First-Principles Study on the CO2 Reduction Reaction (CO2RR) Performance of h-BN-Based Single-Atom Catalysts Modified with Transition Metals
Abstract
1. Introduction
2. Materials and Methods
3. Results
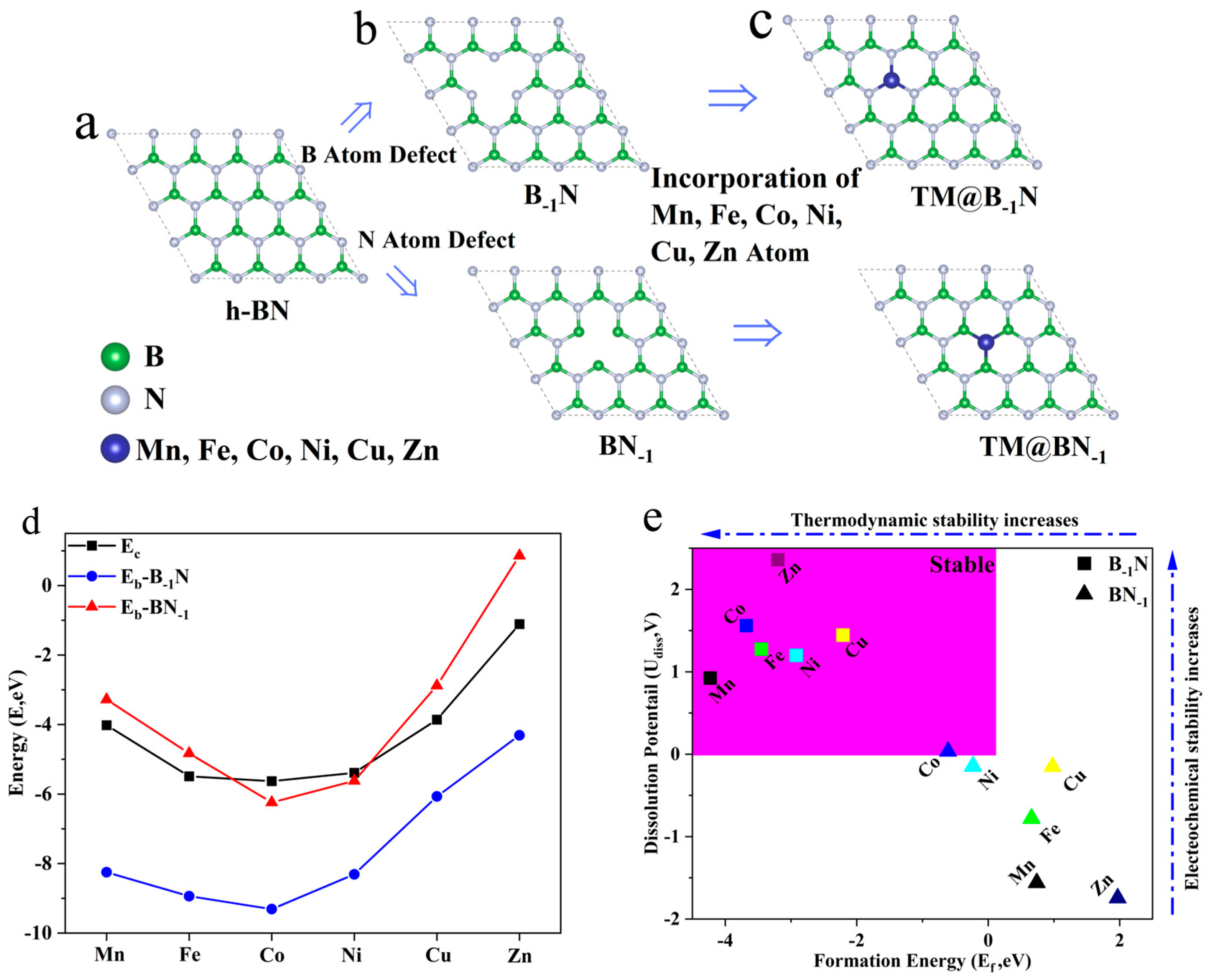
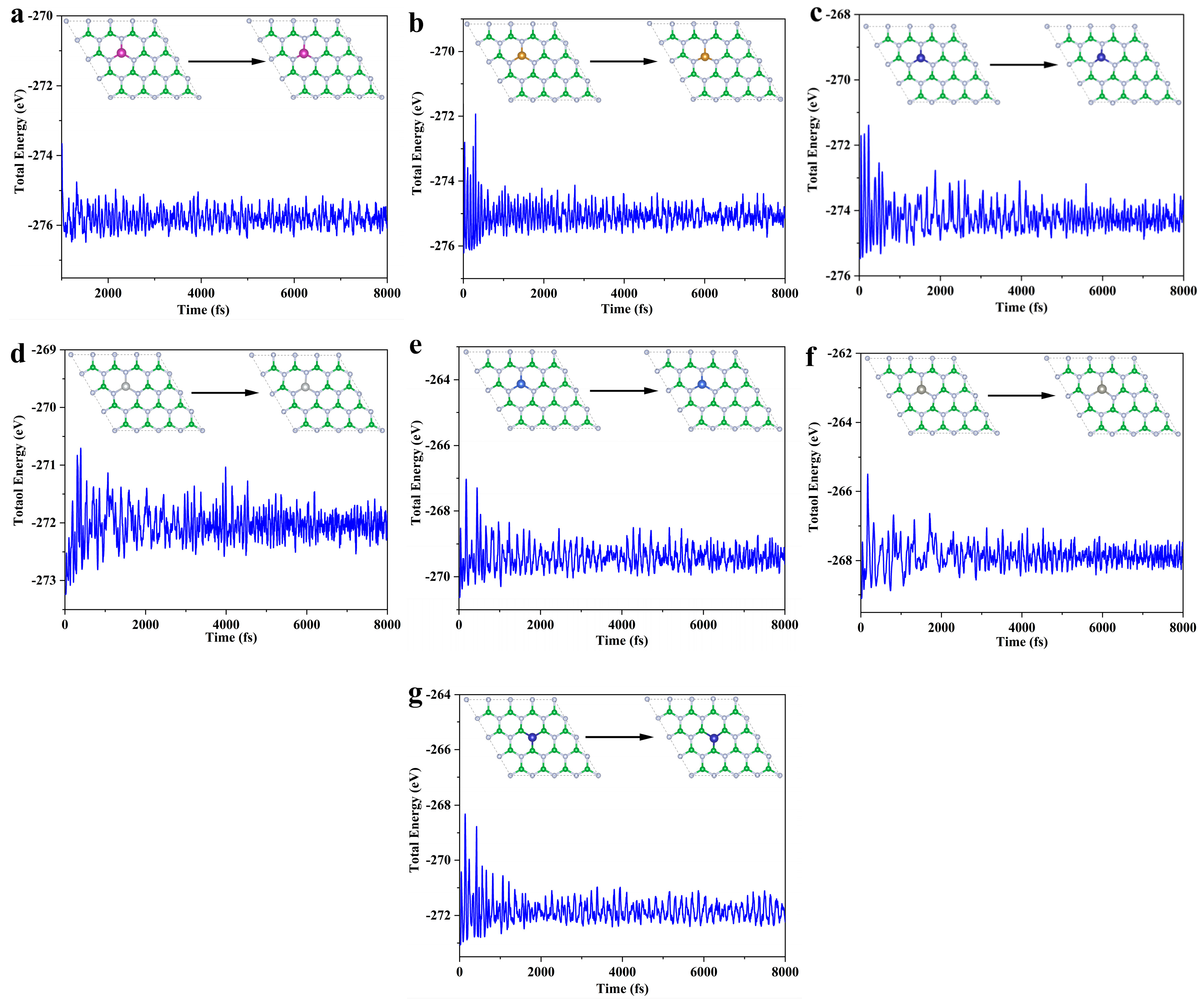
3.1. Subsection
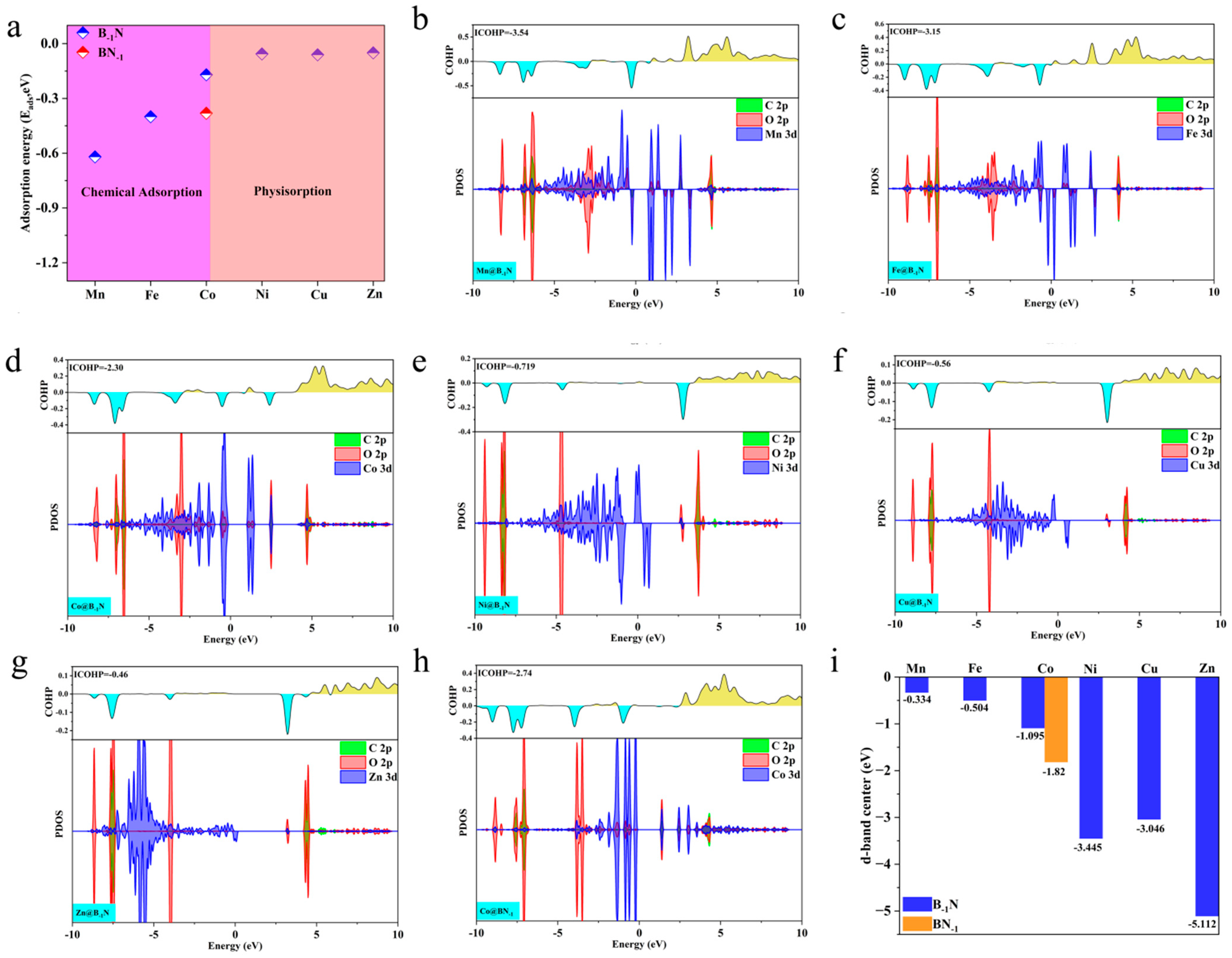
3.2. CO2 Adsorption and Activation
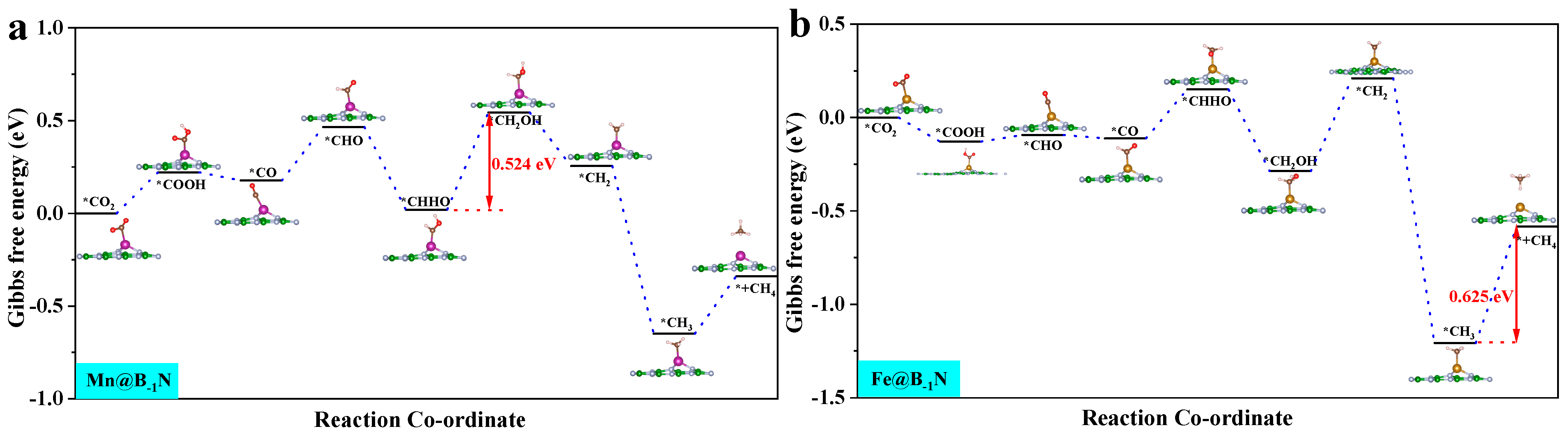
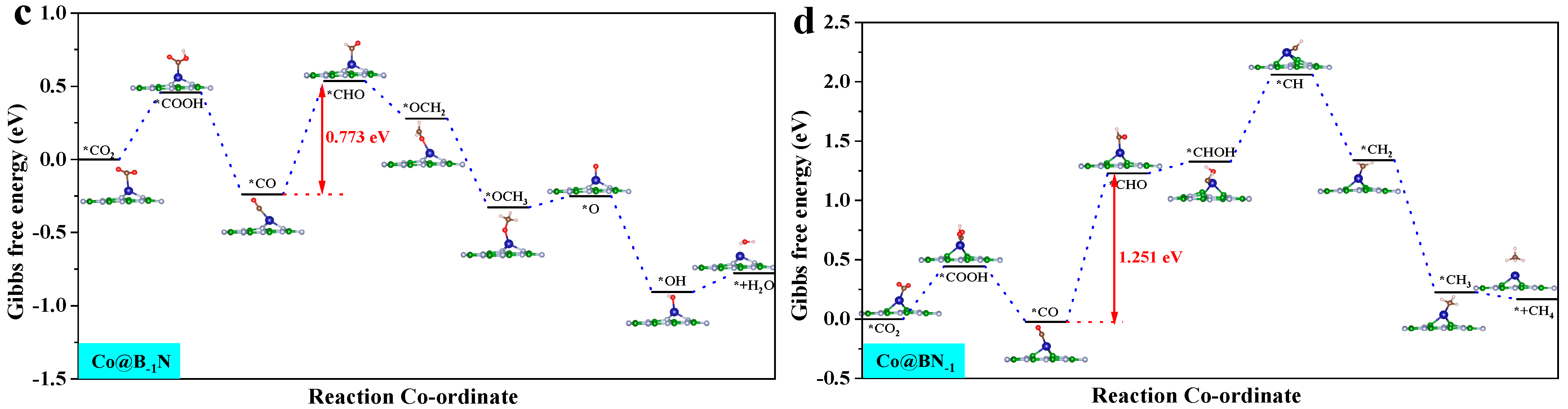
3.3. CO2 Reduction Reaction (CO2RR) Performance
3.4. Competition Between CO2RR and HER
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Li, C.W.; Kanan, M.W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 19969–19972. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shui, J.; Jia, Y.; Zhang, H.; Cen, W.; Tang, S.; Han, Y. Design principles of two-dimentional transition metal carbides based CO2RR catalysts. Chem. Eng. J. 2025, 507, 160716. [Google Scholar] [CrossRef]
- Tu, Q.; Parvatker, A.; Garedew, M.; Harris, C.; Eckelman, M.; Zimmerman, J.B.; Anastas, P.T.; Lam, C.H. Electrocatalysis for chemical and fuel production: Investigating climate change mitigation potential and economic feasibility. Environ. Sci. Technol. 2021, 55, 3240–3249. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, T.; Zhang, Y.; Hu, J. Synergistic modulation of non-precious-metal electrocatalysts for advanced water splitting. Acc. Chem. Res. 2020, 53, 1111–1123. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Hu, B.; Li, Z.; Wang, B.; Chen, L.; Wang, X.; Hu, X.; Bai, Z.; Li, Y.; Chen, G.; Luo, X. Construction of Co-In dual single-atom catalysts for photocatalytic CO2 reduction into CH4. Appl. Catal. B Environ. Energy 2025, 371, 125196. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Dai, L.; Li, B.; Li, Z. High-curvature single-atom catalysts for electrocatalysis: A review. Appl. Catal. A: General. 2025, 694, 120160. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Li, H.; Tian, F.; Li, D.; Cui, T. Unraveling electrochemical CO reduction of the single-atom transition metals supported on N-doped phosphorene. Appl. Surf. Sci. 2021, 545, 148953. [Google Scholar] [CrossRef]
