023, 39, 100490. [Google Scholar] [CrossRef]
- Lu, X.; Lin, R.; Zhu, S.; Lin, Z.; Song, X.; Huang, F.; Zheng, W. Ultralarge elastic deformation (180° Fold) of cubic-natBP microwires for wearable flexible strain sensors. ACS Mater. Lett. 2023, 5, 2282–2291. [Google Scholar] [CrossRef]
- Mou, S.Y.; Wu, T.W.; Xie, J.F.; Zhang, Y.; Ji, L.; Huang, H.; Wang, T.; Luo, Y.L.; Xiong, X.L.; Tang, B.; et al. Boron phosphide nanoparticles: A nonmetal catalyst for high-selectivity electrochemical reduction of CO2 to CH3OH. Adv. Mater. 2019, 31, 1903499. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Somogyi, B.; Nakamura, T.; Zhou, H.; Ichihashi, Y.; Nishiyama, S.; Gali, A.; Fujii, M. Size-dependent photocatalytic activity of cubic boron phosphide nanocrystals in the quantum confinement regime. J. Phys. Chem. C 2019, 123, 23226–23235. [Google Scholar] [CrossRef]
- Kocinski, P.; Zbroszczyk, M. Calculated structural and electronic properties of boron phosphide under pressure. Semicond. Sci. Technol. 1995, 10, 1452–1457. [Google Scholar] [CrossRef]
- Dong, J.C.; Li, H.; Li, L. Multi-functional nano-electronics constructed using boron phosphide and silicon carbide nanoribbons. NPG Asia Mater. 2013, 5, e56. [Google Scholar] [CrossRef][Green Version]
- Tahan, Y.; Rapaud, O.; Pradeilles, N.; Carles, P.; Maître, A.; Dine, S.; Vrel, D.; Moutaabbid, H.; Le Godec, Y.; Genevois, C.; et al. Investigating the thermal decomposition of BP into B12P2: Experimental insights and kinetic modelling at high temperatures. Acta Mater. 2025, 283, 120495. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, S.; Li, C.; Lv, Y.; Liu, X.; Huang, P.Y.; Broido, D.A.; Lv, B.; Cahill, D.G. High thermal conductivity in isotopically enriched cubic boron phosphide. Adv. Funct. Mater. 2018, 28, 1805116. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Bushlya, V. Mechanical properties of boron phosphides. J. Superhard Mater. 2019, 41, 84–89. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, J.; Zhou, H.; Jiang, P.; Zhang, D.; Wang, L.; Chen, S. Facile preparation of boron phosphide particles with high purity and high structural stability. Mater. Res. Express 2019, 6, 125922. [Google Scholar] [CrossRef]
- Sahin, H.; Cahangirov, S.; Topsakal, M.; Bekaroglu, E.; Akturk, E.; Senger, R.T.; Ciraci, S. Monolayer honeycomb structures of group-IV elements and III-V binary compounds: First-principles calculations. Phys. Rev. B 2009, 80, 155453. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Nilsson, O.; Mehling, H.; Horn, R.; Fricke, J.; Hofmann, R.; Muller, S.G.; Eckstein, R.; Hofmann, D. Determination of the thermal diffusivity and conductivity of monocrystalline silicon carbide (300–2300 K). High Temp. High Press. 1997, 29, 73–79. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.O.; Vandersande, J.W. The intrinsic thermal conductivity of AlN. J. Phys. Chem. Solids 1987, 48, 641–647. [Google Scholar] [CrossRef]
- Wei, L.H.; Kuo, P.K.; Thomas, R.L.; Anthony, T.R.; Banholzer, W.F. Thermal conductivity of isotopically modified single crystal diamond. Phys. Rev. Lett. 1993, 70, 3764–3767. [Google Scholar] [CrossRef]
- Qian, X.; Jiang, P.; Yang, R. Anisotropic thermal conductivity of 4H and 6H silicon carbide measured using time-domain thermoreflectance. Mater. Today Phys. 2017, 3, 70–75. [Google Scholar] [CrossRef]
