Toxicological Effects of Silver-Modified Bentonite Nanocomposites on Microalgae: Impact on Cell Growth, Antioxidant Enzymes, and Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
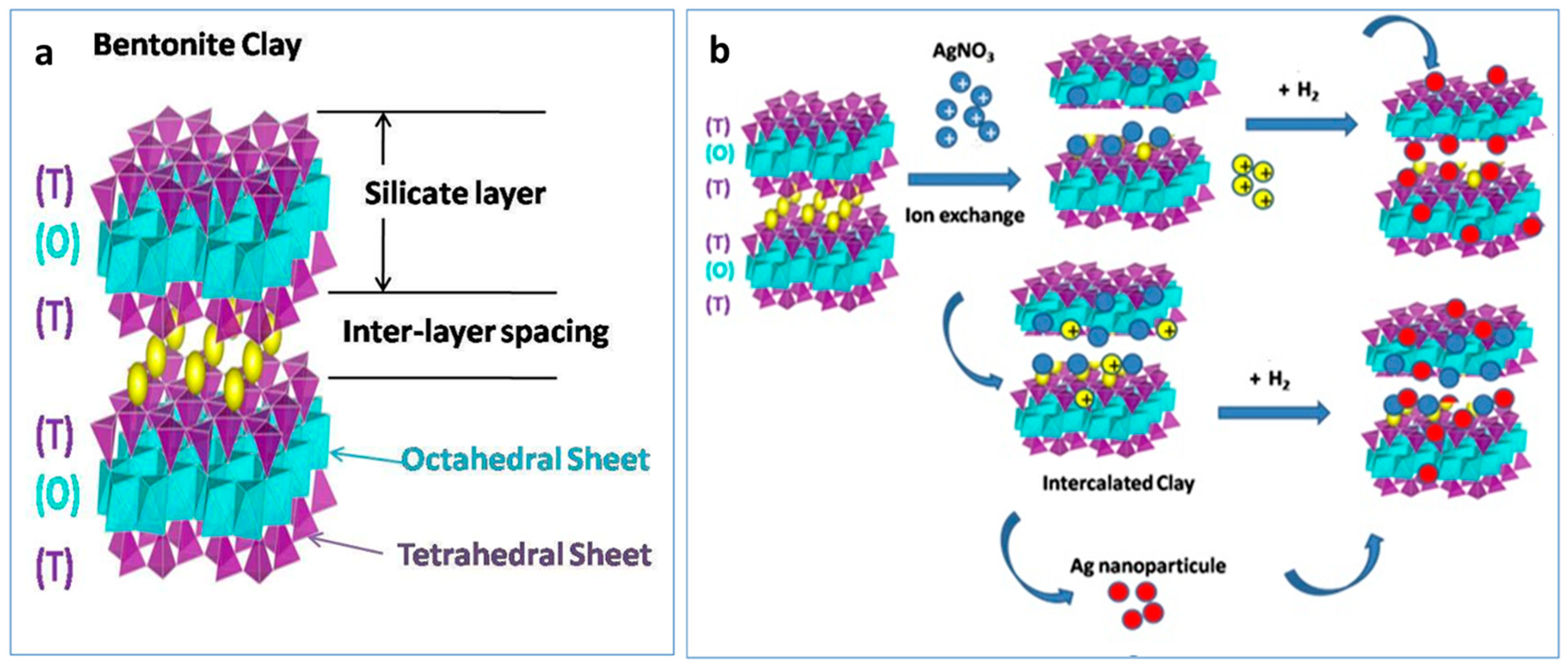
2.1. Preparation of Bentonite Clay–AgNO3 Powder
2.2. Gravimetric Quantification of Ag(I) by Precipitation
2.3. Preparation of Bentonite with Ag Treated with Gaseous Hydrogen
2.4. Characterization
2.5. Toxicity Studies
2.5.1. Algal Cultures
2.5.2. Growth Kinetics
2.5.3. Determination of IC50 Value
2.5.4. Microalga Cell Exposure Procedure
2.5.5. Photosynthetic Pigment Quantification
2.5.6. Cytotoxicity Analysis
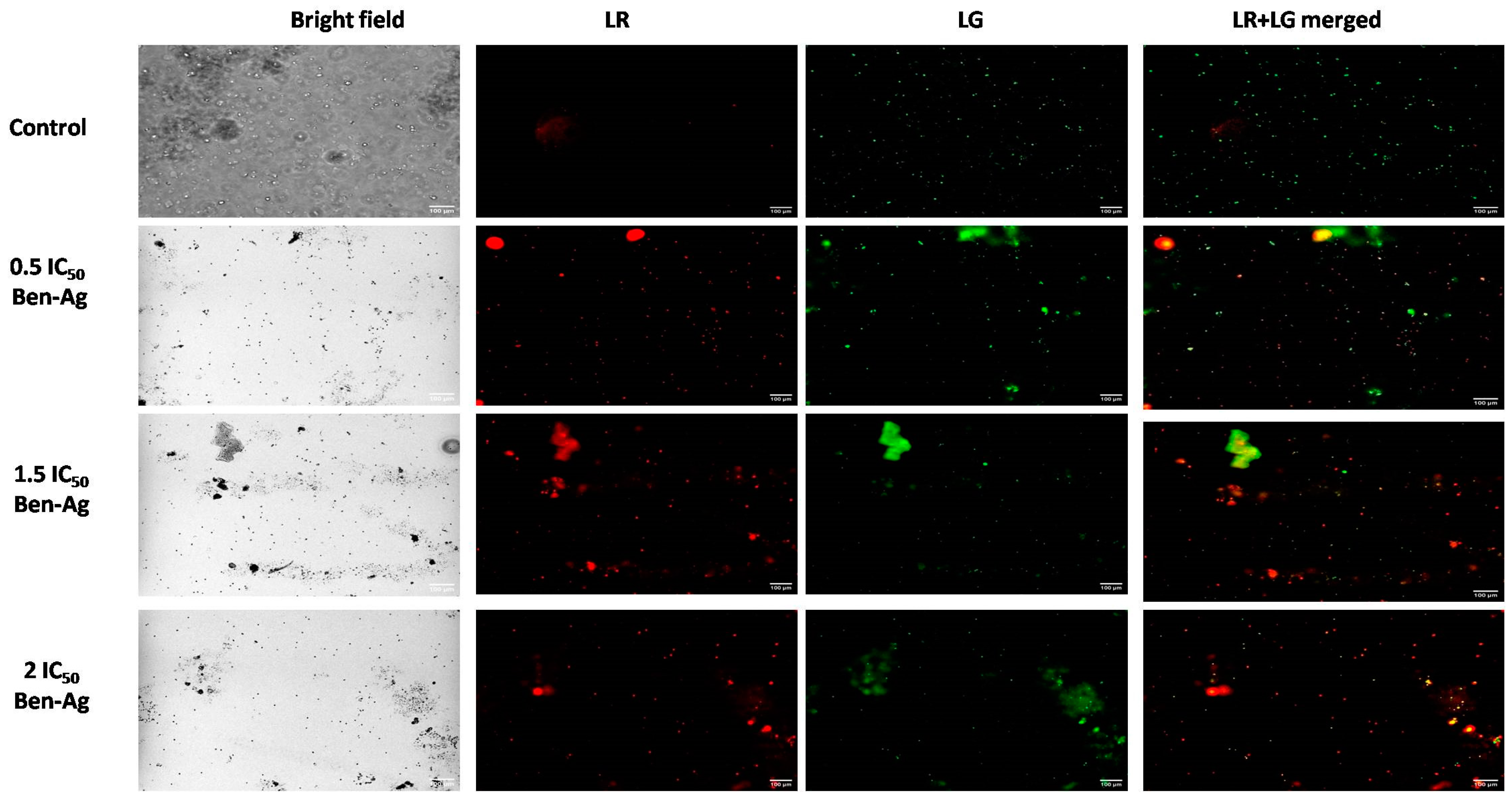
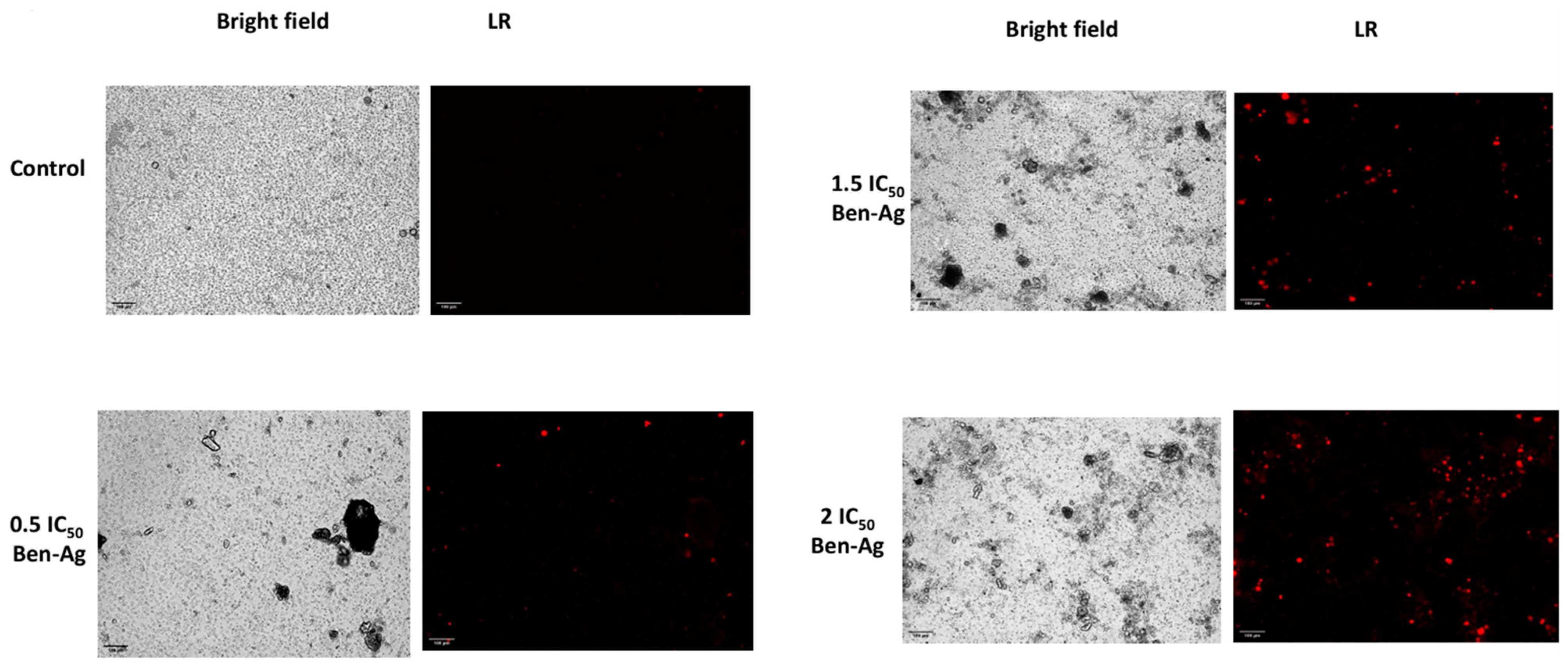
2.5.7. Genotoxicity Assay and Apoptotic Potential Determination
2.5.8. Protein Extraction
2.5.9. Estimation of Enzymatic Antioxidant Activities
2.5.10. Total Phenol Assay
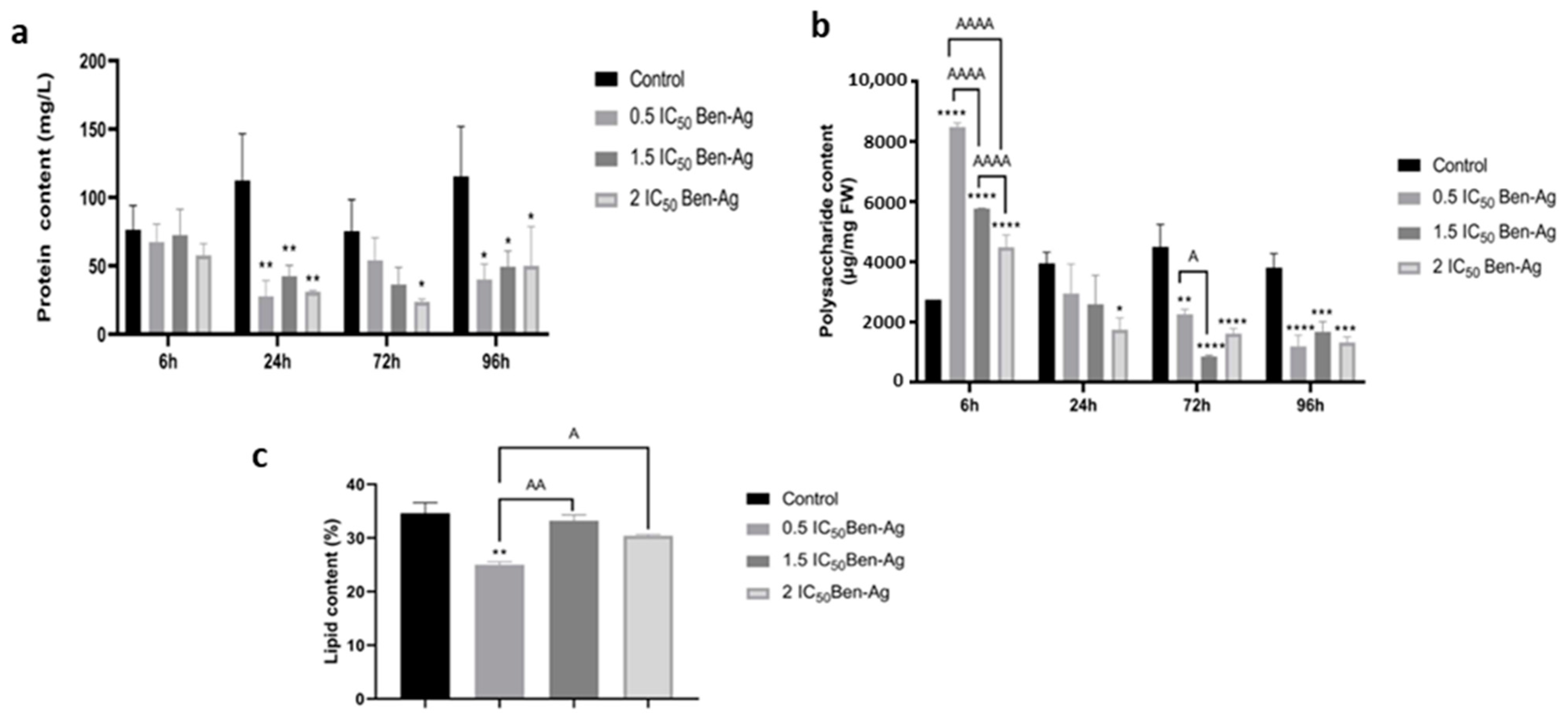
2.6. Polysaccharide Content
2.7. Lipid Content
2.8. FTIR Analysis
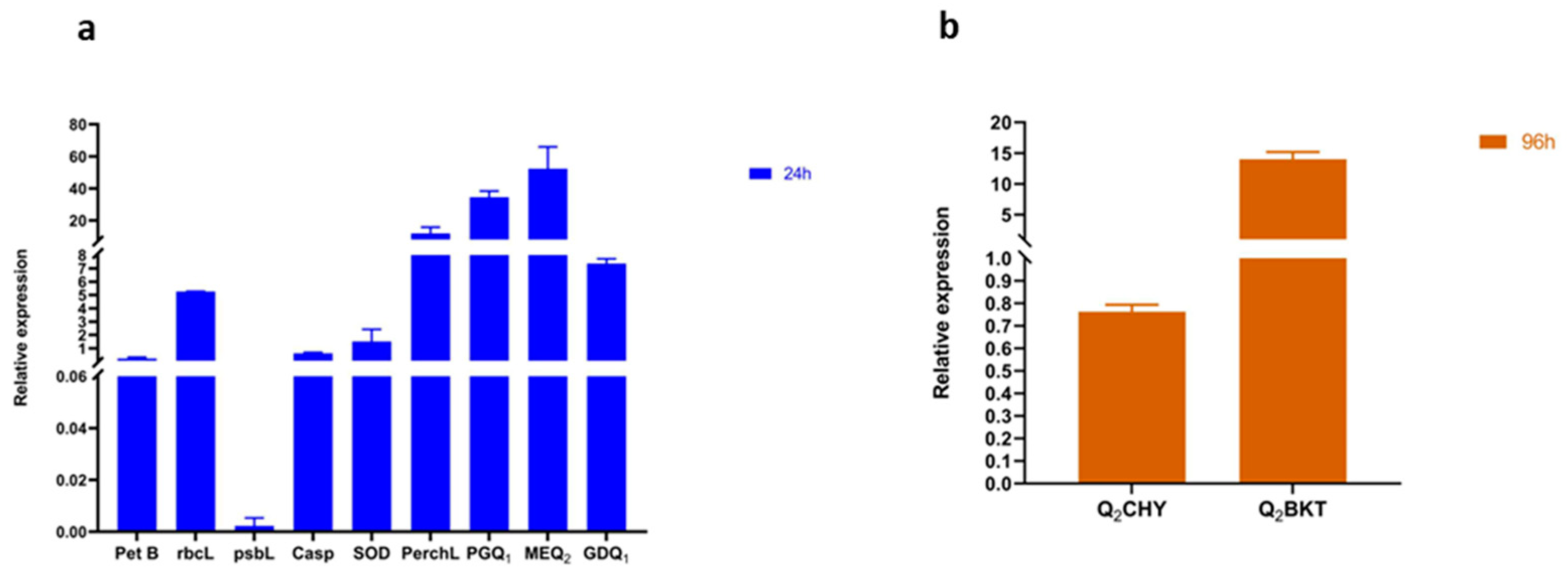
2.9. Gene Expression Assessment
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Nanocomposites
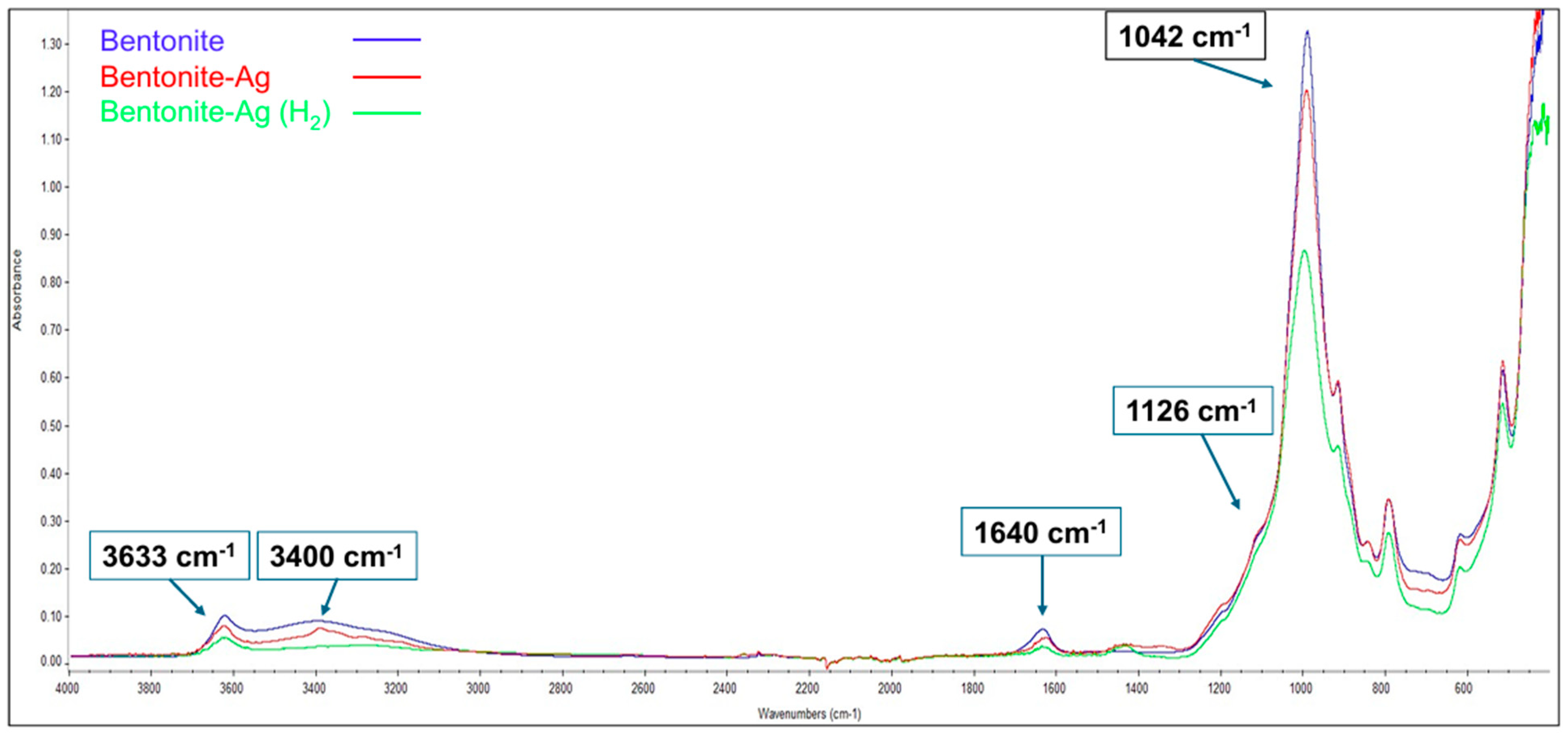
3.1.1. Chemical Composition and Characterization
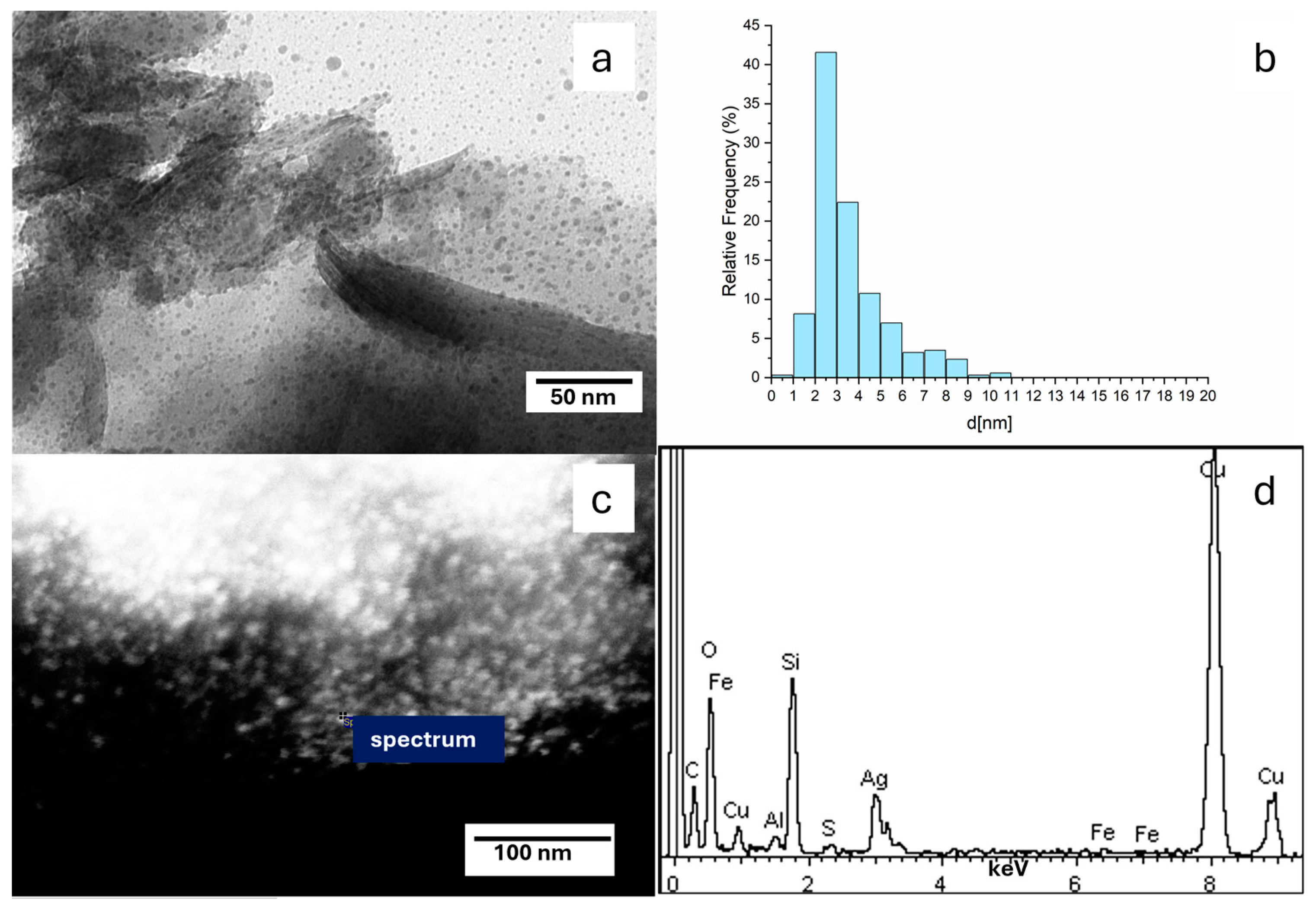
3.1.2. EDX Analysis
3.1.3. TEM Micrograph Study
3.2. Cytotoxicity Assays
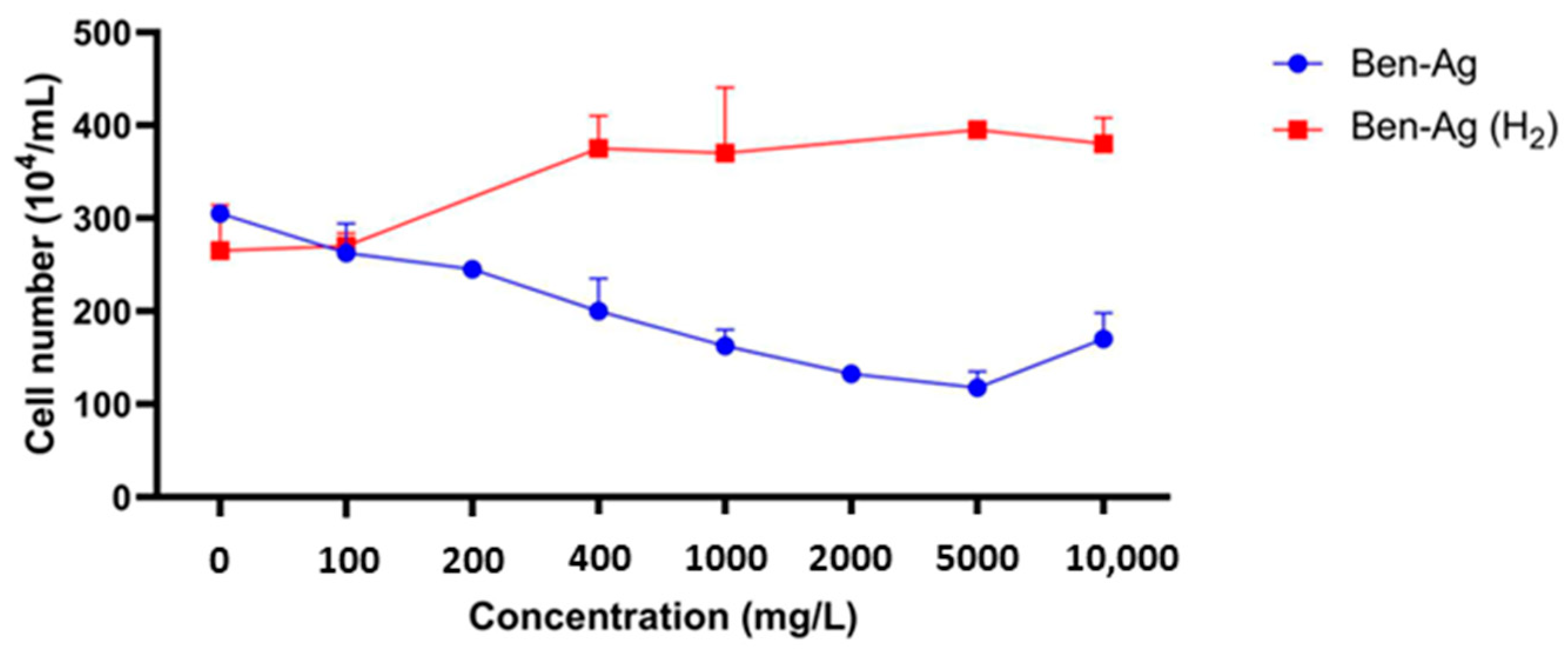
3.2.1. IC50 Value Determination
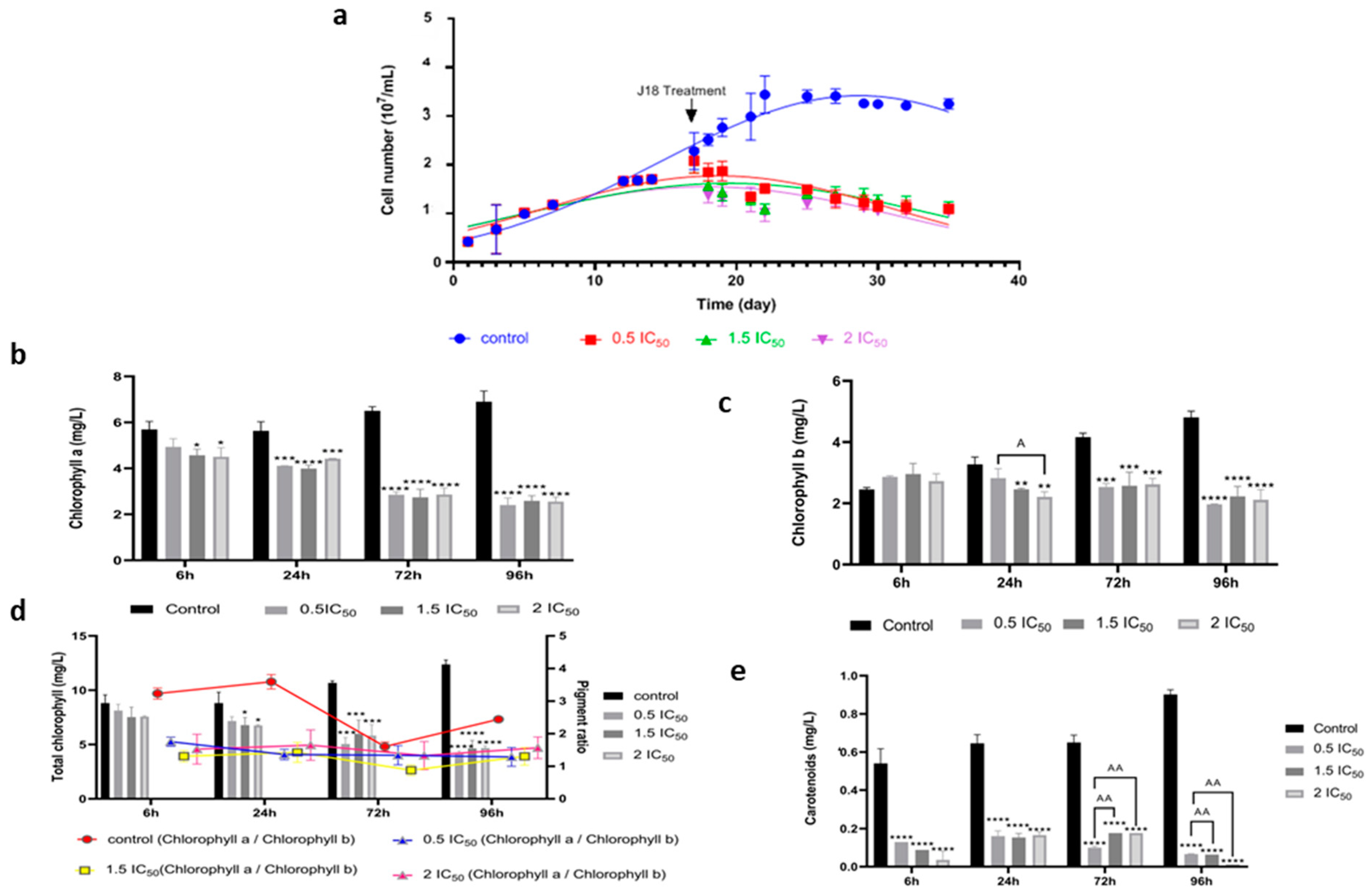
3.2.2. Effects of Ben-Ag on Chlamydomonas sp. Growth and Photosynthesis
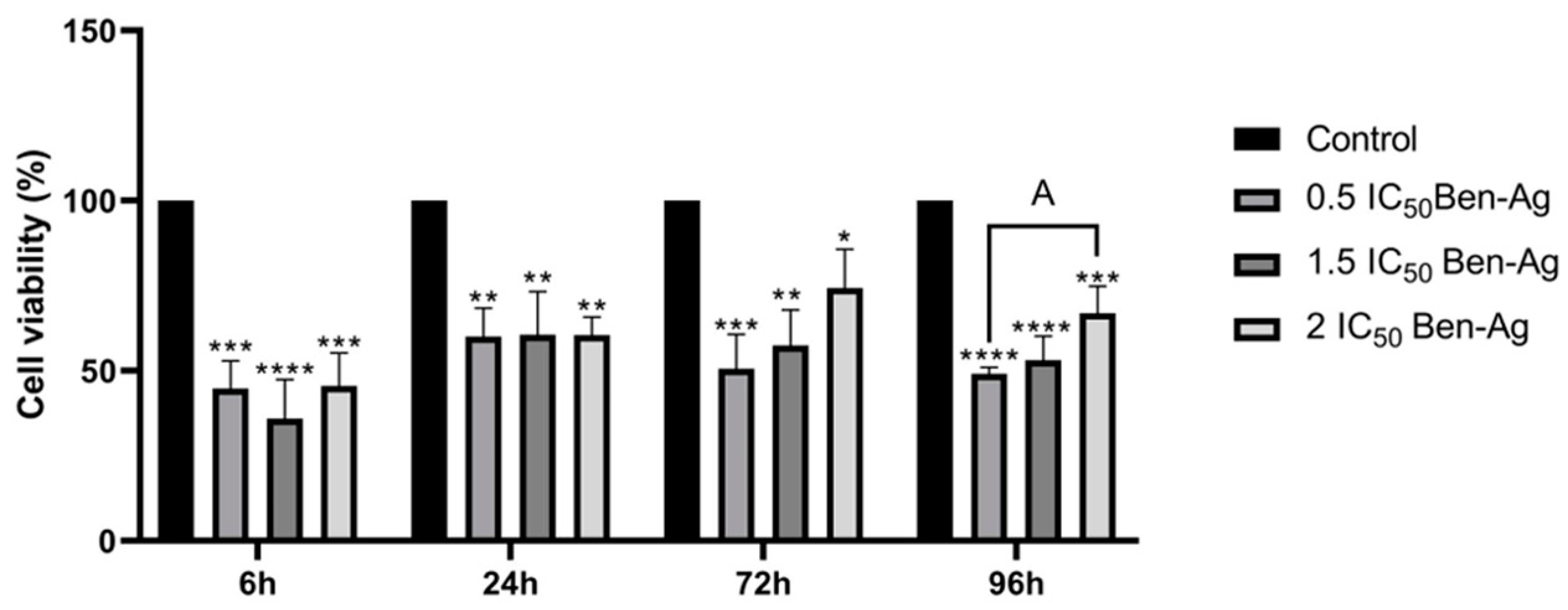
3.2.3. Effects of Ben-Ag on Cell Viability
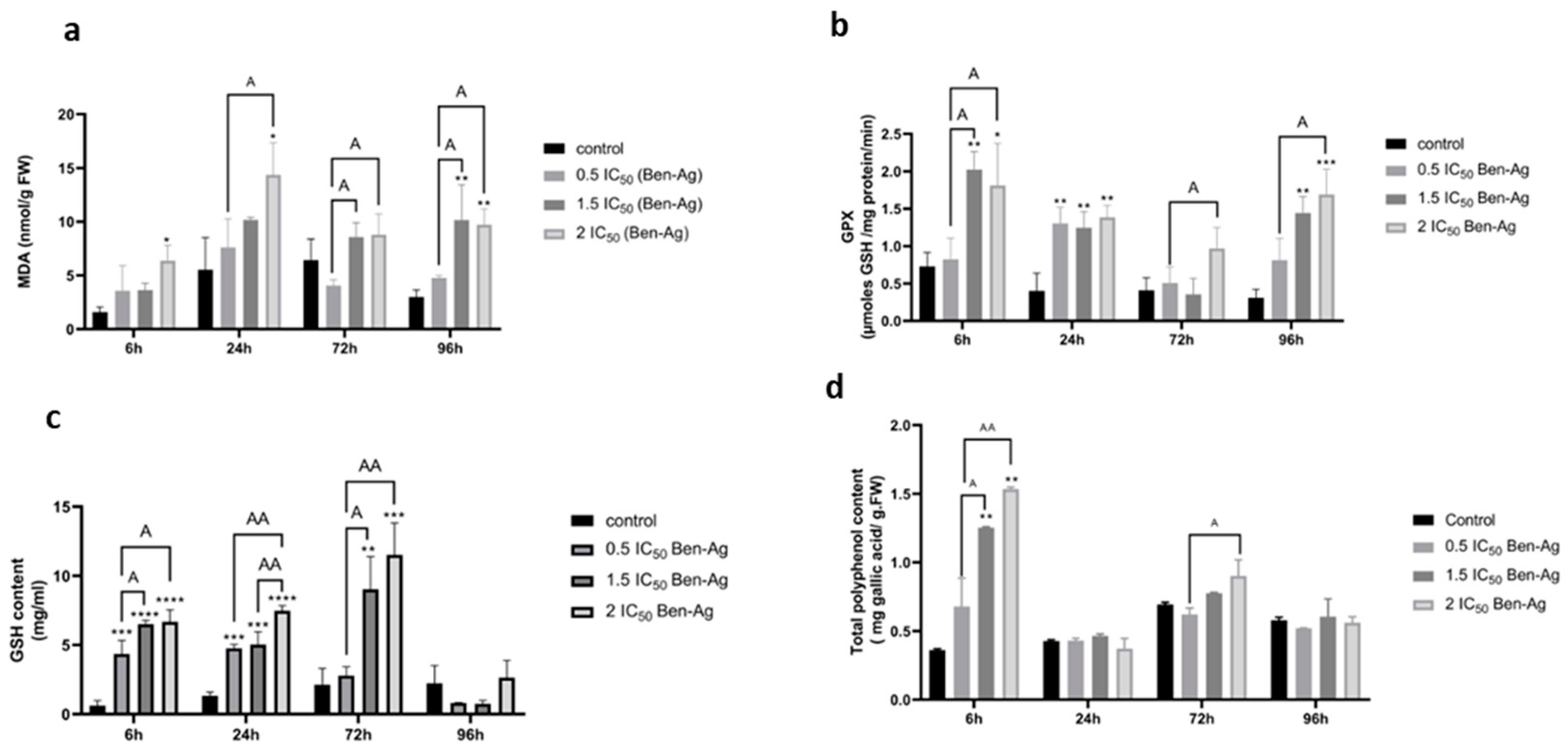
3.3. Effects of Ben-Ag on Enzymatic Antioxidant Activities and Non-Enzymatic Antioxidants
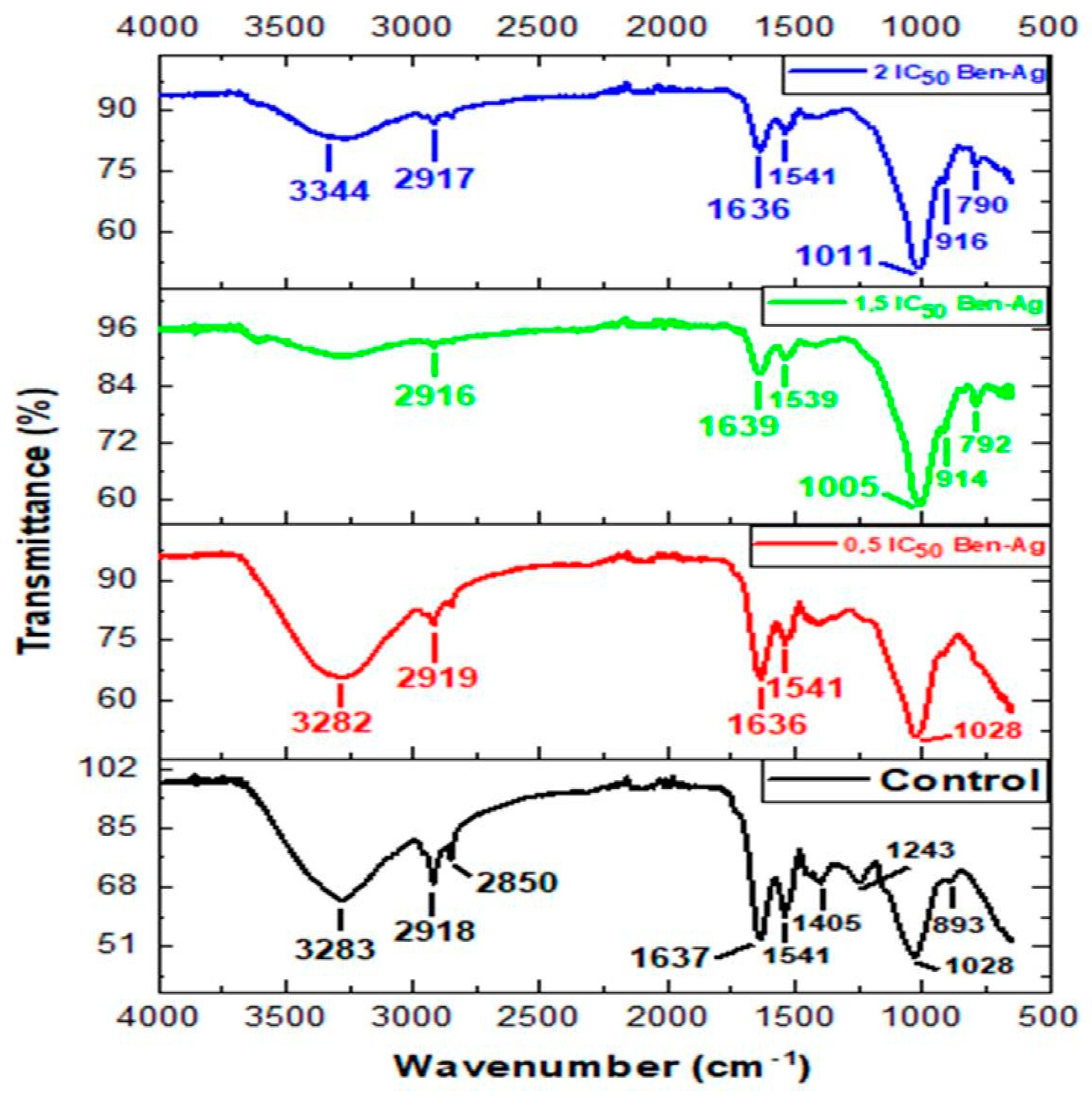
3.4. FTIR Spectra of Chlamydomonas sp. After Exposure to Ben-Ag
3.5. Genotoxic and Apoptotic Potential of Ben-Ag Against Chlamydomonas sp.
3.6. Effect of Ben-Ag Exposure on Protein, Polysaccharide, and Lipid Contents
3.7. Responses of Genes Linked to Oxidative Stress, Photosynthesis, Astaxanthin, and Carbohydrate Biosynthesis Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Manikandan, D.B.; Arumugam, M.; Sridhar, A.; Perumalsamy, B.; Ramasamy, T. Sustainable fabrication of hybrid silver-copper nanocomposites (Ag-CuO NCs) using Ocimum americanum L. as an effective regime against antibacterial, anticancer, photocatalytic dye degradation and microalgae toxicity. Environ. Res. 2023, 228, 115867. [Google Scholar] [CrossRef] [PubMed]
- López de Dicastillo, C.; Patiño Vidal, C.; Falcó, I.; Sánchez, G.; Márquez, P.; Escrig, J. Antimicrobial Bilayer Nanocomposites Based on the Incorporation of As-Synthetized Hollow Zinc Oxide Nanotubes. Nanomaterials 2020, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Malatjie, T.S.; Botha, T.L.; Kuvarega, A.T.; Madima, N.; de Bruyn, K.; Tekere, M.; Nkambule, T.T.I.; Mamba, B.B.; Msagati, T.A.M. Toxicity evaluation of TiO2/MWCNT-CNF hybrid nanocomposites with enhanced photocatalytic activity toward freshwater microalgae: Pseudokirchneriella subcapitata. Chemosphere 2022, 291, 132891. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef]
- Nazari, F.; Jafarirad, S.; Movafeghi, A.; Kosari-Nasab, M.; Kazemi, E.M. Toxicity of microwave-synthesized silver-reduced graphene oxide nanocomposites to the microalga Chlorella vulgaris: Comparison with the hydrothermal method synthesized counterparts. J. Environ. Sci. Health A 2020, 55, 639–649. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of coated silver nanoparticles to Daphnia carinata and trophic transfer from alga Raphidocelis subcapitata. PLoS ONE 2019, 14, e0214398. [Google Scholar] [CrossRef]