- Fan, J.; Yang, L.; Zhu, W. Transition metal-anchored BN tubes as single-atom catalysts for NO reduction reaction: A study of DFT and deep learning. Fuel 2025, 386, 134302. [Google Scholar] [CrossRef]
- Tang, T.; Bai, X.; Wang, Z.; Guan, J. Structural engineering of atomic catalysts for electrocatalysis. Chem. Sci. 2024, 15, 5082–5112. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Zhang, H.; Lv, H.; Li, Q.; Michalsky, R.; Peterson, A.A.; Sun, S. Active and selective conversion of CO2 to CO on ultrathin Au nanowires. J. Am. Chem. Soc. 2014, 136, 16132–16135. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Z. Platinum single-atom catalysts: A comparative review towards effective characterization. Catal. Sci. Technol. 2019, 9, 4821–4834. [Google Scholar] [CrossRef]
- Liu, X.; Xu, M.; Wan, L.; Zhu, H.; Yao, K.; Linguerri, R.; Chambaud, G.; Han, Y.; Meng, C. Superior catalytic performance of atomically dispersed palladium on graphene in CO oxidation. Acs Catal. 2020, 10, 3084–3093. [Google Scholar] [CrossRef]
- Tanna, H.P.; Baraiya, B.A.; Jha, P.K. Co Implanted Ψ-graphene: A Non-Noble Metal Single-Atom Catalyst for Proficient CO Oxidation Reaction. Mol. Catal. 2024, 556, 113907. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Chang, B.; Wu, S.; Wang, Y.; Sun, T.; Cheng, Z. Emerging single-atom iron catalysts for advanced catalytic systems. Nanoscale Horiz. 2022, 7, 1340–1387. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Q.; Fu, Y.; Lei, C.; Yang, B.; Li, Z.; Lei, L.; Wu, G.; Hou, Y. Single Atom Electrocatalysts: Carbon-Rich Nonprecious Metal Single Atom Electrocatalysts for CO2 Reduction and Hydrogen Evolution. Small Methods 2019, 3, 1970033. [Google Scholar] [CrossRef]