- Kang, J.S.; Li, M.; Wu, H.; Nguyen, H.; Hu, Y. Experimental observation of high thermal conductivity in boron arsenide. Science 2018, 361, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, Q.; Lv, Y.; Liu, X.; Wang, X.; Huang, P.Y.; Cahill, D.G.; Lv, B. High thermal conductivity in cubic boron arsenide crystals. Science 2018, 361, 579–581. [Google Scholar] [CrossRef]
- Tian, F.; Song, B.; Chen, X.; Ravichandran, N.K.; Lv, Y.; Chen, K.; Sullivan, S.; Kim, J.; Zhou, Y.; Liu, T.H.; et al. Unusual high thermal conductivity in boron arsenide bulk crystals. Science 2018, 361, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Lan, Y.; Wang, X.; Zhang, Q.; Hu, Y.; Jacobson, A.J.; Broido, D.; Chen, G.; Ren, Z.; Chu, C.W. Experimental study of the proposed super-thermal-conductor: BAs. Appl. Phys. Lett. 2015, 106, 074105. [Google Scholar] [CrossRef]
- Kim, J.; Evans, D.A.; Sellan, D.P.; Williams, O.M.; Ou, E.; Cowley, A.H.; Shi, L. Thermal and thermoelectric transport measurements of an individual boron arsenide microstructure. Appl. Phys. Lett. 2016, 108, 201905. [Google Scholar] [CrossRef]
- Tian, F.; Song, B.; Lv, B.; Sun, J.; Huyan, S.; Wu, Q.; Mao, J.; Ni, Y.; Ding, Z.; Huberman, S.; et al. Seeded growth of boron arsenide single crystals with high thermal conductivity. Appl. Phys. Lett. 2018, 112, 031903. [Google Scholar] [CrossRef]
- Nishinaga, T.; Ogawa, H.; Watanabe, H.; Arizumi, T. Vapor growth of boron monophosphide using open and closed tube processes. J. Cryst. Growth 1972, 13, 346–349. [Google Scholar] [CrossRef]
- Shohno, K.; Takigawa, M.; Nakada, T. Epitaxial growth of BP compounds on Si substrates using the B2H6-PH3-H2 system. J. Cryst. Growth 1974, 24, 193–196. [Google Scholar] [CrossRef]
- Sharma, S.; Soni, A.; Sahariya, J. First-principles calculations to investigate impact of Ga and In dopants on the electronic and optical features of boron phosphide. Opt. Quantum Electron. 2024, 56, 428. [Google Scholar] [CrossRef]
- Varley, J.B.; Miglio, A.; Ha, V.A.; van Setten, M.J.; Rignanese, G.M.; Hautier, G. High-throughput design of non-oxide p-type transparent conducting materials: Data mining, search strategy, and identification of boron phosphide. Chem. Mater. 2017, 29, 2568–2573. [Google Scholar] [CrossRef]
- Williams, F.V.; Ruehrwein, R.A. The preparation and properties of boron phosphides and arsenides1. J. Am. Chem. 1960, 82, 1330–1332. [Google Scholar] [CrossRef]
- Woo, K.; Lee, K.; Kovnir, K. BP: Synthesis and properties of boron phosphide. Mater. Res. Express 2016, 3, 074003. [Google Scholar] [CrossRef]
- Kumashiro, Y.; Yao, T.; Gonda, S. Crystal growth of boron phosphide by high pressure flux method. J. Cryst. Growth 1984, 70, 515–518. [Google Scholar] [CrossRef]
- Kumashiro, Y. Refractory semiconductor of boron phosphide. J. Mater. Res. 1990, 5, 2933–2947. [Google Scholar] [CrossRef]
- Petersen, K.E. Silicon as a mechanical material. Proc. IEEE Inst. Electr. Electron. Eng. 1982, 70, 420–457. [Google Scholar] [CrossRef]
- Li, S.; Qin, Z.; Wu, H.; Li, M.; Kunz, M.; Alatas, A.; Kavner, A.; Hu, Y. Anomalous thermal transport under high pressure in boron arsenide. Nature 2022, 612, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S. GaAs, AlAs, and AlxGa1−xAs: Material parameters for use in research and device applications. J. Appl. Phys. 1985, 58, R1–R29. [Google Scholar] [CrossRef]