- Terzioğlu, E.; Arslan, M.; Balaban, B.G.; Çakar, Z.P. Microbial silver resistance mechanisms: Recent developments. World J. Microbiol. Biotechnol. 2022, 38, 158. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mortensen, A.; Schmidt, B.; Frandsen, H.; Hadrup, N.; Larsen, E.H.; Binderup, M.-L. In-vivo study of genotoxic and inflammatory effects of the organo-modified Montmorillonite Cloisite® 30B. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 770, 66–71. [Google Scholar] [CrossRef]
- Krishnan, B.; Mahalingam, S. Ag/TiO2/bentonite nanocomposite for biological applications: Synthesis, characterization, antibacterial and cytotoxic investigations. Adv. Powder Technol. 2017, 28, 2265–2280. [Google Scholar] [CrossRef]
- Zewudie, A.; Zereffa, E.; Segne, T.; Murthy, H.; Ravikumar, C.; Muniswamy, D.; Binagdie, B. Biosynthesis of Ag/bentonite, ZnO/bentonite, and Ag/ZnO/bentonite nanocomposites by aqueous leaf extract of Hagenia abyssinica for antibacterial activities. Rev. Adv. Mater. Sci. 2023, 62, 20220307. [Google Scholar] [CrossRef]
- Zhirong, L.; Azhar Uddin, M.; Zhanxue, S. FT-IR and XRD analysis of natural Na-bentonite and Cu(II)-loaded Na-bentonite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.-M.; Santomauro, G.; Bill, J.; Sitti, M. Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef]
- Ghariani, O.; Elleuch, J.; Gargouri, B.; Fakhfakh, F.; Bisio, C.; Fendri, I.; Guidotti, M.; Abdelkafi, S. Toxicity potential assessment of silicon dioxide (SiO2) and zinc oxide (ZnO) on green microalgae Chlamydomonas sp. strain GO1. Int. Microbiol. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Trigub, A.; Ipatova, V. Influence of nanocomposite Ag/AgCl on the culture of microalgae Scenedesmus quadricauda and Phaeodactylum tricornutum. Toxicol. Rev. 2018, 2, 44–50. [Google Scholar] [CrossRef]
- Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. The comparative toxic impact assessment of carbon nanotubes, fullerene, graphene, and graphene oxide on marine microalgae Porphyridium purpureum. Toxics 2023, 11, 491. [Google Scholar] [CrossRef]
- Thabet, J.; Elleuch, J.; Martínez, F.; Abdelkafi, S.; Hernández, L.E.; Fendri, I. Characterization of cellular toxicity induced by sub-lethal inorganic mercury in the marine microalgae Chlorococcum dorsiventrale isolated from a metal-polluted coastal site. Chemosphere 2023, 338, 139391. [Google Scholar] [CrossRef]
- Elleuch, J.; Thabet, J.; Ghribi, I.; Jabeur, H.; Hernández, L.E.; Fendri, I.; Abdelkafi, S. Responses of Dunaliella sp. AL-1 to chromium and copper: Biochemical and physiological studies. Chemosphere 2024, 364, 143133. [Google Scholar] [CrossRef]
- Starodub, N.F.; Taran, M.V.; Katsev, A.M.; Bisio, C.; Guidotti, M. Nanomaterials: Structural peculiarities, biological effects, and some aspects of application. In Intelligent Nanomaterials, 2nd ed.; Tiwari, A., Mishra, Y.K., Kobayashi, H., Turner, A.P.F., Eds.; Wiley: Hoboken, NJ, USA, 2016; Volume II. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Moon, J.-Y.; Lee, Y.-C. Microalgal ecotoxicity of nanoparticles: An updated review. Ecotoxicol. Environ. Saf. 2020, 201, 110781. [Google Scholar] [CrossRef]