- Ju, L.; Tan, X.; Mao, X.; Gu, Y.; Smith, S.; Du, A.; Chen, Z.; Chen, C.; Kou, L. Controllable CO2 electrocatalytic reduction via ferroelectric switching on single atom anchored In2Se3 monolayer. Nat. Commun. 2021, 12, 5128. [Google Scholar] [CrossRef]
- Liu, J.; Kong, X.; Zheng, L.; Guo, X.; Liu, X.; Shui, J. Rare earth single-atom catalysts for nitrogen and carbon dioxide reduction. Acs Nano 2020, 14, 1093–1101. [Google Scholar] [CrossRef]
- Han, Q.; Chen, N.; Zhang, J.; Qu, L. Graphene/graphitic carbon nitride hybrids for catalysis. Mater. Horiz. 2017, 4, 832–850. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, H.; Abbas, Q.; Liu, J.; Shi, J.; Xia, X.; Zhang, H.; Du, G. Growth and characterization of porous sp2-BN films with hollow spheres under hydrogen etching effect via borazane thermal CVD. Appl. Surf. Sci. 2018, 452, 314–321. [Google Scholar] [CrossRef]
- Kawrani, S.; Nada, A.A.; Bekheet, M.F.; Boulos, M.; Viter, R.; Roualdes, S.; Miele, P.; Cornu, D.; Bechelany, M. Enhancement of calcium copper titanium oxide photoelectrochemical performance using boron nitride nanosheets. Chem. Eng. J. 2020, 389, 124326. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, X.; Wang, J.; Shang, N.; Feng, T.; Gao, Z.; Zhang, H.; Ding, X.; Gao, S.; Feng, C. Boron nitride supported NiCoP nanoparticles as noble metal-free catalyst for highly efficient hydrogen generation from ammonia borane. Int. J. Hydrogen Energ. 2019, 44, 4764–4770. [Google Scholar] [CrossRef]
- Ngome Okello, O.F.; Doh, K.; Kang, H.S.; Song, K.; Kim, Y.; Kim, K.H.; Lee, D.; Choi, S. Visualization of Transition Metal Decoration on h-BN surface. Nano Lett. 2021, 21, 10562–10569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, J.; Li, P.; Zhao, G.; Wang, G.; Jiang, Y.; Chen, J.; Dou, S.X.; Pan, H.; Sun, W. Hexagonal boron nitride as a multifunctional support for engineering efficient electrocatalysts toward the oxygen reduction reaction. Nano Lett. 2020, 20, 6807–6814. [Google Scholar] [CrossRef]
- Li, D.; Li, W.; Zhang, J. Fe doped BN monolayer: A promising low-cost single atom catalyst for promoted CO oxidation activity. Appl. Surf. Sci. 2020, 525, 146567. [Google Scholar] [CrossRef]