- Aparna, A.R.; Brahmajirao, V.; Karthikeyan, T.V. Review on synthesis and characterization of gallium phosphide. Procedia Mater. Sci. 2014, 6, 1650–1657. [Google Scholar] [CrossRef][Green Version]
- Massidda, S.; Continenza, A.; Freeman, A.J.; de Pascale, T.M.; Meloni, F.; Serra, M. Structural and electronic properties of narrow-band-gap semiconductors: InP, InAs, and InSb. Phys. Rev. B Condens. Matter 1990, 41, 12079–12085. [Google Scholar] [CrossRef] [PubMed]
- Harima, H. Properties of GaN and related compounds studied by means of raman scattering. J. Phys. Condens. Matter 2002, 14, R967–R993. [Google Scholar] [CrossRef]
- Su, H.W.; Guo, Q.; Li, H.T.; Li, A.L.; Zhong, Y.L.; Zhang, X.; Geng, L.; Liu, S.; Dong, L.Q.; Pan, X.H.; et al. Boron phosphide microwires based on-chip electrocatalytic oxygen evolution microdevice. iScience 2025, 28, 111859. [Google Scholar] [CrossRef]
- Kumashiro, Y.; Okada, Y.; Okumura, H. Isotope effects on boron phosphide single-crystal wafers. J. Cryst. Growth 1993, 132, 611–613. [Google Scholar] [CrossRef]
- Ohsawa, J.; Nishinaga, T.; Uchiyama, S. Si contamination in epitaxial boron monophosphide. J. Appl. Phys. 1978, 17, 1579–1586. [Google Scholar] [CrossRef]
- Takenaka, T.; Takigawa, M.; Shohno, K. Diffusion layers formed in Si substrates during the epitaxial growth of BP and application to devices. J. Electrochem. Soc. 1978, 125, 633–637. [Google Scholar] [CrossRef]
- Kim, C.J.; Shono, K. Deviation from stoichiometry of boron monophosphide. J. Electrochem. Soc. 1984, 131, 120–122. [Google Scholar] [CrossRef]
- Schroten, E.; Goossens, A.; Schoonman, J. Photo- and electroreflectance of cubic boron phosphide. J. Appl. Phys. 1998, 83, 1660–1663. [Google Scholar] [CrossRef]
- He, J.; Wang, H.; Gong, Y.; Tian, X.Y.; Zhang, Z.L.; He, J.Y. A novel three-dimensional boron phosphide network for thermal management of epoxy composites. Compos. Part B-Eng. 2022, 233, 109662. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Lei, S.Y.; Wang, M.Y. S-scheme boron phosphide/MoS2 heterostructure with excellent light conversion ability for solar cells and water splitting photocatalysts. ACS Appl. Mater. Interfaces 2024, 16, 30521–30533. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tong, D.G. Amorphous boron phosphide nanosheets: A highly efficient capacitive deionization electrode for uranium separation from seawater with superior selectivity. Sep. Purif. Technol. 2020, 250, 117175. [Google Scholar] [CrossRef]
- Jiang, H.R.; Shyy, W.; Liu, M.; Wei, L.; Wu, M.C.; Zhao, T.S. Boron phosphide monolayer as a potential anode material for alkali metal-based batteries. J. Mater. Chem. A 2017, 5, 672–679. [Google Scholar] [CrossRef]
- Yu, T.T.; Gao, P.F.; Zhang, Y.; Zhang, S.L. Boron-phosphide monolayer as a potential anchoring material for lithium-sulfur batteries: A first-principles study. Appl. Surf. Sci. 2019, 486, 281–286. [Google Scholar] [CrossRef]
- Zhang, N.A.; Zhang, G.K.; Tian, Y.; Guo, Y.L.; Chu, K. Boron phosphide as an efficient metal-free catalyst for nitrate electroreduction to ammonia. Dalton Trans. 2023, 52, 4290–4295. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, X.; Chen, F.; Chen, Z.; Fan, X.; Lau, W. Single atoms on a nitrogen-doped boron phosphide monolayer: A new promising bifunctional electrocatalyst for ORR and OER. ACS Appl. Mater. Interfaces 2020, 12, 52549–52559. [Google Scholar] [CrossRef]