- Wang, F.; Guan, W.; Xu, L.; Ding, Z.; Ma, H.; Ma, A.; Terry, N. Effects of nanoparticles on algae: Adsorption, distribution, ecotoxicity and fate. Appl. Sci. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: New York, NY, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Elleuch, J.; Drira, M.; Ghribi, I.; Hadjkacem, F.; Pierre, G.; Causserand, C.; Abdelkafi, S. Amphora coffeiformis extracellular polymeric substances and their potential applications in lead removal. Antonie Leeuwenhoek 2025, 118, 51. [Google Scholar] [CrossRef]
- Elleuch, J.; Ben Amor, F.; Chaaben, Z.; Frikha, F.; Michaud, P.; Fendri, I.; Abdelkafi, S. Zinc biosorption by Dunaliella sp. AL-1: Mechanism and effects on cell metabolism. Sci. Total Environ. 2021, 773, 145024. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Advances in Photosynthesis Research; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; pp. 9–12. [Google Scholar] [CrossRef]
- Kumar, P.; Ramakritinan, C.M.; Kumaraguru, A.K. Solvent extraction and spectrophotometric determination of pigments of some algal species from the shore of Puthumadam, Southeast Coast of India. Int. J. Oceans Oceanogr. 2010, 1, 29–34. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Prathna, T.C.; Trivedi, S.; Myneni, R.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of aluminium oxide nanoparticles towards fresh water algal isolate at low exposure concentrations. Aquat. Toxicol. 2013, 132–133, 34–45. [Google Scholar] [CrossRef]
- Manzo, S.; Buono, S.; Rametta, G.; Miglietta, M.; Schiavo, S.; Di Francia, G. The diverse toxic effect of SiO2 and TiO2 nanoparticles toward the marine microalgae Dunaliella tertiolecta. Environ. Sci. Poll. Res. 2015, 22, 15941–15951. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, S.; Oliviero, M.; Miglietta, M.; Rametta, G.; Manzo, S. Genotoxic and cytotoxic effects of ZnO nanoparticles for Dunaliella tertiolecta and comparison with SiO2 and TiO2 effects at population growth inhibition levels. Sci. Total Environ. 2016, 550, 619–627. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae Chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Derbel, H.; Elleuch, J.; Mahfoudh, W.; Michaud, P.; Fendri, I.; Abdelkafi, S. In vitro antioxidant and anti-inflammatory activities of bioactive proteins and peptides from Rhodomonas sp. Appl. Sci. 2023, 13, 3202. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Derbel, H.; Elleuch, J.; Tounsi, L.; Nicolo, M.S.; Rizzo, M.G.; Michaud, P.; Fendri, I.; Abdelkafi, S. Improvement of biomass and phycoerythrin production by a strain of Rhodomonas sp. isolated from the Tunisian coast of Sidi Mansour. Biomolecules 2022, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Abdel Baky, H.; El-Baz, F.; El Baroty, G. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects in vitro toward hepatotoxicity model. Afr. J. Pharm. Pharmacol. 2009, 3, 133–139. [Google Scholar]
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ben Mohamed, J.; Elleuch, J.; Drira, M.; Esteban, M.Á.; Michaud, P.; Abdelkafi, S.; Fendri, I. Characterization and biotechnological potential of two native marine microalgae isolated from the Tunisian coast. Appl. Sci. 2021, 11, 5295. [Google Scholar] [CrossRef]
- Drira, M.; Elleuch, J.; Hadjkacem, F.; Hentati, F.; Drira, R.; Pierre, G.; Gardarin, C.; Delattre, C.; El Alaoui-Talibi, Z.; El Modafar, C.; et al. Influence of the sulfate content of the exopolysaccharides from Porphyridium sordidum on their elicitor activities on date palm vitroplants. Plant Physiol. Biochem. 2022, 186, 99–106. [Google Scholar] [CrossRef]
- Déniel, M.; Errien, N.; Lagarde, F.; Zanella, M.; Caruso, A. Interactions between polystyrene nanoparticles and Chlamydomonas reinhardtii monitored by infrared spectroscopy combined with molecular biology. Environ. Pollut. 2020, 266, 115227. [Google Scholar] [CrossRef]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef]