- Wang, S.; Xin, Y.; Yuan, J.; Wang, L.; Zhang, W. Direct conversion of methane to methanol on boron nitride-supported copper single atoms. Nanoscale 2022, 14, 5447–5453. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: A computational study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef]
- Konopatsky, A.; Kustov, A.; Evdokimenko, N.; Shesterkina, A.; Varlamova, L.A.; Teplyakova, T.; Leybo, D.; Antipina, L.Y.; Sorokin, P.B.; Fang, X. Layered Ferrihydrite and BN Nanoparticle Heterostructures Doped with Ag for CO2 Hydrogenation. ACS Appl. Nano Mater. 2024, 7, 10257–10267. [Google Scholar] [CrossRef]
- Wong, H.; Tang, T.W.; Chen, H.; Xu, M.; Wang, J.; Cai, Y.; Goddard, W.A.; Luo, Z. Dual role of h-BN as an artificial solid-electrolyte interface layer for safe zinc metal anodes. J. Mater. Chem. A 2024, 12, 4195–4203. [Google Scholar] [CrossRef]
- Lu, Y.; Li, B.; Xu, N.; Zhou, Z.; Xiao, Y.; Jiang, Y.; Li, T.; Hu, S.; Gong, Y.; Cao, Y. One-atom-thick hexagonal boron nitride co-catalyst for enhanced oxygen evolution reactions. Nat. Commun. 2023, 14, 6965. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wu, D.; Su, Y. The effect of grain boundary in hexagonal boron nitride on catalytic activity of nitrogen reduction reaction. Appl. Surf. Sci. 2022, 593, 153468. [Google Scholar] [CrossRef]
- Li, Y.; Gao, D.; Zhao, S.; Xiao, Y.; Guo, Z.; Fang, Y.; Lin, J.; Liu, Z.; Huang, Y.; Guo, K. Carbon doped hexagonal boron nitride nanoribbon as efficient metal-free electrochemical nitrogen reduction catalyst. Chem. Eng. J. 2021, 410, 128419. [Google Scholar] [CrossRef]
- Leybo, D.; Firestein, K.L.; Evdokimenko, N.D.; Ryzhova, A.A.; Baidyshev, V.S.; Chepkasov, I.V.; Popov, Z.I.; Kustov, A.L.; Konopatsky, A.S.; Golberg, D.V. Ball-milled processed, selective Fe/h-BN nanocatalysts for CO2 hydrogenation. ACS Appl. Nano Mater. 2022, 5, 16475–16488. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, Y.; Kang, L.; Zhang, C.; Wang, D.; Wang, C.; Zhang, M.; Wu, X. First-principles investigation of hydrogen storage capacity of Y-decorated porous graphene. Appl. Surf. Sci. 2017, 399, 463–468. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Bader, R.F.; Nguyen-Dang, T.T. Quantum theory of atoms in molecules–Dalton revisited. Adv. Quantum Chem. 1981, 14, 63–124. [Google Scholar]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comp. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Wyckoff, R.W. Structure of Crystals; The Chemical Catalog Company, Inc.: New York, NY, USA, 1931. [Google Scholar]
- Sun, M.; Wang, S.; Du, Y.; Yu, J.; Tang, W. Transition metal doped arsenene: A first-principles study. Appl. Surf. Sci. 2016, 389, 594–600. [Google Scholar] [CrossRef]
- Kropp, T.; Lu, Z.; Li, Z.; Chin, Y.C.; Mavrikakis, M. Anionic single-atom catalysts for CO oxidation: Support-independent activity at low temperatures. Acs Catal. 2019, 9, 1595–1604. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, L.; Wang, Y.; Liu, J.; Muravev, V.; Alexopoulos, K.; Filot, I.A.; Vlachos, D.G.; Hensen, E.J. Stability of heterogeneous single-atom catalysts: A scaling law mapping thermodynamics to kinetics. Npj Comput. Mater. 2020, 6, 144. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K. Electrochemical dissolution of surface alloys in acids: Thermodynamic trends from first-principles calculations. Electrochim. Acta 2007, 52, 5829–5836. [Google Scholar] [CrossRef]
- Karmodak, N.; Vijay, S.; Kastlunger, G.; Chan, K. Computational screening of single and di-atom catalysts for electrochemical CO2 reduction. Acs Catal. 2022, 12, 4818–4824. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gu, J.; Lin, S.; Zhang, S.; Chen, Z.; Huang, S. Tackling the activity and selectivity challenges of electrocatalysts toward the nitrogen reduction reaction via atomically dispersed biatom catalysts. J. Am. Chem. Soc. 2020, 142, 5709–5721. [Google Scholar] [CrossRef]