| Material | Thermal Conductivity (W/m·K) | Hardness (GPa) | Electron Mobility (cm2/V·s) | Hole Mobility (cm2/V·s) | Bandgap (eV) | Notes |
|---|---|---|---|---|---|---|
| BP | ~500 | 38 | 140 | 500 | ~2.0 | Indirect gap |
| Si [81] | 149 | 13 | 1400 | 450 | ~1.12 | Indirect gap |
| Bas [82] | 1300 | 22 | 1400–1740 | 1600 | ~1.82 | Indirect gap |
| GaAs [83] | 45 | 5.5 | 8500 | 400 | ~1.42 | Direct gap |
| GaP [84] | 75.2 | 8.8 | 250 | 150 | ~2.24 | Indirect gap |
| InP [85] | 68 | 5.0 | <5400 | <200 | ~1.35 | Direct gap |
| GaN [86] | 130 | 10.2 | <1000 | <350 | ~3.4 | Direct gap |
| Method | Morphology | Conditions | Advantages | Limitations | Yield/Size Control | Ref. |
|---|---|---|---|---|---|---|
| High-pressure co-crystal melting | Bulk | 5.0 GPa, 3000 °C | Produces millimeter-scale single crystals | Extreme conditions limit scalability | ≤1 mm crystals | [42] |
| Molten salt (Ni catalyst) | Bulk | 1150 °C, slow cooling | Lower temperature, enables isotopically pure BP | Requires catalyst, slow cooling for large crystals | µm- to mm-scale crystals | [45] |
| Solid-phase reaction ball-milling | Nanoparticles | Ball-milling post-synthesis | High purity, avoids high-pressure setups | Requires post-processing for nanoparticles | Submicron particles (no yield data) | [29] |
| Molten salt | Nanoparticles | NaCl:KCl:LiCl, 1100 °C | Simplified process, high yield | Limited size control for ultrafine particles | 74% yield | [46] |
| CVD#1 | Nanowires | 800–1000 °C | Tunable n-/p-type nanowires | Requires precise gas-phase control | Uniform nanowires (no yield) | [44] |
| VLS growth | Nanowires | 1050 °C, Ni catalyst | High aspect ratio (104), elastic microwires | Catalyst residue may affect applications | Length > 250 µm, diameter > 250 nm | [87] |
| CVD#2 | Film | 1000–1100 °C, low pressure | High crystallinity, preferred orientation | Lattice mismatch with Si/Sapphire | µm-thick films | [43] |
| SiO2-assisted solid-state reaction | Film | Annealing in sealed ampoule | Ultrathin nanosheets (~4 nm), water-dispersible | Lateral size inhomogeneity, limited thickness control | Sub-10 nm thickness | [49] |
| Reactive sputtering | Film | Annealing at 1000 °C | Amorphous-to-crystalline transition, doping control | High annealing temperature required | – | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Wu, J.; Xu, M.; Liu, Y.; Tian, Q.; Hou, C.; Tai, G. Boron Phosphide: A Comprehensive Overview of Structures, Properties, Synthesis, and Functional Applications. Nanomaterials 2025, 15, 654. https://doi.org/10.3390/nano15090654
Wu Q, Wu J, Xu M, Liu Y, Tian Q, Hou C, Tai G. Boron Phosphide: A Comprehensive Overview of Structures, Properties, Synthesis, and Functional Applications. Nanomaterials. 2025; 15(9):654. https://doi.org/10.3390/nano15090654
Chicago/Turabian StyleWu, Qilong, Jiamin Wu, Maoping Xu, Yi Liu, Qian Tian, Chuang Hou, and Guoan Tai. 2025. "Boron Phosphide: A Comprehensive Overview of Structures, Properties, Synthesis, and Functional Applications" Nanomaterials 15, no. 9: 654. https://doi.org/10.3390/nano15090654
APA StyleWu, Q., Wu, J., Xu, M., Liu, Y., Tian, Q., Hou, C., & Tai, G. (2025). Boron Phosphide: A Comprehensive Overview of Structures, Properties, Synthesis, and Functional Applications. Nanomaterials, 15(9), 654. https://doi.org/10.3390/nano15090654