- Hmani, R.; Elleuch, J.; Elleuch, F.; Drira, M.; Michaud, P.; Aleya, L.; Abdelkafi, S.; Fendri, I. Molecular and enzymatic responses of Chlorococcum dorsiventrale to heavy metal exposure: Implications for their removal. Appl. Sci. 2024, 14, 8551. [Google Scholar] [CrossRef]
- Baccari, O.; Elleuch, J.; Barkallah, M.; Boukedi, H.; Ayed, N.B.; Hammami, A.; Fendri, I.; Abdelkafi, S. Development of a new TaqMan-based PCR assay for the specific detection and quantification of Simkania negevensis. Mol. Cell. Probes 2020, 53, 101645. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, J.; Ben Amor, F.; Barkallah, M.; Haj Salah, J.; Smith, K.F.; Aleya, L.; Fendri, I.; Abdelkafi, S. q-PCR-based assay for the toxic dinoflagellate Karenia selliformis monitoring along the Tunisian coasts. Environ. Sci. Pollut. Res. 2021, 28, 57486–57498. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Rodríguez, M.A.; Suarez, M.; Bañares-Muñoz, M.A.; de Dios Lopez-Gonzalez, J. Comparative FT-IR study of the removal of octahedral cations and structural modifications during acid treatment of several silicates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1996, 52, 1685–1694. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Mehdipour Moghaddam, M.J.; Salavatiyan, T. Synthesis and characterization of CuO-montmorillonite nanocomposite by thermal decomposition method and antibacterial activity of nanocomposite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 125, 73–78. [Google Scholar] [CrossRef]
- Yu, L.; He, T.; Yao, J.; Xu, W.; Peng, S.; Feng, P.; Shuai, C. Cu ions and cetyltrimethylammonium bromide loaded into montmorillonite: A synergistic antibacterial system for bone scaffolds. Mater. Chem. Front. 2022, 6, 103–116. [Google Scholar] [CrossRef]
- Kamp, J.; Kraume, M. Coalescence efficiency model including electrostatic interactions in liquid/liquid dispersions. Chem. Eng. Sci. 2015, 126, 132–142. [Google Scholar] [CrossRef]
- Pham, T.-L. Effect of silver nanoparticles on tropical freshwater and marine microalgae. J. Chem. 2019, 2019, e9658386. [Google Scholar] [CrossRef]
- Książyk, M.; Asztemborska, M.; Stęborowski, R.; Bystrzejewska-Piotrowska, G. Toxic effect of silver and platinum nanoparticles toward the freshwater microalga Pseudokirchneriella subcapitata. Bull. Environ. Contam. Toxicol. 2015, 94, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Costa, E.; Piazza, V.; Fabbrocini, A.; Magi, E.; Faimali, M.; Garaventa, F. Effect of silver nanoparticles on marine organisms belonging to different trophic levels. Mar. Environ. Res. 2015, 111, 41–49. [Google Scholar] [CrossRef]
- Ha, M.K.; Shim, Y.J.; Yoon, T.H. Effects of agglomeration on in vitro dosimetry and cellular association of silver nanoparticles. Environ. Sci. Nano 2018, 5, 446–455. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, W.; Qiao, K.; Feng, J.; Zhu, L.; Zhu, X. Bioavailability and toxicity of silver nanoparticles: Determination based on toxicokinetic-toxicodynamic processes. Water Res. 2021, 204, 117603. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Judd, L.W.; Howe, E.N.W.; Withecombe, A.M.; Soto-Cerrato, V.; Li, H.; Busschaert, N.; Valkenier, H.; Pérez-Tomás, R.; Sheppard, D.N.; et al. Nonprotonophoric Electrogenic Cl−Transport Mediated by Valinomycin-like Carriers. Chem 2016, 1, 127–146. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Strużyński, W.; Strużyńska, L. Prolonged Exposure to Silver Nanoparticles Results in Oxidative Stress in Cerebral Myelin. Neurotox Res. 2019, 35, 495–504. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Rogers, N.J.; Franklin, N.M.; Apte, S.C.; Batley, G.E.; Angel, B.M.; Lead, J.R.; Baalousha, M. Physico-chemical behaviour and algal toxicity of nanoparticulate CeO2 in freshwater. Environ. Chem. 2010, 7, 50. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity Assessments of Multisized Gold and Silver Nanoparticles in Zebrafish Embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Samei, M.; Sarrafzadeh, M.-H.; Faramarzi, M.A. The impact of morphology and size of zinc oxide nanoparticles on its toxicity to the freshwater microalga, Raphidocelis subcapitata. Environ. Sci. Pollut. Res. Int. 2019, 26, 2409–2420. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Engineered nanoparticles in aquatic systems: Toxicity and mechanism of toxicity in fish. Emerg. Contam. 2023, 9, 100212. [Google Scholar] [CrossRef]
- Osterheld, K.; Millour, M.; Pelletier, E.; Magesky, A.; Doiron, K.; Lemarchand, K.; Gagné, J.-P. Nanotoxicity of Silver Nanoparticles: From Environmental Spill to Effects on Organisms. In Nanotoxicology: From Environmental Spill to Effects on Organisms; CRC Press: Boca Raton, FL, USA, 2018; pp. 191–240. [Google Scholar] [CrossRef]
- Dedman, C.J.; Newson, G.C.; Davies, G.-L.; Christie-Oleza, J.A. Mechanisms of silver nanoparticle toxicity on the marine cyanobacterium Prochlorococcus under environmentally-relevant conditions. Sci. Total Environ. 2020, 747, 141229. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Hao, Z.; Fadare, O.O.; Hanachi, P.; Chen, Y.; Liu, J. Toxicity of biosynthesized silver nanoparticles to aquatic organisms of different trophic levels. Chemosphere 2020, 258, 127346. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.; Patiño-Ruiz, D.; Tejeda-Benitez, L.; Herrera, A. Metal-and metal/oxide-based engineered nanoparticles and nanostructures: A review on the applications, nanotoxicological effects, and risk control strategies. Environ. Sci. Pollut. Res. 2021, 28, 16962–16981. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.R.R.; Bernardes, L.E.; Barbetta, M.F.S.; Da Veiga, M.A.M.S. Iron oxide nanoparticle phytotoxicity to the aquatic plant Lemna minor: Effect on reactive oxygen species (ROS) production and chlorophyll a/chlorophyll b ratio. Environ. Sci. Pollut. Res. 2019, 26, 24121–24131. [Google Scholar] [CrossRef]
- Talebzadeh, F.; Valeo, C. Evaluating the Effects of Environmental Stress on Leaf Chlorophyll Content as an Index for Tree Health. IOP Conf. Ser. Earth Environ. Sci. 2022, 1006, 012007. [Google Scholar] [CrossRef]
- Busch, A.W.U.; Montgomery, B.L. Interdependence of tetrapyrrole metabolism, the generation of oxidative stress and the mitigative oxidative stress response. Redox Biol. 2015, 4, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Millour, M.; Gagné, J.-P.; Doiron, K.; Lemarchand, K.; Pelletier, É. Silver nanoparticles aggregative behavior at low concentrations in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125191. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef]
- Dong, F.; Zhou, Y. Distinct mechanisms in the heteroaggregation of silver nanoparticles with mineral and microbial colloids. Water Res. 2020, 170, 115332. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Zakupszky, D.; Boka, E.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Vágvölgyi, C.; Kiricsi, M.; Kónya, Z. Are Smaller Nanoparticles Always Better? Understanding the Biological Effect of Size-Dependent Silver Nanoparticle Aggregation Under Biorelevant Conditions. Int. J. Nanomed. 2011, 16, 3021–3040. [Google Scholar] [CrossRef] [PubMed]