- Ma, J.; Shang, S.; Kim, H.; Liu, Z. An ab initio molecular dynamics exploration of associates in Ba-Bi liquid with strong ordering trends. Acta Mater. 2020, 190, 81–92. [Google Scholar] [CrossRef]
- Lv, L.; Shen, Y.; Liu, J.; Meng, X.; Gao, X.; Zhou, M.; Zhang, Y.; Gong, D.; Zheng, Y.; Zhou, Z. Computational screening of high activity and selectivity TM/g-C3N4 single-atom catalysts for electrocatalytic reduction of nitrates to ammonia. J. Phys. Chem. Lett. 2021, 12, 11143–11150. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Shao, Y.; Zhou, S.; Wu, X.; Zhu, J.; Tang, D. Relationship between the electric structures calculated by the first principles calculation method and the photoelectrocatalysis degradation of Ir-doped SnO2 electrodes. Appl. Surf. Sci. 2017, 422, 891–899. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Lou, F.; Yu, Z. Theoretical study of single transition metal atom catalysts supported on two-dimensional Nb2NO2 for efficient electrochemical CO2 reduction to CH4. J. CO2 Util. 2022, 62, 102069. [Google Scholar] [CrossRef]
- Araujo, R.B.; Rodrigues, G.L.; Dos Santos, E.C.; Pettersson, L.G. Adsorption energies on transition metal surfaces: Towards an accurate and balanced description. Nat. Commun. 2022, 13, 6853. [Google Scholar] [CrossRef] [PubMed]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-Wave Based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
- Gao, Z.; Li, A.; Liu, X.; Ma, C.; Li, X.; Yang, W.; Ding, X. Density functional study of the adsorption of NO on Nin (n= 1, 2, 3 and 4) clusters doped functionalized graphene support. Appl. Surf. Sci. 2019, 481, 940–950. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, D.; Huang, B.; Xie, T.; Cai, L.; Hu, W. Highly effective Ru-based Heusler alloy catalysts for N2 activation: A theoretical study. Appl. Surf. Sci. 2022, 575, 151658. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Xu, C.; Li, Q.