- Nazari, F.; Movafeghi, A.; Jafarirad, S.; Kosari-Nasab, M.; Divband, B. Synthesis of Reduced Graphene Oxide-Silver Nanocomposites and Assessing Their Toxicity on the Green Microalga Chlorella vulgaris. BioNanoScience 2018, 8, 997–1007. [Google Scholar] [CrossRef]
- Soto, K.F.; Carrasco, A.; Powell, T.G.; Garza, K.M.; Murr, L.E. Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. J. Nanopart. Res. 2005, 7, 145–169. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interac. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular Redox Compartmentation and ROS-Related Communication in Regulation and Signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef]
- Yin, J.; Dong, Z.; Liu, Y.; Wang, H.; Li, A.; Zhuo, Z.; Feng, W.; Fan, W. Toxicity of reduced graphene oxide modified by metals in microalgae: Effect of the surface properties of algal cells and nanomaterials. Carbon 2020, 169, 182–192. [Google Scholar] [CrossRef]
- Bensoltane, S.; Djekoun, M. Oxidative stress and histological changes induced by the nano-Fe2O3 in Helix aspersa. Sci. Study Res. Chem. Chem. Eng. 2021, 20, 119–133. [Google Scholar]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.H.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc Oxide Nanoparticles Boost Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Pantelica, A.; Demcak, S.; Mitu, A.; Apostol, A. Lithium biosorption by Arthrospira (Spirulina) platensis biomass. Ecol. Chem. Eng. 2020, 27, 271–280. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ravindran, B.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Phukan, M.M.; Chutia, R.S.; Konwar, B.K.; Kataki, R. Microalgae Chlorella as a potential bio-energy feedstock. Appl. Energy 2011, 88, 3307–3312. [Google Scholar] [CrossRef]
- Bataller, B.G.; Capareda, S.C. A rapid and non-destructive method for quantifying biomolecules in Spirulina platensis via Fourier transform infrared–Attenuated total reflectance spectroscopy. Algal Res. 2018, 32, 341–352. [Google Scholar] [CrossRef]
- Sukarni; Sudjito; Hamidi, N.; Yanuhar, U.; Wardana, I.N.G. Potential and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. Int. J. Energy Environ. Eng. 2014, 5, 279–290. [Google Scholar] [CrossRef]
- Fazelian, N.; Movafeghi, A.; Yousefzadi, M.; Rahimzadeh, M.; Zarei, M. Impact of silver nanoparticles on the growth, fatty acid profile, and antioxidative response of Nannochloropsis oculata. Acta Physiol. Plant. 2020, 42, 126. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; De Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011, 2, 326–333. [Google Scholar] [CrossRef]
- Liang, S.X.T.; Djearamane, S.; Tanislaus Antony Dhanapal, A.C.; Wong, L.S. Impact of silver nanoparticles on the nutritional properties of Arthrospira platensis. PeerJ 2022, 10, e13972. [Google Scholar] [CrossRef] [PubMed]
- Gnanakani, P.E.; Santhanam, P.; Premkumar, K.; Kumar, K.E.; Dhanaraju, M.D. Nannochloropsis Extract–Mediated Synthesis of Biogenic Silver Nanoparticles, Characterization and in vitro Assessment of Antimicrobial, Antioxidant and Cytotoxic Activities. Asian Pac. J. Cancer Prev. 2019, 20, 2353–2364. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.-Z.; Sun, X.-Y.; Bhadja, P.; Yao, X.-Q.; Ouyang, J. Reinjury risk of nano-calcium oxalate monohydrate and calcium oxalate dihydrate crystals on injured renal epithelial cells: Aggravation of crystal adhesion and aggregation. Int. J. Nanomed. 