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Modulating the d-band centers by coordination environment regulation of single-atom Ni on porous carbon fibers for overall water splitting. Nano Energy 2022, 98, 107266. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Dong, S.; Li, Y.; Zhang, X.; Hu, H.; Yan, B.; Ren, J.; Wan, L.; Zhao, H.; Sun, F. Pt single-atom loaded on nonmetallic elements (C, N, P, S) doped ZrO2 in photocatalytic hydrogen evolution: First principles. Mater. Des. 2023, 231, 112068. [Google Scholar] [CrossRef]
- Bronsted, J.N. Acid and Basic Catalysis. Chem. Rev. 1928, 5, 231–338. [Google Scholar] [CrossRef]
- Wang, M.; Shi, H.; Tian, M.; Chen, R.; Shu, J.; Zhang, Q.; Wang, Y.; Li, C.; Wan, N.; Lei, S. Single nickel atom-modified phosphorene nanosheets for electrocatalytic CO2 reduction. ACS Appl. Nano Mater. 2021, 4, 11017–11030. [Google Scholar] [CrossRef]
- Lv, P.; Wu, D.; He, B.; Li, X.; Zhu, R.; Tang, G.; Lu, Z.; Ma, D.; Jia, Y. An efficient screening strategy towards multifunctional catalysts for the simultaneous electroreduction of NO3−, NO2− and NO to NH3. J. Mater. Chem. A 2022, 10, 9707–9716. [Google Scholar] [CrossRef]
- Xie, L.; Huang, W.; Chen, J.; Chen, H.; Hou, C.; Ni, Q.; Huang, T.; Gui, L.; Wang, X. Selective oxidation of β-keto ester modulated by the d-band centers in DA conjugated microporous metallaphotoredox catalysts containing M− salen (MZn, Cu and Co) and triazine monomers. J. Colloid. Interf. Sci. 2024, 665, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, H.; Chan, S.H.; Sun, Q. CO2 electroreduction performance of transition metal dimers supported on graphene: A theoretical study. Acs Catal. 2015, 5, 6658–6664. [Google Scholar] [CrossRef]
- Back, S.; Lim, J.; Kim, N.; Kim, Y.; Jung, Y. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 2017, 8, 1090–1096. [Google Scholar] [CrossRef] [PubMed]

| CO2 Adsorption | UL of CO2RR (V) | UL of HER (V) | d Band Center (eV) | ICOHP (eV) | Stability | CO2RR vs. HER | |
|---|---|---|---|---|---|---|---|
| Mn@B−1N | chemisorption | −0.524 | −3.631 | −0.334 | −3.54 | stable | CO2RR (16.4 times) |