2016, 11, 2839. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, J.; Yi, J. Impact of silver nanoparticles on marine diatom Skeletonema costatum. J. Appl. Toxicol. 2016, 36, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Behzadi Tayemeh, M.; Esmailbeigi, M.; Shirdel, I.; Joo, H.S.; Johari, S.A.; Banan, A.; Nourani, H.; Mashhadi, H.; Jami, M.J.; Tabarrok, M. Perturbation of fatty acid composition, pigments, and growth indices of Chlorella vulgaris in response to silver ions and nanoparticles: A new holistic understanding of hidden ecotoxicological aspect of pollutants. Chemosphere 2020, 238, 124576. [Google Scholar] [CrossRef]
- Tikhonov, A.N. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 2020, 81, 163–183. [Google Scholar] [CrossRef]
- Shayan, S.; Norouzi, M.; Moghaddam Vahed, M.; Mohammadi, S.A.; Toorchi, M. Leaf proteome pattern of two bread wheat varieties under water deficit stress conditions. Curr. Plant Biol. 2020, 23, 100146. [Google Scholar] [CrossRef]
- Ren, S.; Ma, K.; Lu, Z.; Chen, G.; Cui, J.; Tong, P.; Wang, L.; Teng, N.; Jin, B. Transcriptomic and Metabolomic Analysis of the Heat-Stress Response of Populus tomentosa Carr. Forests 2019, 10, 383. [Google Scholar] [CrossRef]
- Alafari, H.A.; Abd-Elgawad, M.E. Differential expression gene/protein contribute to heat stress-responsive in Tetraena propinqua in Saudi Arabia. Saudi J. Biol. Sci. 2021, 28, 5017–5027. [Google Scholar] [CrossRef]
- Guan, X.; Tang, Y.; Zha, S.; Han, Y.; Shi, W.; Ren, P.; Yan, M.; Pan, Q.; Hu, Y.; Fang, J.; et al. Exogenous Ca2+ mitigates the toxic effects of TiO2 nanoparticles on phagocytosis, cell viability, and apoptosis in haemocytes of a marine bivalve mollusk, Tegillarca granosa. Environ. Poll. 2019, 252, 1764–1771. [Google Scholar] [CrossRef]
- Kulasza, M.; Skuza, L. Changes of Gene Expression Patterns from Aquatic Organisms Exposed to Metal Nanoparticles. Int. J. Environ. Res. Public Health 2021, 18, 8361. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xian, J.-A.; Li, B.; Ye, C.-X.; Wang, A.-L.; Miao, Y.-T.; Liao, S.-A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2013, 157, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Jan, C.-R.; Su, J.-A.; Teng, C.-C.; Sheu, M.-L.; Lin, P.-Y.; Chi, M.-C.; Chang, C.-H.; Liao, W.C.; Kuo, C.-C.; Chou, C.-T. Mechanism of maprotiline-induced apoptosis: Role of [Ca2+]i, ERK, JNK and caspase-3 signaling pathways. Toxicology 2013, 304, 1–12. [Google Scholar] [CrossRef]
- Al-Azab, A.J.S.; Aoki, Y.; Sarian, F.D.; Sori, Y.; Widyaningrum, D.; Yamasaki, T.; Kong, F.; Ohama, T. Chronological transcriptome changes induced by exposure to cyanoacrylate resin nanoparticles in Chlamydomonas reinhardtii with a focus on ROS development and cell wall lysis-related genes. Algal Res. 2022, 68, 102884. [Google Scholar] [CrossRef]
- Abideen, Z.; Hanif, M.; Munir, N.; Nielsen, B.L. Impact of Nanomaterials on the Regulation of Gene Expression and Metabolomics of Plants under Salt Stress. Plants 2022, 11, 691. [Google Scholar] [CrossRef] [PubMed]


| Metabolic Pathways | Target Genes | Primer Sequences | References |
|---|---|---|---|
| Oxidative stress | Caspase (Casp) | F: 5′-GCAAGAAGGCTGTCCTCATC-3′ R: 5′-GCTCTTGTCCGTGTCAATCA-3′ | [43,44] |
| Superoxide dismutase (SOD) | F: 5′-ATGAACATCCACCACACCAA-3′ R: 5′-CCTTCCAGAAGAAGCTGTGG-3′ | ||
| Peroxidase (PerchL) | F: 5′-CAGCTTTCCTGCAGACCTTT-3′ R: 5′-TCCACAAACTCCTCCTCCAC-3′ | ||
| Photosynthesis | Cytochrome b(N-terminal)/b6 (petB) | F: 5′-GGATTGCATCAGGAACACCT-3′ R: 5′-AGGTGGTTTCAAACGTCCAG-3′ | [44] |
| Ribulose bisphosphate carboxylase, large chain (rbcL) | F: 5′-AGCTTCAGCAACGAAAAGGA-3′ R: 5′-ATTCGTAGGTCCTCCACACG-3′ | ||
| P700 chlorophyll a apoproteins of the Photosystem I complex (psbL) | F: 5′-ATGGCTAGACCAAATCCAAA-3′ R: 5′-TAGAGAAAAGAACAGCTAATACGAAAA-3′ | ||
| Astaxanthin biosynthesis | β-carotene hydrolase (Q2 CHY) | F: 5′-GAGCTCAACGACATCTACGC -3′ R: 5′-TTGGTGTGGTGGATCTGATG -3′ | [43] |
| β-carotene ketolase (Q2 BKT) | F: 5′-TACCACTTCGACCTGCACTG-3′ R: 5′-GAGGCGGAGGAAGCTGAC-3′ | ||
| Carbohydrate biosynthesis | GDP mannose 3-5 epimerase galactose (ME Q2) | F: 5′-GTCCTTCGACGACAAGAAGC-3′ R: 5′-TGCTGTGGCTGTACTTGGTT-3′ | [43] |
| UDP-glucoronate decarboxylase xylose (GDQ1) | F: 5′-GTGACTACCTGGTGGCTCGT-3′ R: 5′-GATTTGGTCCACCTCCAAGA-3′ | ||
| Housekeeping gene | β-tubulin | F: 5′-TGTACGACATCTGCTTCCGC-3′ R: 5′-AGCCGACCATGAAGAAGTGC-3′ | [45] |
| Nanomaterials | Main Size Average (nm) | Standard Deviation (nm) | PDI |
|---|---|---|---|
| Ben | 154.1 | 63.9 | 0.571 |
| Ben-Ag | 791.8 | 172.5 | 0.467 |
| Ben-Ag (H2) | 160.9 | 45.65 | 0.695 |
| Bentonite | Ben-Ag | Ben-Ag (H2) | |
|---|---|---|---|
| C (%) | 0.23 | 0.17 | 0.24 |
| H (%) | 1.54 | 1.18 | 0.97 |
| N (%) | 0 | 1.22 | 0.94 |
| Culture Condition | ||||
|---|---|---|---|---|
| Control (%) | 0.5 IC50 Ben-Ag (%) | 1.5 IC50 Ben-Ag (%) | 2 IC50 Ben-Ag (%) | |
| Myristic acid (C14:0) | 4.07 | 2.70 | 2.51 | 2.71 |
| Palmitic acid (C16:0) | 24.10 | 19.65 | 26.77 | 13.70 |
| Hepthadecanoic acid (C17:0) | 10.94 | 16.41 | 8.85 | 26.01 |
| Stearic acid (C18:0) | 5.81 | 11.11 | 10.77 | 4.73 |
| SFAs | 44.92 | 49.87 | 48.9 | 47.15 |
| Palmitoleic acid (C16:1) | 28.11 | 26.49 | 14.55 | 23.42 |
| Hepthadecenoic acid (C17:1) | 2.06 | 2.36 | 1.14 | 3.25 |
| Oleic acid (C18:1) | 12.01 | 7.10 | 24.69 | 6.02 |
| Gadoleic acid (C20:1) | 3.58 | 1.45 | 1.07 | 1.93 |
| MUFAs | 45.76 | 37.4 | 41.45 | 34.62 |
| Linoleic acid (C18:2) | 3.86 | 2.25 | 5.37 | 2.10 |
| Linolenic acid (C18:3) | 5.46 | 10.51 | 4.28 | 16.13 |
| PUFAs | 9.32 | 12.46 | 9.65 | 18.23 |
| UFAs | 55.08 | 49.86 | 51.1 | 52.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghariani, O.; Elleuch, J.; Ferretti, A.M.; Econdi, S.; Bisio, C.; Michaud, P.; Fendri, I.; Guidotti, M.; Abdelkafi, S. Toxicological Effects of Silver-Modified Bentonite Nanocomposites on Microalgae: Impact on Cell Growth, Antioxidant Enzymes, and Gene Expression. Nanomaterials 2025, 15, 629. https://doi.org/10.3390/nano15080629
Ghariani O, Elleuch J, Ferretti AM, Econdi S, Bisio C, Michaud P, Fendri I, Guidotti M, Abdelkafi S. Toxicological Effects of Silver-Modified Bentonite Nanocomposites on Microalgae: Impact on Cell Growth, Antioxidant Enzymes, and Gene Expression. Nanomaterials. 2025; 15(8):629. https://doi.org/10.3390/nano15080629
Chicago/Turabian StyleGhariani, Oumayma, Jihen Elleuch, Anna Maria Ferretti, Stefano Econdi, Chiara Bisio, Philippe Michaud, Imen Fendri, Matteo Guidotti, and Slim Abdelkafi. 2025. "Toxicological Effects of Silver-Modified Bentonite Nanocomposites on Microalgae: Impact on Cell Growth, Antioxidant Enzymes, and Gene Expression" Nanomaterials 15, no. 8: 629. https://doi.org/10.3390/nano15080629
APA StyleGhariani, O., Elleuch, J., Ferretti, A. M., Econdi, S., Bisio, C., Michaud, P., Fendri, I., Guidotti, M., & Abdelkafi, S. (2025). Toxicological Effects of Silver-Modified Bentonite Nanocomposites on Microalgae: Impact on Cell Growth, Antioxidant Enzymes, and Gene Expression. Nanomaterials, 15(8), 629. https://doi.org/10.3390/nano15080629