| Fe@B−1N | chemisorption | −0.625 | −3.635 | −0.504 | −3.15 | stable | CO2RR |
| Co@B−1N | chemisorption | −0.773 | −0.721 | −1.095 | −2.30 | stable | CO2RR |
| Ni@B−1N | physisorption | / | −3.599 | −3.445 | −0.72 | stable | No CO2RR activity |
| Cu@B−1N | physisorption | / | −0.263 | −3.046 | −0.56 | stable | No CO2RR activity |
| Zn@B−1N | physisorption | / | −3.775 | −5.112 | −0.46 | stable | No CO2RR activity |
| Co@BN−1 | chemisorption | −1.251 | −0.217 | −1.82 | −2.74 | stable | HER |
| ΔG(*H)/eV | ΔG(*COOH)/eV | |
|---|---|---|
| Mn@B−1N | 3.631 | 0.221 |
| Fe@B−1N | 3.635 | −0.129 |
| Co@B−1N | 0.721 | 0.456 |
| Ni@B−1N | 3.599 | / |
| Cu@B−1N | −0.263 | / |
| Zn@B−1N | 3.775 | / |
| Co@BN−1 | 0.217 | 0.445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhao, C.; Chen, Q.; Wei, L.; Zhao, X.; Zhang, L.; Wu, L.; Huang, Y. First-Principles Study on the CO2 Reduction Reaction (CO2RR) Performance of h-BN-Based Single-Atom Catalysts Modified with Transition Metals. Nanomaterials 2025, 15, 628. https://doi.org/10.3390/nano15080628
Yu X, Zhao C, Chen Q, Wei L, Zhao X, Zhang L, Wu L, Huang Y. First-Principles Study on the CO2 Reduction Reaction (CO2RR) Performance of h-BN-Based Single-Atom Catalysts Modified with Transition Metals. Nanomaterials. 2025; 15(8):628. https://doi.org/10.3390/nano15080628
Chicago/Turabian StyleYu, Xiansheng, Can Zhao, Qiaoyue Chen, Lai Wei, Xucai Zhao, Lili Zhang, Liqian Wu, and Yineng Huang. 2025. "First-Principles Study on the CO2 Reduction Reaction (CO2RR) Performance of h-BN-Based Single-Atom Catalysts Modified with Transition Metals" Nanomaterials 15, no. 8: 628. https://doi.org/10.3390/nano15080628
APA StyleYu, X., Zhao, C., Chen, Q., Wei, L., Zhao, X., Zhang, L., Wu, L., & Huang, Y. (2025). First-Principles Study on the CO2 Reduction Reaction (CO2RR) Performance of h-BN-Based Single-Atom Catalysts Modified with Transition Metals. Nanomaterials, 15(8), 628. https://doi.org/10.3390/nano15080628







